The Water Cycle (Water Science for Schools)
The Water Cycle ¦ Water Science for Schools ¦ Contact us ¦ Back
 Sublimation is the conversion between the solid and the gaseous phases of matter, with no intermediate liquid stage. For those of us interested in the water cycle, sublimation is most often used to describe the process of snow and ice changing into water vapor in the air without first melting into water. The opposite of sublimation is "deposition", where water vapor changes directly into ice—such a snowflakes and frost.
Sublimation is the conversion between the solid and the gaseous phases of matter, with no intermediate liquid stage. For those of us interested in the water cycle, sublimation is most often used to describe the process of snow and ice changing into water vapor in the air without first melting into water. The opposite of sublimation is "deposition", where water vapor changes directly into ice—such a snowflakes and frost.
It is not easy to actually see sublimation occuring, at least not with ice. One way to see the results of sublimation is to hang a wet shirt outside on a below-freezing day. Eventually the ice in the shirt will disappear. Actually, the best way to visualize sublimation is to not use water at all, but to use carbon dioxide instead. If you don't know what I mean, then look at this picture of dry ice. "Dry ice" is actually solid, frozen carbon dioxide, which happens to sublimate, or turn to gas, at a chilly -78.5 °C (-109.3°F). The fog you see is actually a mixture of cold carbon dioxide gas and cold, humid air, created as the dry ice "melts" ... oops, I mean sublimates.
 Sublimation occurs more readily when certain weather conditions are present, such as low relative humidity and dry winds. Sublimation also occurs more at higher altitudes, where the air pressure is less than at lower altitudes. Energy, such as strong sunlight, is also needed. If I was to pick one place on Earth where sublimation happens a lot, I might choose the south face of Mt. Everest. Low temperatures, strong winds, intense sunlight, very low air pressure—just the recipe for sublimation to occur.
Sublimation occurs more readily when certain weather conditions are present, such as low relative humidity and dry winds. Sublimation also occurs more at higher altitudes, where the air pressure is less than at lower altitudes. Energy, such as strong sunlight, is also needed. If I was to pick one place on Earth where sublimation happens a lot, I might choose the south face of Mt. Everest. Low temperatures, strong winds, intense sunlight, very low air pressure—just the recipe for sublimation to occur.
Dave Thurlow of the Mount Washington Observatory offers a good explanation of sublimation in The Weather Notebook:
"There's more than one way for Mother Nature to get rid of a fresh blanket of snow. The most common way, of course, is by melting-which gives everyone the pleasure of trudging through slush, mud, and water. But in the western U.S., there's a wind called the Chinook, or "snow eater," that vaporizes snow before it even has a chance to melt."
"Chinook winds are westerlies from the Pacific whose moisture gets wrung out as it passes over the Rocky Mountains. Once these winds come down from the mountains onto the high plains, they can be quite mild and extremely dry-as warm as 60 or 70 degrees Fahrenheit -- over 15 Celsius -- with a relative humidity of 10% or less. The air is so dry that when it hits a snowpack, the frozen water evaporates, going directly from the ice to vapor and bypassing the liquid phase entirely. This is called sublimation, and it's a common way for snow to disappear in the arid West."
 Without the addition of energy (heat) to the process, ice would not sublimate into vapor. That is where sunlight plays a large role in the natural world. Water has a physical property called the "heat of vaporization," which is the amount of heat required to vaporize water. If you want an exact amount of heat, the heat of vaporization of water is 540 calories/gram, or 2,260 kilojoules/kilogram. That is a lot more energy than is needed to convert water to ice (the latent heat of fusion), which is 80 calories/gram. And, it is also about five times the energy needed for heating water from the freezing point to the boiling point. In summary, energy is needed for the sublimation of ice to vapor to occur, and most of the energy is needed in the vaporiazation phase. A cubic centimeter (1 gram) of water in ice form requires 80 calories to melt, 100 calories to rise to boiling point, and another 540 calories to vaporize, a total of 720 calories. Sublimation requires the same energy input, but bypasses the liquid phase.
Without the addition of energy (heat) to the process, ice would not sublimate into vapor. That is where sunlight plays a large role in the natural world. Water has a physical property called the "heat of vaporization," which is the amount of heat required to vaporize water. If you want an exact amount of heat, the heat of vaporization of water is 540 calories/gram, or 2,260 kilojoules/kilogram. That is a lot more energy than is needed to convert water to ice (the latent heat of fusion), which is 80 calories/gram. And, it is also about five times the energy needed for heating water from the freezing point to the boiling point. In summary, energy is needed for the sublimation of ice to vapor to occur, and most of the energy is needed in the vaporiazation phase. A cubic centimeter (1 gram) of water in ice form requires 80 calories to melt, 100 calories to rise to boiling point, and another 540 calories to vaporize, a total of 720 calories. Sublimation requires the same energy input, but bypasses the liquid phase.
![]() The Weather Notebook, Mount Washington Observatory
The Weather Notebook, Mount Washington Observatory
![]() Sublimation, Wikipedia, the free encyclopedia
Sublimation, Wikipedia, the free encyclopedia
![]() Dry ice, Wikipedia, the free encyclopedia
Dry ice, Wikipedia, the free encyclopedia
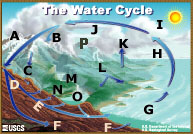 |
A - Storage in ice and snow B - Precipitation C - Snowmelt runoff to streams D - Infiltration E - Ground-water discharge F - Ground-water storage G - Water storage in oceans H - Evaporation |
I - Condensation J - Water storage in the atmosphere K - Evapotranspiration L - Surface runoff M - Streamflow N - Springs O - Freshwater storage P - Sublimation |