|
CDER 2007 Update
Generic Drug Review
Generic drugs are not required to repeat the extensive clinical trials used in the development of the original, brand-name drugs. For many products such as tablets and capsules, generics must show bioequivalence to the brand-name reference listed drug. This means that the generic version must deliver the same amount of active ingredient into a patient’s bloodstream over the same time period as the brand-name reference listed drug.
The rate and extent of absorption of a drug is called its bioavailability. The bioavailability of the generic drug is compared to that of the brand-name drug. If the bioavailability of the two is similar, the drugs are bioequivalent.
Brand-name drugs are subject to the same bioequivalency tests as generics when their manufacturers reformulate them.
Generic drug 2007 approvals
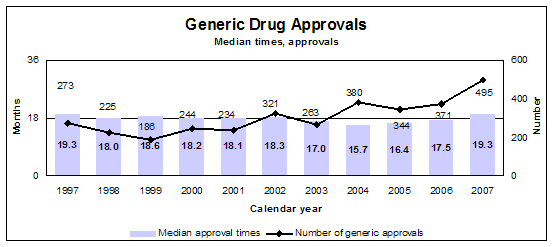
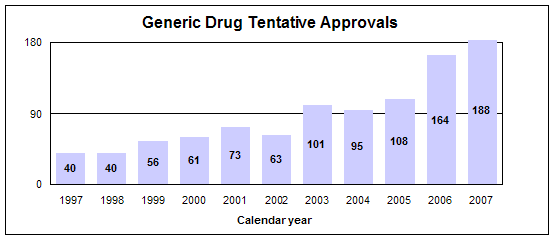
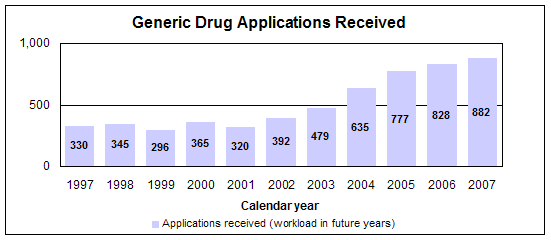
We had 683 approval actions with 495 fully approved and 188 tentatively approved generic drug products in calendar year 2007. This included a substantial number of products that represent the first time a generic drug was available for the brand-name product.
The median statistic for total approval time showed an increase this year due, in part, to the high numbers of new applications for the last two years. We have made several changes to improve the efficiency of our generic drug review process in order to try to keep up with the dramatic increase in applications. These efforts will continue.
- 495 generic drugs
- Median approval time 19.26 months
- 188 tentative approvals
- 882 receipts
Click image for larger view. Accessible text.
Tentative vs. full approval
The difference between a full approval and a tentative approval is that the final approval of these applications is delayed due to an existing patent or exclusivity on the innovator drug product. The FDA review of an application that is tentatively approved requires the same amount of work as a review that results in a full approval. Tentative approvals are displayed in our approvals chart only when they are converted to full approvals.
Tentative approvals key to affordable, worldwide AIDS relief
Tentative approval is a key regulatory mechanism to support the availability of drugs for the President’s Emergency Plan for AIDS Relief.
Click image for larger view. Accessible text.
Click image for larger view. Accessible text.
Notable 2007 generic drug approvals
- Alendronate tablets. Used to treat certain types of osteoporosis and Paget’s disease.
- Carvedilol tablets. A beta blocking agent used to treat congestive heart failure and hypertension.
- Cetirizine tablets. An antihistamine for treatment of various allergic conditions.
- Granisetron tablets. For the prevention and treatment of nausea or vomiting related to cancer therapy and postoperative nausea and vomiting.
- Irinotecan injection. Used for treatment of colon and rectal cancer.
- Zolpidem Tablets. Used for the short-term treatment of insomnia.
Generic drug review efficiencies
The dramatic increase of generic drug applications makes it imperative that we process applications more efficiently. Our steps to improve our processes and to improve the content and completeness of generic drug applications include:
- The Generic Initiative for Value and Efficiency which focuses on using existing resources to help FDA modernize and streamline the generic approval process.
- Question-based Review to assist sponsors in providing information that demonstrates their understanding of the manufacture of the product.
- Posting bioequivalence information, including data tables, information about laboratory tests and necessary studies.
- Focused hiring whichincrease staff in critical review components.
- Holding joint meetings and workshops with academia and industry to improve knowledge of the submission process and quality of applications.
- Encouraging electronic submission of applications.
Reducing hurdles to generic drug availability
We expedite the review of applications that, at the time of submission, represent the first generic application for an innovator product that had no patent or exclusivity protection.
We are working to implement recently passed legislation aimed at reducing certain delays in acting on generic drug applications.
Next Page
 Back
to Top
Back
to Top  CDER 2007 Update Table of Contents CDER 2007 Update Table of Contents
 PDF requires the free
Adobe Acrobat Reader PDF requires the free
Adobe Acrobat Reader
Date created: July 31, 2008 |