 |
CDER 2007 Update
Over-the-Counter Drug Review
How we regulate OTC drugs
Although many drugs are approved for OTC use through the new drug review process, other OTC medicines are regulated under the OTC drug review process. This process relies on published monographs created by public rule making. We publish monographs that establish acceptable ingredients, doses, formulations and consumer labeling for OTC drugs. Products that conform to a final monograph may be marketed without prior FDA clearance. FDA maintains an online library of all OTC monographs which can be found at http://www.fda.gov/cder/otcmonographs/rulemaking_index.htm.
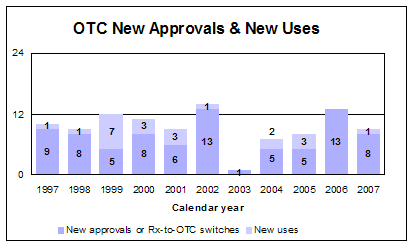
In 2007, we approved one new drug application for first-time over-the-counter sale, eight prescription-to-OTC switches and one new strength.
OTC approval statistics
- 1 first-time OTC
- 8 Rx-to-OTC switches
- 1 new strength
Click image for larger view. Accessible text.
First-time OTC:
- Orlistat 60 mg (Alli) capsules for weight loss in overweight adults, 18 years and older, when used along with a reduced-calorie, low-fat diet and exercise program.
Rx-to-OTC switches:
- Cetirizine HCl 5 mg/pseudoephedrine HCl 120 mg (Zyrtec-D) tablets for the temporary relief of symptoms of hay fever or other upper respiratory allergies: runny nose, sneezing, itchy, watery eyes, itching of the nose or throat, and nasal congestion in adults and children 12 years of age and older.
- Cetirizine HCl 1 mg/ml (Children’s Zyrtec Allergy) syrup for the temporary relief of symptoms of hay fever or other upper respiratory allergies: runny nose, sneezing, itchy, watery eyes, itching of the nose or throat in adults and children 2 years of age and older.
- Cetirizine HCl 1 mg/ml (Children’s Zyrtec Hives Relief) syrup for the relief of itching due to hives (urticaria) in adults and children 6 years of age and older.
- Cetirizine HCl 5 mg and 10 mg (Children’s Zyrtec Allergy) chewable tablets for the temporary relief of symptoms of hay fever or other upper respiratory allergies: runny nose, sneezing, itchy, watery eyes, itching of the nose or throat in adults and children 6 years of age and older.
- Cetirizine HCl 5 mg and 10 mg (Children’s Zyrtec Hives Relief) chewable tablets for the relief of itching due to hives (urticaria) in adults and children 6 years of age and older.
- Cetirizine HCl 5 mg and 10 mg (Zyrtec Allergy) tablets for the temporary relief of symptoms of hay fever or other upper respiratory allergies: runny nose, sneezing, itchy, watery eyes, itching of the nose or throat in adults and children 6 years of age and older.
- Cetirizine HCl 5 mg and 10 mg (Hives Relief) tablets for the relief of itching due to hives (urticaria) in adults and children 6 years of age and older.
- Omeprazole 20 mg delayed-release tablets for the treatment of frequent heartburn in adults 18 years of age and older.
New strength:
- We approved a new 20 mg famotidine strength formulation of Pepcid AC chewable tablet for the treatment or prevention of meal-induce heartburn, acid indigestion and sour stomach for adults and children 12 years of age and older replacing the old 10 mg strength chewable tablet.
Next Page
 Back
to Top
Back
to Top  CDER 2007 Update Table of Contents CDER 2007 Update Table of Contents
 PDF requires the free
Adobe Acrobat Reader PDF requires the free
Adobe Acrobat Reader
Date created: July 31, 2008 |
 |