Accomplishments under the Food Quality Protection Act (FQPA)
August 3, 2006 -- 10th Anniversary of the Food Quality Protection Act
Quick Resources
FQPA
Related Pesticides Resources
Overview
With the 1996 enactment of the Food Quality Protection Act (FQPA), Congress presented EPA with the enormous challenge of implementing the most comprehensive and historic overhaul of the Nation's pesticide and food safety laws in decades. The centerpiece of FQPA was the requirement to complete within a decade the massive review and reassessment of the tolerances (maximum permitted residues) for all food use pesticides. On the tenth anniversary of FQPA enactment, we have completed over 99% of the required tolerance reassessments, and we celebrate the cumulative public health progress achieved by the thousands of individual protective actions taken under this law. This degree of success for such an ambitious, controversial and complex undertaking is unprecedented.
When it passed, House Commerce Committee Chairman Bliley noted the bill was a "landmark bipartisan agreement that will bring Federal regulation of the Nation's food producers into the 21st century." Recognizing the formidable charge Congress was placing on the Agency, Agriculture Committee Chairman Roberts stated that "the ultimate success of this reform will rest with the professionalism and the common sense of EPA."
Over this 10-year period, EPA and its public and private sector partners have met FQPA's challenge and achieved significant enhancements in public health and environmental protection for the American people. This tremendous accomplishment required persistence and commitment to the strategic FQPA principles of sound, science-based decisions, open government, timely action, and sensible public policy.
By successfully implementing the Food Quality Protection Act, EPA is ensuring that all pesticides used on food in the United States meet FQPA's more stringent safety standard. To carry out the pesticide regulatory program under FQPA, EPA has used groundbreaking science and provided extensive opportunities for public involvement, while maintaining a commitment to timeliness. As a result, the Agency and its partners have upgraded the protective framework of integrated programs and actions ensuring that safe and effective pesticides are available to support production of one of the most abundant, affordable, and healthy food supplies in the world and to safely meet America's other pest control needs.
On this page:
Notable Achievements
FQPA dramatically changed the safety standards EPA uses in evaluating potential pesticide risks, especially to infants and children. Since FQPA was enacted, effective protection of children, already a priority, received additional emphasis through the addition of an extra tenfold Children's Safety Factor. This additional factor is now standard in dietary risk assessments, unless reliable data support a different factor.
Other new protective measures require EPA to assess the aggregate impact of exposure to pesticides in the food we eat and water we drink, along with exposures resulting from residential pesticide uses and other non-occupational sources of exposure. Finally, FQPA mandated that EPA's safety assessments consider the cumulative effects on health from exposures to multiple different pesticides that cause the same biological effects in humans.
Timely Reassessments Despite Massive Number of Actions
The 1996 FQPA required EPA to reassess the safety of thousands of existing tolerances and tolerance exemptions by August 3, 2006, while simultaneously making determinations about the reregistration of existing pesticides and reviewing the registrations of thousands of pesticide end-use products. EPA has succeeded in meeting these goals beyond all reasonable expectations.
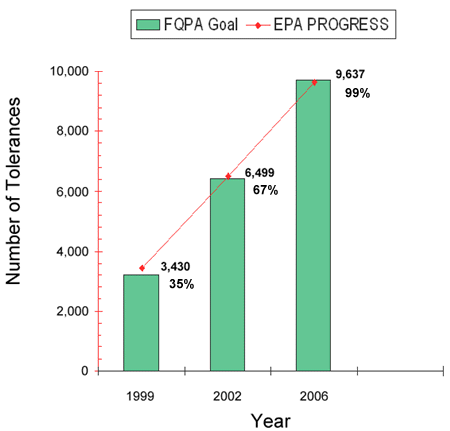
FQPA required the Agency to complete 33 percent of the required tolerance reassessment decisions within three years, 66 percent within six years, and 100 percent within 10 years, giving priority to the review of pesticides that pose the greatest risk to public health. EPA readily met the first two statutory deadlines, and has completed nearly all the remaining tolerance reassessment decisions within the 10-year timeframe. This tolerance reassessment effort has led, among other things, to EPA decisions to revoke or modify thousands of existing tolerances, and to require the establishment of many new tolerances, improving food safety and human health protection in the U.S.
The reregistration program, while not a formal part of FQPA, is the critical mechanism used by EPA to implement its provisions. FQPA presented new challenges that strengthened our existing reregistration program. Thus, EPA made it our goal to complete reregistration of all the food use pesticides as we completed their tolerance reassessment. Reregistering food use pesticides meant not only that EPA reassessed their tolerances but also that EPA evaluated the safety of those pesticides for workers and the environment. This effort entailed review of tens of thousands of new studies – a significant amount of additional work to accomplish in 10 years. EPA has completed nearly all of this work:
- Completed 9,637, or over 99% of the 9,721 tolerance reassessment decisions required by FQPA, please see figure 1
- Recommended the revocation of 3,200 tolerances
- Recommended the modification of 1,200 tolerances
- Confirmed the safety of 5,237 tolerances
Figure 1
The Food Quality Protection Act of 1996 required EPA to review the safety of all existing tolerances that were in effect as of August 1996. Of the 9,721 existing tolerances, EPA was requred to reassess 33% by August 3, 1999, 66% by August 3, 2002, and 100% by August 3, 2006.In completing these tolerance reassessment decisions, the Agency also has completed:
- Reregistration actions or eligibility decisions for 559, or almost 99 percent of the 566 reregistration eligibility decisions due by August 3, 2006.
- These actions include the cancellation of nearly 4,400 individual pesticide end-use product registrations out of a current universe of 17,592.
- EPA plans to complete reregistration eligibility decisions for the remaining 47 non-food use pesticide reregistration cases by October 3, 2008, as required by the 2004 amendments to FIFRA contained in the Pesticide Registration Improvement Act (PRIA).
Implementation of FQPA required the development of more refined pesticide risk assessments that better reflect real-world situations and that provide an adequate margin of safety for children and infants. To meet this challenge, EPA worked diligently to enhance and develop innovative risk assessment tools and methods for identifying those chemicals that have the greatest potential to harm human health and the environment.
Aggregate Exposure and Risk Assessment
One critical risk assessment advancement was the development of aggregate exposure and risk assessment methods that account for exposure to a pesticide by multiple routes and from multiple sources, including food, drinking water, residential, and other non-occupational sources. In developing aggregate risk assessment methods, EPA developed and refined complex models that evaluate multiple exposures from each source via oral, dermal, and inhalation routes.
Each model is highly specific to the source and considers almost all possible scenarios of exposure. For instance, EPA has models for up to 30 different residential pesticide exposure scenarios, covering uses ranging from lawn and garden care and household insect control, to exposures to humans due to pet treatments and wearing clothes impregnated with insect repellents. Accurately assessing the complex pesticide risks from all these varied use patterns required the creation of new "aggregate exposure" models capable of combining exposures received by an individual from food, water, and residential uses, through oral, dermal, or inhalation routes. These cutting edge scientific models now provide more accurate assessments of the risks of using pesticides, ensuring better informed and protective Agency decisions.
Cumulative Exposure Risk Assessment
In addition to the aggregate risk assessment tools, EPA has also developed tools for performing cumulative risk assessment, in which groups of pesticides that share a common mechanism of toxicity are evaluated together. This approach combines the estimates of aggregate exposure for individual chemicals with the same toxic effect and generates a cumulative risk assessment. EPA has conducted these extraordinarily intricate assessments for four pesticide groups:
- Organophosphate insecticides
- Carbamate insecticides
- Triazine herbicides
- Chloracetanilide herbicides
Developing models and algorithms to calculate these assessments required persistence, motivation, and cooperation from all science disciplines as well as input from outside experts.
To evaluate the 870 tolerance exemptions for pesticide inert ingredients that were part of the FQPA reassessment effort, the Office of Pesticide Programs (OPP) used available in-house data and existing publicly available assessments conducted by other U.S. Federal agencies such as the Agency for Toxic Substances and Disease Registry, National Toxicology Program, and international organizations such as the Organization for Economic Cooperation and Development and World Health Organization. A wealth of data and information exists on many of these chemicals because the majority of their uses are in industrial processes and the manufacture of consumer goods. OPP also consulted with experts in EPA's Office of Pollution Prevention and Toxics on Structure Activity Relationships (SAR) to ensure the proper use of available data on similar chemicals. Also, OPP developed models to assist in the characterization of risk from the use of inert ingredients on food commodities.
Coordination and Advice
OPP worked closely with other government agencies and other EPA program offices in developing new scientific approaches. The program's work with the Office of Research and Development to obtain data, develop policies, and formulate models and with the Office of Water on water modeling are just two examples.
OPP typically seeks the advice of the FIFRA Scientific Advisory Panel (SAP) whenever it encounters complex or novel scientific issues. The increase in the number of SAP consultations since FQPA demonstrates the large number of complex scientific issues tackled by the Agency. In the last 10 years, EPA has consulted the SAP 58 times.
To help implement FQPA, EPA created a transparent, collaborative process through which the public could participate directly and regularly, and EPA could obtain valuable input and information. Before FQPA, opportunities for the public to be actively involved in the development of regulatory decisions on pesticides were limited. EPA recognized that public participation and open discussion, at all stages of the process, enhances everyone's understanding of both science and policy issues and provides a sound basis for risk management decisions.
Advisory Committees One way EPA has ensured stakeholder consultation and public involvement was by creating a number of federal advisory committees. These committees include:
- The Food Safety Advisory Committee
- The Tolerance Reassessment Advisory Committee (TRAC)
- The Committee to Advise on Reassessment and Transition (CARAT)
- The Endocrine Disruptors Screening and Testing Advisory Committee (EDSTAC)
Public Participation Process EPA, following the recommendations of TRAC, one of its advisory committees, established a formal public participation process. This process was first piloted for the organophosphate pesticides starting in 1998, and it was soon extended to all pesticides undergoing reregistration and tolerance reassessment. Since then, public input has helped shape the outcome of reregistration and tolerance reassessment decisions. Input from the public has also helped illuminate and refine pesticide risks, leading to more effective regulatory decisions and better health protection.
Pesticide Web site Further enhancing transparency of public process is the role the Internet has played in FQPA implementation. EPA's pesticides topic area Web site with its substantive pesticide topic area offers the public ready access to information on regulatory decisions, risk assessments, advisory committees, and a host of other subjects and issue areas. The American public now expects this kind of service from the government, and uses the Internet more than ever before. According to a Pew Foundation poll, for example, more than 40 million people went on-line to look at federal, state and local government policies, and over 20 million used the Internet to send their views to governments about those policies. During the FQPA implementation period, the public has grown to rely on the internet as a major channel of communication and an information resource. EPA has used this technology to its fullest to provide services and information that is centered on its stakeholders.
Establishing Partnerships with All Stakeholders
FQPA created momentum for fuller participation by all stakeholders. EPA's accomplishment in meeting the goals and mandates of FQPA is based on strong partnerships with other federal agencies, international organizations, states, tribes, and many other stakeholders.
As with any major change in the law and the regulatory process, numerous constituencies became concerned about how the new law would be implemented. There had been broad consensus supporting the strengthened protection of the public that Congress mandated in FQPA, but there were also corresponding concerns about potential impacts on those whose livelihood and practices would potentially be affected as EPA implemented the law.
Registrants and User Groups Addressing FQPA's mandates has been an information-intensive exercise for all parties. Pesticide registrants have conducted tens of thousands of new scientific studies on pesticides, and user groups have provided an enormous amount of information needed to better characterize the real-world use of pesticide products.
Regulation of antimicrobial pesticides engages a diverse and often unique group of stakeholders, including consumer product companies, infection control specialists, public health agencies at the federal, state, and local levels, and professional and other interest groups. EPA has a staff dedicated to antimicrobial issues and has taken several steps to develop strong relationships with this distinct group of stakeholders. The Agency currently enjoys several constructive partnerships in the antimicrobial arena.
Federal Agencies From the outset, the U.S. Department of Agriculture (USDA) has also provided critical data through food consumption surveys and the Pesticide Data Program, which help refine risk estimates. The Agency has worked with USDA to characterize the benefits of pesticide use on specific crop-pesticide combinations where limited crop protection alternatives exist.
FQPA has caused many changes in the way pesticides are used. In some cases, rather than develop new data to address questions about safety, registrants voluntarily withdrew tolerances and registrations for crop uses. This, in turn, spurred companies to develop and growers to pursue reduced-risk alternatives. EPA and several partner agencies that coordinate regulatory actions at the national level have assisted in these transitions. USDA, in particular, has been an important partner in developing and evaluating alternative pest control tools and reaching out to the agricultural community and ensuring that their concerns are addressed. USDA's Office of Pest Management Policy:
- Serves as a liaison to EPA and the agricultural community
- Supports minor use registrations through the Inter-Regional Research Project No. 4

- Conducts training for pesticide applicators through its cooperative extension service
The Department of Health and Human Services (HHS) and EPA together have advanced efforts to achieve mutual environmental public health goals and strengthened the bridge between the environmental and public health communities, with a special emphasis on public health use pesticides. The outcome has been a better understanding of the linkages between environmental hazards, ensuing human exposure, and potential health outcomes that better inform environmental and public health decisions and improve our ability to assess the efficacy of such policies and decisions. Examples of consultative activities include:
- Sharing the science of mosquito and other vector control with the Centers for Disease Control and Prevention
- Assisting the Food and Drug Administration to improve detection of pesticide residues in food at far lower levels than previously possible
International Partners FQPA also set the stage for major changes in our collaboration with other countries in pesticide evaluation and control. Some countries, such as Canada, have adopted similar standards through domestic regulation. EPA has also worked on a range of pesticide issues with regulators in Europe, Japan, and other countries in the Organization for Economic Cooperation and Development, as well as with international organizations such as the Food and Agriculture Organization (FAO)
, the World Health Organization (WHO)
, and Codex
. This cooperation has helped to improve food safety and environmental protection and is an integral factor in resolving trade problems and streamlining regulatory processes.
Public interest advocacy groups are also important stakeholders with a strong interest in our implementation of FQPA. They have provided valuable input to strengthen the risk assessment and risk management process.
The states and tribes are very much our regulatory partners and have an essential role in FQPA implementation through their delegated authority to enforce pesticide regulations within their jurisdiction. As the people "on the ground," state and tribal regulators interact directly with pesticide dealers and commercial applicators, as well with farmers and others who use pesticide products. Their unique position helps us collect information on pest control needs, current pesticide use practices, and the potential impacts of changes in pesticide availability that may result from FQPA-based decisions. State and tribal agencies also have the role of communicating regulatory decisions to the user community and in providing information and training in the use of alternative pest control methods to replace products that may not meet FQPA safety standards.
Advisory Committees and Workgroups Throughout implementation, EPA sought to bring key FQPA policy and implementation issues to a broad coalition of stakeholders. As noted previously, EPA established or used several federal advisory committees since passage of FQPA to ensure an open and transparent decision-making process. Membership to these advisory committees and work groups included a broad representation of stakeholders, including pesticide companies, environmental/public interest groups, pesticide users and growers, farmworker representatives, public health officials, academia, Federal representatives, state officials, and tribal government representatives.
- The Food Safety Advisory Committee (FSAC) was established immediately after FQPA passage (through December 1996); it developed interim decision policies, which are still being employed.
- The Tolerance Reassessment Advisory Committee (TRAC) followed FSAC and gave useful advice on the development and communication of critical science policies that EPA used in its FQPA risk assessments, and has piloted the public participation process used in reregistration.
- The Agency created the Committee to Advise on Reassessment and Transition (CARAT) as follow-on to TRAC's efforts, with a particular focus on strategic approaches for pest management planning and tolerance reassessment.
In addition to these specific FQPA advisory committees, the following committees and organizations also addressed FQPA issues:
- The Pesticide Program Dialogue Committee, a permanent, broadly representative advisory committee, meets with EPA on a regular basis to discuss issues associated with FQPA implementation.
- EPA involved its FIFRA Scientific Advisory Panel of independent scientists in developing approaches for implementing many of the more technically challenging FQPA scientific assessment policies.
- The Endocrine Screening and Testing Advisory Committee (EDSTAC) and the Endocrine Disruptor Methods Validation Advisory Committee (EDMVAC) are two advisory committees of scientists and stakeholders that have offered guidance on the development and implementation of the Endocrine Disruptor Program mandated by FQPA.
- The State FIFRA Issues Research and Evaluation Group (SFIREG), which consists primarily of state pesticide regulatory officials, continues to work with EPA to improve the development, guidance, and approval of state pesticide programs and policies.
- The Tribal Pesticide Program Council (TPPC) brings together regulatory officials responsible for tribal pesticide programs, and offer useful perspectives on how EPA and tribes can work more effectively. Advice from TPPC has led to the creation of new risk assessment models that capture differences in pesticide risk for tribal members.
Meeting Agriculture's Need for Safe, Effective Pest Control Products
FQPA acknowledges the importance of "reduced-risk pesticides" and supports expedited review to help these pesticides reach the market sooner and replace older and potentially riskier chemicals. The law defines a reduced risk pesticide as one which may reasonably be expected to accomplish one or more of the following:
- Reduce pesticide risks to human health
- Reduce pesticide risks to non-target organisms
- Reduce the potential for contamination of valued, environmental resources
- Broaden adoption of IPM or make it more effective
EPA developed procedures and guidelines on expedited review of applications for registration or amendments for a reduced risk pesticide. The Agency expanded the pesticide program to include consideration of new active ingredients, new uses of active ingredients already deemed to be reduced risk, and amendments to all uses deemed to be reduced risk. EPA gives priority of review to reduced risk pesticides and worked with the regulated community and user groups to refine review and registration procedures.
Minor uses of pesticides are defined as uses for which pesticide product sales are low enough to make it difficult for a manufacturer to justify the costs of developing and maintaining EPA registrations. Collectively, such "minor" crops are important to a healthy diet, and include many fruits and vegetables. Minor uses also include use on commercially grown flowers, trees and shrubs, certain applications to major crops such as wheat or corn where the pest problem is not widespread, and many public health applications. Since many of these uses produce smaller revenues for pesticide registrants than do major uses, the registrants are sometimes reluctant to support and maintain registrations and associated tolerances. Some minor uses have been lost through lack of registrant support during the reregistration process, resulting in grower concerns that adequate pest control tools will no longer be available for many minor crops.
Registration of minor uses of pesticides is a priority for the Agency, and both USDA and EPA have worked to alleviate minor use problems. EPA has a staff dedicated to minor use registrations and the Agency works closely with USDA's IR-4 program
to generate residue data for tolerances on minor crops. Data requirements to support minor uses are carefully considered to minimize the burden of data generation. EPA and USDA operate early alert systems to notify growers when a pesticide use for a minor crop is about to be canceled. EPA also provides advance public notice of a proposed cancellation to allow time for another registrant to consider maintaining the pesticide use.
Addressing Major Public Policy Challenges
Effective FQPA implementation required not only new cutting-edge science policies and operational procedures, but also the resolution of significant public policy issues. Notably, concern about the potential use of data obtained from human studies was a barrier to taking final protective action for a number of pesticides. Over a period of years, the Agency evaluated the competing considerations and sought public input and expert advice on the ethical and scientific issues associated with human testing from its own advisory committees and the National Academy of Sciences. This put EPA in the position to act swiftly to meet a 2005 Congressional directive to establish a final Human Studies regulation before it could review or rely on data from human research in tolerance reassessment. In a matter of months, the Agency developed regulations to strengthen and expand significantly the protections for subjects of human research. These regulations:
- Prohibit new research for pesticides involving intentional exposure of children and pregnant and nursing women
- Extend ethical protections in the Common Rule to other human research involving intentional exposure of non-pregnant, non-nursing adults
- Require submission to EPA of protocols and related information to ensure any future studies meet the highest ethical safeguards
- Require the establishment of an independent Human Studies Review Board (HSRB) to obtain expert peer review of both proposals for new research and completed third-party intentional dosing
EPA established the independent Human Studies Review Board (HSRB), which is made up of world class scientists, and will take into serious consideration the scientific and ethical advice and recommendations of HSRB concerning use of human research data in our pesticide risk assessments.
Providing Tailored Attention to Unique Classes of Pesticides
Antimicrobials and biopesticides present different and special challenges from the typical agricultural pesticide. In recognition of these differences FQPA directed EPA to regulate these products in a manner consistent with their unique characteristics. Although not formally required by FQPA, EPA undertook a major organizational overhaul to deal with these challenges. The new structure dedicated separate divisions to the regulation of antimicrobials and biopesticides. The creation of the Antimicrobials Division and the Biopesticides and Pollution Prevention Division ensured that these unique materials would be evaluated by highly educated specialists who would make sure that these products receive timely and appropriate scientific and regulatory reviews.
Conclusion
A Bright Future – After a decade of groundbreaking accomplishments, FQPA provisions, principles, and innovative scientific approaches have become an integral part of the Agency's work. This bedrock foundation will sustain effective pesticide regulation and helps ensure that the American people will continue to enjoy one of the most plentiful, wholesome, and reliable food supplies in the world. With the tools of FQPA, the national pesticide program is equipped to meet the challenges of protecting public health and the environment for decades to come.
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20081108181939im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)