Chapter 7: Health Effects Evaluation: Screening Analysis
- (Section 7.1) What Are Comparison Values?
- (Section 7.2) Conducting Environmental Guideline Comparisons
- (Section 7.3) Conducting Health Guideline Comparisons
- (Section 7.4) Other Factors That Influence the Screening Analysis
- (Section 7.5) Presenting Screening Analysis Findings in the Public Health Assessment Document
As you gather information for the exposure evaluation and gain an understanding of the site and community health concerns (Chapters 3 and 4), the nature and extent of contamination (Chapter 5), and exposure pathways (Chapter 6), you will begin performing the other scientific component of the public health assessment process—the health effects evaluation. The health effects evaluation consists of two pieces: a screening analysis (described in this chapter) and, at some sites, based on the results of the screening analysis and community health concerns, a more in-depth analysis to determine possible public health implications of site-specific exposures (described in Chapter 8).
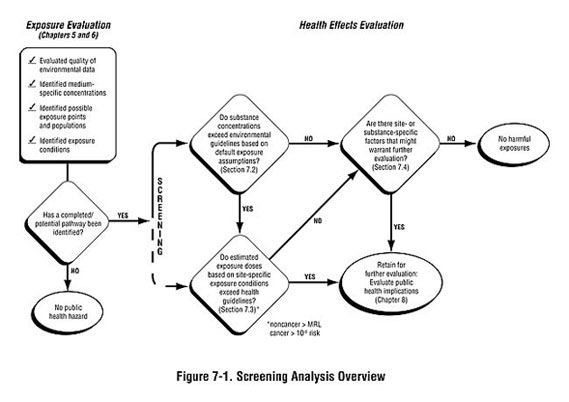
During the public health assessment process, you typically need to review large volumes of environmental data and evaluate these data in the context of the site-specific exposure assessment. The screening analysis, described in this chapter, enables you to sort through the data in a consistent manner to identify substances within completed and potential exposure pathways that may need to be evaluated more closely. This is achieved through the use of health-based "comparison values."
As shown in Figure 7-1, the screening analysis is generally conducted in a step-wise manner:
- Step #1: The environmental guideline comparison involves comparing detected
substance concentrations to medium-specific comparison values derived from
standard exposure default values.
- Step #2: The health guideline comparison involves looking more closely as site-specific exposure conditions, estimating exposure doses, and comparing them to dose-based comparison values. (Some health assessors may begin with this step recognizing substance- or site-specific concerns.)
After completing a screening analysis, you will have divided substances identified at the site into two categories:
- Those not exceeding comparison values and usually requiring no further analysis.
- Those exceeding comparison values and requiring further analysis to evaluate the likelihood of possible harmful effects.
This chapter first briefly describes what comparison values are and how they are used in the screening analysis (Section 7.1) and then describes when and how to conduct environmental (Section 7.2) and health guideline (Section 7.3) comparisons. Other factors that you also may need to be consider during the screening analysis are discussed in Section 7.4. Lastly, guidance is provided on how to best incorporate the findings of the screening analysis into your public health assessment documents (Section 7.5).
|
7.1 What Are Comparison Values?
Comparison values are doses (health guidelines) or substance concentrations (environmental guidelines) set well below levels that are known or anticipated to result in adverse health effects. ATSDR and other government agencies have developed these values to help health assessors make consistent decisions about what substance concentrations or dose levels associated with site exposures might require a closer look.
Health guidelines are derived based on data drawn from the epidemiologic and toxicologic literature with many uncertainty or safety factors applied to ensure that they are amply protective of human health. ATSDR's minimal risk level (MRL) and EPA's reference doses, reference concentrations, and cancer slope factors are the health guidelines most commonly used in the public health assessment screening process (see Section 7.3.2).
Environmental guidelines are derived from the health guidelines and represent concentrations of a substance (e.g., in water, soil, and air) to which humans may be exposed via a particular exposure route during a specified period of time without experiencing adverse health effects. ATSDR's environmental guidelines include environmental media evaluation guides (EMEGs) and cancer risk evaluation guides (CREGs). Section 7.2.1 describes available environmental guidelines in more detail.
In general, comparison values are derived for substances for which adequate toxicity data exist for the exposure route of interest. Where possible, comparison values are generally available for three specified exposure periods: acute (14 days or less), intermediate (15 to 365 days), and chronic (more than 365 days). Comparison values are also generally available for two exposure routes: ingestion and inhalation. No comparison values have been established for dermal contact exposures. Comparison values are available for many, but not all, substances you may find at a site. Appendix F details the derivation and applicability of available comparison values.
In the overall context of the public health assessment process, you need to clearly understand what comparison values represent and what they do not represent. Such an understanding will help you use the comparison values appropriately and clearly communicate the role they play in the public health assessment. The following sections describe when and how to conduct environmental guideline (Section 7.2) and health guideline comparisons (Section 7.3), including direction on selecting the most appropriate comparison value.
|
7.2 Conducting Environmental Guideline Comparisons
The environmental guideline comparison is a quick, easy way of choosing the contaminants that require further evaluation at your site. You will likely use environmental guidelines throughout the exposure evaluation process as you study the nature and extent of contamination at a site and begin to evaluate the potential for harmful exposures. Use of comparison values, along with background concentrations, will help you quickly gauge the relative magnitude of site contamination.
When screening against environmental guidelines, generally you begin with the list of substances found in potential or completed exposure pathways (see Chapter 6). You then need to select the most appropriate environmental guideline as well as the most appropriate substance concentration. (1) Typically, the quickest and easiest way to screen data is by selecting the maximum detected concentration in the environmental medium of interest and the lowest available comparison value. Remember that this method provides an appropriate initial screen, but does not incorporate site-specific exposure scenarios that will need to be considered during the health guideline screening.
To conduct the screening itself, just compare detected concentrations to the most appropriate comparison value. This will allow you to identify (1) substances whose concentrations are below environmental guidelines and likely pose no health hazards, and (2) substances whose concentrations are above environmental guidelines and may require further evaluation. For those substances whose concentrations are above environmental guidelines, you will proceed to the health guideline comparison. At some sites, you may find that none of the detected substances are identified as needing further evaluation. Therefore, public health conclusions are drawn based on the results of the environmental guideline comparison. However, before excluding all substances detected at concentrations below environmental guidelines from further consideration in a public health assessment, you need to consider the factors described in Section 7.4.
The following subsections describe elements to consider when selecting environmental guidelines for screening and interpreting the results, including which environmental guideline to use and what to do when no guideline is available. Be sure to clearly state all assumptions and methods used throughout the screening process in your public health assessment.
7.2.1 Selecting Environmental Guidelines
ATSDR has developed environmental guidelines for substances in drinking water, soil, and air. ATSDR's environmental guidelines include environmental media evaluation guides (EMEGs), cancer risk evaluation guides (CREGs), and reference dose media evaluation guides (RMEGs). These guidelines are derived in a uniform way using health guidelines and standard default exposure assumptions. These default exposure assumptions generally represent high estimates of exposure (greater than the mean, approaching the 90th percentile), based on observed ranges of human activity patterns (e.g., water ingestion rates, residence times). Guidelines are available to evaluate both child and adult exposures. The text box below provides brief definitions of these environmental guidelines. Again, see Appendix F for a detailed description of how medium-specific environmental guidelines are derived.
|
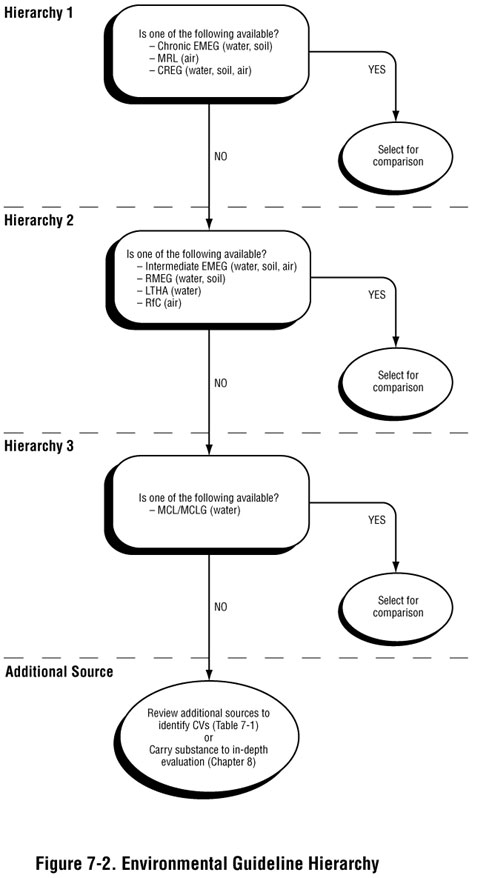
When determining what environmental guideline value to use, follow ATSDR's general hierarchy, as shown in Figure 7-2. Hierarchy 1 environmental guidelines (such as CREGs and chronic EMEGs), are developed based on ATSDR analyses of substance-specific toxicity data. In the absence of these values, Hierarchy 2 intermediate EMEGs or RMEGs or lifetime health advisories (LTHAs), which are based on EPA analyses of toxicity data, may be selected. For drinking water exposures, Hierarchy 3 maximum contaminant levels (MCLs) or maximum contaminant level goals (MCLGs) may be selected for comparison in the absence of other comparison values in the hierarchy.
Typically, you select the lowest environmental guideline consistent with the conditions at or near the site for screening purposes. However, be sure to use judgment in selecting the environmental guideline that best applies to site conditions in terms of time frames and populations that might be exposed. Consideration of the following issues may lead you to stray from the hierarchy presented in Figure 7-2, but will help you select the most appropriate values for conducting screening:
- Exposure duration. Always consider exposure duration when selecting the most
appropriate environmental guideline. A one-time exposure to a high contaminant
concentration may result in different health effects than repeated exposure to a lower
contaminant concentration. As noted, ATSDR has developed EMEGs that apply to acute
(14 days or less), intermediate (15–365 days) and chronic (365 days or more) exposures.
Comparison values developed by other organizations may also account for acute,
intermediate, and/or chronic exposures.
- Site-specific exposure conditions. In some instances, the most conservative environmental guideline may not be the most appropriate value to use in screening. Of critical importance in conducting public health assessments is selecting environmental guidelines that are most appropriate and applicable to site-specific conditions. Exposures identified at the site should closely approximate the exposure assumptions used to derive the environmental guideline. For example, including a soil contaminant for further evaluation based on a comparison value for a child would be inappropriate if the contaminant is found in a restricted industrial site where children are prohibited. Be sure to keep in mind, however, past, current, and potential future exposure conditions.
When environmental guidelines listed in the ATSDR hierarchy are unavailable, those from other sources should be considered. For example, to meet their unique mandates, other government agencies, such as EPA, the Food and Drug Administration (FDA), and state and tribal environmental and health departments, have developed their own comparison values. These comparison values may address hazardous substances in water, soil, air, fish, or other biota. Possible sources of additional comparison values are listed in Table 7-1.
Before choosing another environmental guideline, be sure to understand the derivation and use of that guideline to ensure that its use in screening is adequately protective of public health.
Because the mandates of different agencies may not always be strictly health-driven or consistent with the concerns of Superfund sites, fully understanding the derivation, uncertainties, and possible limitations of a comparison value is critical to determining its appropriateness for use in the public health assessment process. For example, some environmental guidelines are derived based on environmental impacts rather than human health concerns. Selecting such guidelines would not necessarily aid in evaluating public health concerns.
Table 7-1. Additional Sources of Environmental Guidelines
|
When evaluating possible health effects from exposures to radionuclides, you should consult a health physicist to evaluate sampling results, select appropriate environmental guidelines, and conduct further analysis of substances found above these guidelines.(2) In some circumstances, you may be able to develop a site-specific environmental guideline with assistance from a toxicologist or health physicist.
7.2.2 What If No Comparison Values Exist?
When no comparison values are available, the contaminant is generally retained for further evaluation (see Chapter 8). Exceptions exist, however. For example, essential nutrients (e.g., calcium, iron, magnesium) are typically not harmful under most environmental exposure scenarios and may not necessarily be retained for further analysis. It may be helpful to compare these and other naturally occurring elements to background concentrations when assessing the need for further examination. Section 5.3 in Chapter 5 provides some guidance on background considerations.
7.3 Conducting Health Guideline Comparisons
Understanding how site conditions may influence the extent to which people come in contact with site contaminants is central to the public health assessment process. Thus, once the simple environmental screening described in Section 7.2 has been completed, the health guideline comparison is designed to evaluate site-specific exposure doses. Exposure doses are estimated and then compared to health guideline values. In doing this, the health assessor begins to consider site-specific conditions rather than the default exposure values considered in the environmental guideline comparison. That is, you examine the likely exposure conditions (e.g., the duration, frequency, and magnitude of exposure) that may be unique to your site. Much of the information learned as part of your exposure evaluation will support this effort.
The health guideline comparison allows you to begin studying possible public health implications of site-specific conditions. Again, because health guidelines do not represent thresholds of toxicity, this process simply identifies substances in completed or potential exposure pathways that require more extensive evaluation. At some sites, however, you may find that none of the detected substances are identified as needing additional evaluation. Therefore, public health conclusions are drawn based on the results of the health guideline comparison.
After completing a health guideline screening, you will have identified (1) substances that are below conservatively derived health guidelines and likely pose no health hazards, and (2) substances that are above health guidelines and may require more in-depth analysis (Chapter 8). Do not forget that the factors described in Section 7.4 should be considered before excluding from further consideration those substances with site-specific exposure doses below health guidelines.
The following subsections describe how to estimate site-specific doses, including the selection of input parameters; how to select health guidelines for screening; and how to interpret the results of the comparison.
7.3.1 Estimating Site-Specific Exposure Doses
Depending on potential health concerns and site conditions, you may estimate doses for past, current, or future exposures. The ability to accurately estimate past and potential future exposure doses, however, may be limited. Information about past contaminant levels or exposures may be incomplete or unavailable. In some cases, exposures are characterized by using mathematical models, which can be used to estimate exposure concentrations. More information about selecting exposure concentrations and modeling data is provided in Chapter 6.
Estimates of exposure doses are generally determined for exposure to a single substance via a single route of exposure. However, at many sites, exposure to a substance may occur through multiple routes of exposure. When this occurs, the exposures from the various pathways can be summed to derive a total exposure dose. More information about approaches to assessing doses to multiple chemicals is presented in Chapter 8.
The procedures outlined in this section do not pertain to estimating the doses from exposure to radioactivity or radioactive materials. The terms "exposure dose" and "radiological dose" are not interchangeable as there are subtle differences in the methods of calculation. ATSDR recommends that the health assessor contact a trained health physicist or radiation specialist for assistance in evaluating radiological exposures.
7.3.1.1 How Are Exposure Doses Estimated?
|
An exposure dose (generally expressed as milligrams of chemical per kilogram of body weight per day or "mg/kg/day") is an estimate of how much of a substance a person may contact based on their actions and habits. Estimating an exposure dose requires identifying how much, how often, and how long a person or population may come in contact with some concentration of a substance (e.g., maximum or mean) in a specific medium.
You should strive to estimate exposure doses by using site-specific or population-specific exposure information. Doses are calculated using the following general equation:
Exposure Dose = (C x IR x AF x EF) / BW
Where:
| C | = Substance concentration (milligrams/liter, milligrams/kilogram, or parts per million) |
| IR | = Intake rate (liters/day or kilograms/day) |
| AF | = Bioavailability Factor (unitless) [usually considered as part of the more in-depth evaluation (see Chapter 8)] |
| EF | = Exposure factor (unitless) |
| BW | = Body weight (kilograms) |
The exposure factor is an expression of how often and how long a person may be contacting a substance in the environment. The exposure factor is calculated using the following general equation:
Exposure Factor = (F x ED) / AT
Where:
| F | = Frequency of exposure (days/year) |
| ED | = Exposure duration (years) |
| AT | = Averaging time (ED x 365 days/year) |
When estimating shorter-term or acute exposures or in situations where daily exposures are expected over time, the exposure factor term equals one.
Appendix G contains detailed information about estimating exposures from various pathways and medium-specific considerations. The appendix presents the methodology for ingestion, dermal contact, and inhalation using standardized (default) exposure assumptions, but it also provides guidance on how dose estimates can be refined to better represent site-specific exposure conditions. Although the appendix presents the method for estimating doses from dermal contact, you should recognize that ATSDR generally considers that for most exposure scenarios dermal exposure to be a minor contributor to the overall exposure dose relative to the contributions of ingestion and inhalation exposures. If dermal exposures are a particular concern at your site, substance-specific characteristics may need to be examined (e.g., absorption potential). You should consult with the team toxicologist as needed.
7.3.1.2 How Are Input Parameters Selected?
At some sites, the existing conditions may result in exposures that differ from the standard default assumptions, as described in Appendix G. For example, you may learn that the population under study does not rely exclusively on water from private wells for drinking purposes. Using the default assumption of 2 liters per day might therefore overestimate exposures. To ensure that the most reasonable, yet protective, exposure conditions are considered, select input parameters for the dose equation by carefully examining site-specific exposure conditions.
You do not need to limit dose estimates to a single point estimate. Where possible, present a range of doses. Presenting a range of realistic scenarios and doses can provide greater perspective regarding health implications. It can enable concerned community members to understand where their exposures may fit into the overall picture. For example, an exposure to a contaminant in soil may be expected to result in long-term effects to workers regularly exposed to soils. However, no adverse health effects would be expected for people contacting the same soils on an infrequent basis. EPA and others have developed tools for conducting probabilistic risk assessments that evaluate data distributions instead of point estimates (e.g., Monte Carlo analysis); the primary purpose of such tools is to more adequately characterize variability and uncertainty in risk assessments. Such tools can be considered in public health assessments, but you should work with the appropriate experts in these types of analyses to determine their applicability, use, and interpretation at a particular site. More information about probabilistic risk assessment tools can be found through EPA's Web site (http://epa.gov/osa/spc/htm/probpol.htm). ![]()
Remember, the purpose of the public health assessment is to put environmental exposures into proper perspective, and estimating appropriate exposure doses is an important step in this process.
7.3.1.3 What Are Some Sources of Input Parameters?
In the absence of site-specific information, refer to exposure estimates that have been derived
based on population studies, such as those contained in EPA's Exposure Factors Handbook
(http://www.epa.gov/ncea/pdfs/efh/front.pdf)![]() (EPA 1997). EPA's Exposure Factors Handbook
(EFH) provides a summary of population studies and presents a range of exposure estimates
based on the results of these studies. Information that can be obtained from EPA's EFH includes,
for example, drinking water or food intakes, breathing rates, body weights, and time spent at
different activities, such as showering, swimming, or gardening. Select the values that best
represent site conditions and would be adequately protective of the surrounding community.
Note, however, that if doses are estimated using the standard default assumptions, the dose
estimate will exceed its health guideline by the same magnitude by which the substance
concentration exceeded its environmental guideline.
(EPA 1997). EPA's Exposure Factors Handbook
(EFH) provides a summary of population studies and presents a range of exposure estimates
based on the results of these studies. Information that can be obtained from EPA's EFH includes,
for example, drinking water or food intakes, breathing rates, body weights, and time spent at
different activities, such as showering, swimming, or gardening. Select the values that best
represent site conditions and would be adequately protective of the surrounding community.
Note, however, that if doses are estimated using the standard default assumptions, the dose
estimate will exceed its health guideline by the same magnitude by which the substance
concentration exceeded its environmental guideline.
At some sites (e.g., as part of an RI/FS), a risk assessment may have been conducted to determine cleanup goals. In these cases, it may be worthwhile to review the risk assessment to understand what exposure variables and assumptions assessors used to estimate risks and evaluate site exposure conditions. For public health assessment purposes, you should independently choose site-specific exposure input parameters based on the results of your exposure evaluation, though risk assessments can be used as a reference.
7.3.1.4 What Factors Should Be Considered When Selecting Input Parameters?
The following text defines each of the input parameters used in the exposure dose equation and discusses key factors to consider when selecting appropriate variables. To ensure adequate protection of public health, begin by choosing conservative input parameters. As appropriate, refine the analysis by considering more realistic parameters consistent with what is known about site-specific exposures.
- Substance concentration. The maximum detected substance concentration is selected to assess potential exposures from substances in site media, at least as a first screen. You, however, should recognize that use of the maximum detected concentration of a substance to estimate the exposure dose may result in an overestimate of likely exposure. You may determine that the arithmetic or geometric average concentration may be appropriate to assess exposure conditions, especially when concentrations vary temporally or spatially (see text box below).
When reporting exposure concentrations, specify whether the estimates are based on maximum substance concentrations, an average of measurements taken from the same location, or a range of substance concentrations detected.
In general, consider the following questions when selecting an appropriate substance concentration. These questions attempt to determine whether the maximum detected concentration best characterizes actual exposures.
- Where are the highest substance concentrations located?
- Are the most contaminated areas accessible to the public?
- How frequently was the substance(s) detected?
|
- Intake rate. The intake rate is the amount of a contaminated medium to which a
person is exposed during a specified period of time. The amount of water, soil,
and food ingested on a daily basis; the amount of air inhaled; or the amount of
water or soil that a person may contact through dermal exposures are all examples
of intake rates. Select intake rates that best characterize the exposed population.
Usually standard default values represent intake rates that tend to overestimate exposures (see Appendix G for default values). Consider, however, the unique behavior or exposure rates of site populations when selecting an appropriate intake rate. In some instances, using default assumptions may underestimate exposures: using intake rates for the general population (e.g., a recreational fisher) to represent a subsistence fisher population, for example, may lead to underestimates of actual intake. Studies of subsistence populations have found ingestion rates as high as 170 grams of fish per day, whereas studies of the general public have estimated a fish intake rate of 20 grams per day (EPA 1997). It is very important to make sure that consumption rates accurately reflect the habits and consumption behaviors of the local population.
Questions to consider when selecting an appropriate intake rate include: Does the population include unique subpopulations or conditions that may affect intake rates, such as gender, age, health status, cultural practices, climate, site activities, season, region, or urbanization level?
- What behaviors or practices might impact intake rates? For example, are
people gardening in areas of contaminated soil or visiting contaminated
areas of industrial facilities?
- What are the drinking water and food sources in the affected area? Do
people use private wells or municipal water supplies? Do people consume
local or homegrown produce and livestock?
- What behaviors or practices might impact intake rates? For example, are
people gardening in areas of contaminated soil or visiting contaminated
areas of industrial facilities?
- Bioavailability factor. The amount of a substance that is absorbed into a person's
body is expressed as the bioavailability factor. The bioavailability factor is the
percent of the total amount of a substance ingested, inhaled, or contacted that
actually enters the bloodstream and is available to potentially harm a person. For
screening purposes, the bioavailability factor is typically assumed to be 1—that is,
all of a substance to which a person is assumed to be absorbed. Further,
comparison values are often based on exposures, and not absorbed doses. The
bioavailability factor may be revisited if you conduct a more in-depth analysis of
exposures and substance toxicology, as described in Chapter
8.
- Exposure factor. How often and how long a person is exposed to a contaminated
medium is expressed as the exposure factor. The exposure factor is derived by
considering frequency of exposure, exposure duration, and time of exposure.
The frequency of exposure can be estimated as the average number of days in a year in which exposure occurs. ATSDR assumes daily exposures when developing environmental guidelines. Actual pathway- and media-specific exposures may occur with less frequency, such as with recreational use of a site, an occupational setting, or local climate conditions that limit accessibility. You should gather information about the frequency of exposure because the same total dose of a substance can cause different toxic effects depending on whether the dose is administered during a short or prolonged period.
The exposure duration is the length of time a population has been exposed to site contaminants. Examining the site's history will usually allow you to estimate the maximum duration of exposure. Exposure duration can also be based on the activities of the exposed population, which may be exposed only infrequently or for a short duration. For example, patients in a hospital served by a contaminated water supply will have an exposure duration only as long as their stay at the hospital.
The time of exposure is used to express exposure in terms of an average daily dose that can be compared to health guidelines and toxicity study results. For noncarcinogenic substances, the dose is estimated by using a time input parameter equal to the exposure duration. For example, when estimating the dose for a child exposed to a contaminant in a playground for 3 years, the time input parameter would equal 1,095 days (365 days/year x 3 years). For carcinogenic effects, doses are generally estimated by calculating an average daily dose during a lifetime (which is generally assumed to be 70 years). The time input parameter for carcinogenic effects is therefore 25,550 days (365 days/year x 70 years). This approach for carcinogens assumes that a high dose received during a short period is the same as a corresponding low dose during a lifetime (EPA 2003). As with all assumptions, you are cautioned that they may not be applicable in all situations.
Questions to consider when selecting appropriate input parameters for the exposure factor include:
- What is the likelihood that people will actually come in contact with the
highest detected concentrations of substances?
- Are exposures likely to be incidental, frequent/regular, or excessive?
- What is the likelihood that exposures to environmental media will occur at
default levels? Is the level likely to be more or less?
- How might the site-specific climate affect exposure frequency?
- What land use factors, such as the location of the water supply, parks, or
schools, will affect exposure frequency?
- When was contamination first released from the site (e.g., initial and final
dates of operation or receipt of wastes)?
- Are there measures in place that may have ended exposures (e.g., water
treatment systems, site access barriers, or remedial actions)?
- Who are the exposed populations and when could exposures have begun
(e.g., construction date of residential neighborhoods)?
- What is the likelihood that people will actually come in contact with the
highest detected concentrations of substances?
- Body weight. Body weight is used in the exposure dose equation to express doses
that can be compared across a population. When exposed to the same amount of a
substance, people with lower body weights will receive a relatively higher dose of
the substance than people with higher body weights. This effect is best seen when
examining exposures to adults and children. For example, a child weighing 10 kg
receives a higher dose of manganese per kilogram of body weight when drinking
contaminated water than an adult drinking the same water. The default assumption
is that the average adult weighs 70 kg (154 pounds) and a child weighs 10 kg (22
pounds or about the size of a 1-year old child). These default assumptions may not
apply when assessing exposures to a population of women or older children.
Some questions to consider when selecting an appropriate body weight include:
- Does the receptor population represent the average U.S. population?
- What is the age group and respective body weights of the exposed population (e.g, toddlers, young teens, or adults)?
- Does the receptor population represent the average U.S. population?
7.3.2 Selecting Health Guidelines
After site-specific exposure doses are estimated, these doses are then compared with the most appropriate health guideline. This step assists you in screening out substances that are not expected to result in adverse health effects (i.e., below health guidelines) from those that require further evaluation (i.e., above health guidelines). Different health guidelines are available for exposure routes (ingestion, inhalation), exposure durations (acute, subchronic/ intermediate, and chronic), and health endpoints (carcinogenic, noncarcinogenic). Appendix F provides detailed information about available health guidelines and their derivations.
Health guidelines for ingestion exposures are expressed as a dose, in mg/kg/day. For air exposures, the health guidelines are expressed as exposure concentrations (usually in parts per billion [ppb] or micrograms per cubic meter [µg/m3])(3). Health guidelines are protective of human health and are developed for both noncarcinogenic and carcinogenic effects. Health guidelines for noncarcinogenic effects are derived from human or experimental animal data and modified, as necessary, by a series of "uncertainty" factors (also known as safety factors) that ensure that guidelines are set at levels safely below those that could result in adverse health effects. Health guidelines for cancer are derived by the U.S. Environmental Protection Agency (EPA) and represent hypothetical estimates of cancer risk at low levels of exposure.
ATSDR and EPA have developed health guidelines for noncarcinogenic effects resulting from substance exposures. MRLs are the health guidelines derived by ATSDR. Reference doses (RfDs) and reference concentrations (RfCs) are health guidelines derived by EPA. In addition, EPA has derived factors to measure the relative potency of various carcinogens—known as oral cancer slope factors and inhalation [air] unit risks (for oral and inhalation exposures, respectively). ATSDR sometimes uses these EPA-generated values to derive cancer risk evaluation guides (CREGs).
When available, select ATSDR's MRLs. If no MRL is available for a substance, EPA's RfDs or RfCs should be used. Other sources can be consulted if no ATSDR or EPA health guidelines are available (e.g., EPA's National Center for Environmental Assessment [NCEA] provisional values). In general, consider the following when selecting the most appropriate health guidelines:
- Exposure route. If substance-specific health guidelines are not available for the
exposure route of concern at a site, guidelines developed for other exposure routes
may be used. However, care should be exercised when drawing conclusions from
those comparisons. For example, when guidelines are not available for dermal
contact or for inhalation, MRLs for ingestion exposures may be used for screening
purposes. You should consult with a toxicologist and consider the impact of
extrapolating from one route of exposure to another in these cases.
- Exposure duration. ATSDR develops MRLs for acute (14 days or less),
intermediate (15–365 days), and chronic (365 days or more) exposures. EPA's
RfDs and RfCs are developed assuming chronic exposures. MRLs, RfDs, and
RfCs are available for ingestion or inhalation exposures. A health assessor should
take care in selecting the health guidelines that best represent the exposure
duration assumed in their estimation of site-specific dose.
- Health endpoints. For possible noncarcinogenic health effects, the derived site-specific doses are compared to health guidelines for noncarcinogenic health
effects, most commonly ATSDR's MRLs or EPA's RfDs and RfCs.
For possible carcinogenic outcomes, you should generally carry the site-specific doses to a more in-depth evaluation, as described in Chapter 8. However, quantitative risk assessment methods for evaluating theoretical excess cancer risks can be used to provide initial information about a carcinogen, as described in the text box below. Results of such a quantitative assessment should not be used, however, as the sole basis for any health conclusions for a site.
Quantitative Screening Analysis for Carcinogens Under quantitative risk assessment methodology, site-specific cancer doses and concentrations are multiplied by EPA's cancer slope factors (CSFs) or inhalation unit risks (IURs), respectively, to estimate a theoretical cancer risk. The following illustrates this calculation. Theoretical Cancer Risk = Dose (or air concentration) × CSF (or IUR) Where:
This calculation estimates a theoretical excess cancer risk expressed as the proportion of a population that may be affected by a carcinogen during a lifetime of exposure. For example, an estimated cancer risk of 1 x 10-6 predicts the probability of one additional cancer over background in a population of 1 million. Because of conservative models used to derive CSFs and IURs, using this approach provides a theoretical estimate of risk; the true or actual risk is unknown and could be as low as zero (EPA 2003). When considering numerical risk estimates, you should understand that CSFs and IURs are generated using mathematical models applied to epidemiologic or experimental data for carcinogenic effects. The mathematical models extrapolate from higher experimental doses to lower environmental doses. Often, the experimental data represent exposures to chemicals at concentrations orders of magnitude higher than concentrations found in the environment. In addition, these models often assume that there are no thresholds for carcinogenic effects—a single molecule of a carcinogen is assumed to be able to cause cancer. The doses associated with these estimated hypothetical risks may be orders of magnitude lower than doses reported in the toxicology literature to cause carcinogenic effects. As such, a low cancer risk estimate (less than 10-6) may indicate that the toxicology literature would support a finding that no excess cancer risk is likely. A higher cancer risk estimate (greater than 10-6), however, indicates that you should carefully review the toxicology literature before making conclusions about potential cancer risks. Chapter 8 describes the more in-depth evaluation to follow when assessing cancer outcomes. Although ATSDR recognizes the utility of numerical risk estimates in risk analysis, the agency considers such estimates in the context of the variables and assumptions involved in their derivation and in the broader context of biomedical opinion, host factors, and actual exposure conditions. The actual parameters of environmental exposures must be given careful consideration in evaluating the assumptions and variables relating to both toxicity and exposure (ATSDR 1993). |
7.4 Other Factors That Influence the Screening Analysis
Generally, the screening analysis is a simple comparison of exposure point concentrations or exposure doses against environmental or health guidelines, as described in Section 7.2 and 7.3. However, some other site-specific factors may need to be considered before including or excluding a substance from a list for further evaluation. Remembering these issues as the screening analysis progresses will prevent you from inadvertently dismissing a substance that should be identified for further evaluation or doing the opposite—inadvertently conducting a lengthy evaluation of a substance that could have been quickly identified as not likely to cause adverse health effects at detected levels and conditions of likely exposure.
As you proceed with the screening analysis, consider the following site-specific factors:
- Community concerns. As mentioned throughout this manual, community concerns
are important to the public health assessment process. Therefore, when a
community has expressed special concern about a particular substance or
exposure, whether comparison values are exceeded or not, you should include this
substance for evaluation and discussion. Guidance on responding to community
concerns is provided in Chapter 4.
- Specific populations.Although environmental and health guidelines are designed
to be protective for most of the population, including sensitive populations and
children, it is important to remember that they may not apply to all populations of
potential interest. For example, subsistence fishers may be exposed at a higher
rate than the general population for which fish comparison values are derived, or
people in extremely warm climates may ingest extremely high quantities of water.
These factors should be accounted for when estimating site-specific doses. In
addition, some people might be more sensitive to the effects of a substance, such
as asthmatics or the elderly, and should be identified when evaluating site-related
exposures. Consult with your toxicologist to determine which if any of the
substances detected at your site might warrant special attention in light of site
exposure conditions (e.g., detected contaminants and demographics).
- Multiple pathways of exposure. People can be exposed to substances found in
more than one environmental medium (e.g., in both water and soil). Substance
concentrations in a specific medium, however, might not exceed comparison
values. Therefore, consider substances detected in more than one medium that
could compound potential exposures. For example, you may want to further
evaluate the possible combined effects of a particular substance found in drinking
water, surface soil, and air, even though media-specific environmental guidelines
may not be exceeded. You may also want to retain a substance found below its
environmental guideline in one medium (e.g., soil) if this substance was also
found above its environmental guideline in another medium (e.g. water). Be
cautious, however, when assessing chemicals across pathways. Effects are not
always additive. Exposure frequencies and absorption rates for a single substance
can vary by medium and route of exposure.
- Multiple-chemical exposures.Community members are often concerned about exposure to multiple chemicals. Generally, if detected levels of chemicals are individually below conservative screening values, then exposure to these chemicals collectively is not expected to be of health concern. Even so, you may decide that further evaluation of multiple-chemical exposures is necessary. In these instances, you should perform further analysis, as described in Chapter 8, in consultation with a toxicologist, as necessary.
Chapter 8 expands on how you should weigh these factors in your evaluation of site exposures and determining public health implications.
7.5 Presenting Screening Analysis Findings in the Public Health Assessment Document
The environmental guideline comparison and health guideline comparison are screening tools that serve as the first step in assessing and understanding potential harmful effects posed by exposures to site contaminants. It is, therefore, important to clearly and effectively communicate the methods used and the findings of the screening evaluation.
A concise summary of the screening analysis process should be included in PHAs. This summary should be written in nontechnical terms and present the uses and limitations of the screening analysis process. The document should state that these methods are screening tools used to rapidly assess large volumes of data, emphasizing that the process does not identify adverse health outcomes. This concept must be clearly stated and explained: ATSDR has found that in instances where this information is not clearly laid out, it is easy for people to misinterpret comparison values as indicators of illness or harm.
The PHA should also clearly state all assumptions that you used in your evaluation to select the substance concentrations, environmental guidelines, dose estimate variables, or health guidelines. Including an appendix detailing your dose calculations is an effective means of presenting your methods and assumptions.
The Discussion section of the PHA is the most appropriate place to discuss the results of the screening analysis. In presenting the results, provide a discussion of what substances were selected for further evaluation and why they were selected. Also, briefly describe what substances were determined to pose no public health hazards and eliminated from further evaluation. Results of the screening analysis can be easily summarized in a table, as described in Chapter 5. At some sites, no substances will be identified as needing further evaluation. Your public health conclusions will therefore be based on the results of the screening analysis process, and the Discussion section of the PHA should outline the information you used to draw conclusions. At other sites, you will identify substances requiring further evaluation, and the Discussion section of the PHA will be expanded to include the findings of the more in-depth evaluation of those substances (see Chapter 8).
ATSDR. 1993. Cancer policy framework. Atlanta: US Department of Health and Human Services. January 1993. Available at: http://www.atsdr.cdc.gov/cancer.html
EPA. 2003. Draft final guidelines for carcinogen risk assessment final (external review draft). U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC, NCEA-F-0644A. March 2003.
EPA. 1997. Exposure factors handbook. Volumes 1, 2, and 3. Available at: http://www.epa.gov/ncea/pdfs/efh/front.pdf.![]()
Comparison Values
ATSDR. 1996. Minimal risk levels for priority substances and guidance for derivation, republication FR 61, 33511-15. 1996.
NCRP. 1999. Recommended screening limits for contaminated surface soil and review of factors relevant to site-specific studies. NCRP Report 129. Bethesda: National Council on Radiation Protection and Measurements. January 1999.
The Hazardous Substance Database (HazDat) at ATSDR's intranet site lists environmental and health guidelines. Health guidelines are also posted at ATSDR's Web site (http://www.atsdr.cdc.gov/mrls.html). Information in HazDat and posted on ATSDR's Web site is updated regularly.
California's Acute Reference Exposure Levels are available on-line
(http://www.oehha.ca.gov/air/acute_rels/allAcRELs.html).![]()
EPA's Information Risk Information System (IRIS) database lists health guidelines developed by
EPA (http://www.epa.gov/iris/index.html).![]() Information in IRIS is updated regularly.
Information in IRIS is updated regularly.
EPA's maximum contaminant levels (MCLs), maximum contaminant level coals (MCLGs), and
health advisories (HAs) are posted on EPA's Web site (http://www.epa.gov/safewater/mcl.html and http://www.epa.gov/waterscience/drinking/). ![]() Information posted on these Web sites is
updated regularly.
Information posted on these Web sites is
updated regularly.
EPA Region 9's Preliminary Remediation Goals (PRGs) can be found at the EPA Region 9 Web
site (http://www.epa.gov/Region9/waste/sfund/prg/index.htm).![]()
EPA Region 3's Risk-based concentrations (RBCs) list environmental and health guidelines and
can be found at the EPA Region 3 Web site (http://www.epa.gov/reg3hwmd/risk/riskmenu.htm). ![]() These tables are updated in April and October each year.
These tables are updated in April and October each year.
EPA. Proposed Acute Exposure Guideline Values are available on-line
(http://www.epa.gov/fedrgstr/EPA-TOX/2000/March/Day-15/o-t6397.htm).![]()
FDA's guidelines and action levels are available at the FDA Web site (http://www.fda.gov/).![]()
State-derived guidelines may be available. These values may be posted at state Web sites or may be available through the state's health and environmental agencies.
Toxicity Equivalents (TEQs)
ATSDR's approach to toxic equivalency factors (TEFs) and toxicity equivalents (TEQs) for dioxins is outlined in ATSDR 1998. Toxicological Profile for Chlorinated Dibenzo-p-dioxins. Atlanta: US Department of Health and Human Services.
An expert meeting was convened by the World Health Organization (WHO) in 1997 to derive consensus toxic equivalency factors (TEFs) for dioxins/furans and PCBs. The results of this meeting were reported in Van den Berg M et al. 1998. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106:775-792.
EPA released an SAB Review Draft of their Part II: Health Assessment for 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and Related Compounds (SAB Review Draft) in
September 2000. EPA/600/P-00/001. Available at: http://www.epa.gov/ncea/pdfs/dioxin/part2/drich9.pdf![]()
The relevant potency of carcinogenic polycyclic aromatic hydrocarbons (PAHs) is presented in ATSDR. 1995. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs). Atlanta: US Department of Health and Human Services. August 1995.
EPA. 1993. Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons. Office of Research and Development. EP/600/R-93/039. July 1993.
2 In those cases where comparison values for radioactive materials are not available (which is the rule and not the exception), a health physicist should be consulted to determine if the reported results (1) are realistic based on the methods of analysis (equipment used), (2) are plausible based on site history and description, and (3) meet standard, recognized quality control and quality assurance protocols such as minimum detectable activity and uncertainty of the measurement. (See also Chapter 5).
3 Note: In the case of air concentrations, parts per billion (ppb) do not equal micrograms per cubic meter (µg/m3). See Appendix F for more information.