
Volume 6, No. 8, August 2008
Features
American College of Obstetricians and Gynecologists
ACOG Practice Bulletin #95 Anemia in Pregnancy
Introduction: Anemia, the most common hematologic abnormality, is a reduction in the concentration of erythrocytes or hemoglobin in blood. The two most common causes of anemia in pregnancy and the puerperium are iron deficiency and acute blood loss. Iron requirements increase during pregnancy, and a failure to maintain sufficient levels of iron may result in adverse maternal–fetal consequences. The purpose of this document is to provide a brief overview of the causes of anemia in pregnancy, review iron requirements, and provide recommendations for screening and clinical management of anemia during pregnancy.
Summary of Recommendations and Conclusions:
The following conclusion is based on good and consistent scientific evidence (Level A):
- Iron supplementation decreases the prevalence of maternal anemia at delivery.
The following recommendation and conclusions are based on limited or inconsistent scientific data (Level B):
- Iron deficiency anemia during pregnancy has been associated with an increased risk of low birth weight, preterm delivery, and perinatal mortality. Severe anemia with maternal Hgb levels less than 6 g/dL has been associated with abnormal fetal oxygenation resulting in nonreassuring fetal heart rate patterns, reduced amniotic fluid volume, fetal cerebral vasodilatation, and fetal death. Thus, maternal transfusion should be considered for fetal indications.
The following recommendations are based primarily on consensus and expert opinion (Level C):
- All pregnant women should be screened for anemia, and those with iron deficiency anemia should be treated with supplemental iron, in addition to prenatal vitamins.
- Patients with anemia other than iron deficiency anemia should be further evaluated.
- Failure to respond to iron therapy should prompt further investigation and may suggest an incorrect diagnosis, coexisting disease, malabsorption (sometimes caused by the use of enteric-coated tablets or concomitant use of antacids), noncompliance, or blood loss.
Proposed Performance Measure:
Percentage of pregnant patients with iron deficiency anemia treated with supplemental iron in addition to prenatal vitamins.
American College of Obstetricians and Gynecologists. Anemia in pregnancy. ACOG Practice Bulletin No. 95. Obstet Gynecol 2008;112:201–7.
http://www.ncbi.nlm.nih.gov/pubmed/18591330
American Family Physician**
The mirror lies: body dysmorphic disorder
Body dysmorphic disorder is an increasingly recognized somatoform disorder, clinically distinct from obsessive-compulsive disorder, eating disorders, and depression. Patients with body dysmorphic disorder are preoccupied with an imagined deficit in the appearance of one or more body parts, causing clinically significant stress, impairment, and dysfunction. The preoccupation is not explained by any other psychiatric disorder. Patients present to family physicians for primary care reasons and aesthetic or cosmetic procedures. Cosmetic correction of perceived physical deficits is rarely an effective treatment. Pharmacologic treatment with selective serotonin reuptake inhibitors and nonpharmacologic treatment with cognitive behavior therapy are effective. Body dysmorphic disorder is not uncommon, but is often misdiagnosed. Recognition and treatment are important because this disorder can lead to disability, depression, and suicide.
Hunt TJ, Thienhaus O, Ellwood A. The mirror lies: body dysmorphic disorder. Am Fam Physician. 2008;78(2):217-222, 223-224. http://www.aafp.org/afp/20080715/217.html
Patient Handout, “Body Dysmorphic Disorder: What You Should Know” available at:
http://www.aafp.org/afp/20080715/223ph.html
AHRQ
USPSTF Reaffirms Recommendation for Screening Pregnant Women for Asymptomatic Bacteriuria
Summary of Recommendation:
- The USPSTF recommends screening for asymptomatic bacteriuria with urine
culture for pregnant women at 12 to 16 weeks' gestation or at the first prenatal
visit, if later.
Grade: A recommendation - The USPSTF recommends against screening for asymptomatic bacteriuria in
men and nonpregnant women.
Grade: D recommendation.
Background: Asymptomatic bacteriuria is common, and screening for this condition in pregnant women is a well-established, evidence-based standard of current medical practice. Screening other groups of adults has not been shown to improve outcomes.
Purpose: To review new and substantial evidence on screening for asymptomatic bacteriuria, to support the work of the U.S. Preventive Services Task Force.
Data Sources: English-language studies of adults (age >18 years) indexed in PubMed and the Cochrane Library and published from 1 January 2002 through 30 April 2007.
Study Selection: For benefits of screening or treatment for screened populations, systematic reviews; meta-analyses; and randomized, controlled trials were included. For harms of screening, systematic reviews; meta-analyses; randomized, controlled trials; cohort studies; case–control studies; and case series of large multisite databases were included. Two reviewers independently reviewed titles, abstracts, and full articles for inclusion.
Data Extraction: Two reviewers extracted data from studies on benefits of screening and treatment (including decreases in the incidence of adverse maternal and fetal outcomes, symptomatic urinary tract infections, hypertension, and renal function decline).
Data Synthesis: An updated Cochrane systematic review of 14 randomized, controlled trials of treatment supports screening for asymptomatic bacteriuria in pregnant women. A randomized, controlled trial and a prospective cohort study show that screening nonpregnant women with diabetes for asymptomatic bacteriuria is unlikely to produce benefits. No new evidence on screening men for asymptomatic bacteriuria or on harms of screening was found.
Limitation: The focused search strategy may have missed some smaller studies on the benefits and harms of screening for asymptomatic bacteriuria.
Conclusion: The available evidence continues to support screening for asymptomatic bacteriuria in pregnant women, but not in other groups of adults.
Lin K, Fajardo K. Screening for Asymptomatic Bacteriuria in Adults: Evidence for the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement. AHRQ Publication No. 08-05120-EF-3, July 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/uspstf08/asymptbact/asbactart.htmAsk A Librarian - Diane Cooper, IHS National Library Informationist
Health Services Research Library (HSRL) - a branch of the National Institutes of Health Library
Your information partner
As your partner at the HSRL, I’m here to help you meet your information needs. If you need to find information at either patient point-of-care or as background information for a specific project, I can help. I am here to save you time and ensure you get the information you need. As your information needs evolve, I will expand and enhance my skills and work hard to stay on top of the latest information resources useful to the Indian Health Service.
Benefit from a Content Expert
I have provided clinical and health-related information services to healthcare providers and systems in academic and rural settings in Kentucky, Nevada, and California. With a Master’s in Library and Information Science and extensive experience providing information to providers in outreach areas, I am qualified to provide the Indian Health Service with up-to-date and credible information services. As your direct link to the HSRL and partner in your work, I can support you in the following ways:
- Help with complex and difficult literature searches to support direct patient care and patient care activities
- Participate and be a partner in IHS projects and development team activities
- Assist in manuscript preparation (verify references; editing)
- Set up current awareness alerts in your field of interest
- Create customized databases in bibliographic software programs (Endnote; Reference Manager) to organize your information for easy retrieval when you need it
- Provide instruction on how to search literature databases and other information resources more efficiently
Tips to Get You Started
Here’s a tip to make finding information a little easier. Try using PubMed’s “Clinical Queries” for a quick and easy source of evidence-based medicine information. Let me know if I can help you get started.
Call me at 301.594.2449 or email me at cooperd@mail.nih.gov or Diane.Cooper2@ihs.gov for information.
Health Services Research Library (HSRL)
http://hsrl.nihlibrary.nih.gov
Behavioral Health Insights - Peter Stuart, IHS Psychiatry Consultant
QuickStats: Percentage of Adults with Symptoms of Serious Psychological Distress,* by Age Group and Sex - National Health Interview Survey, United States, 2007 †
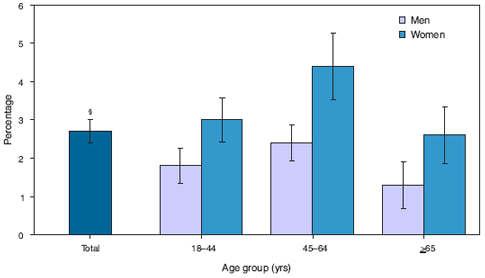
* Results are based on responses to the questions "During the past 30 days, how often did you feel 1) so sad that nothing could cheer you up, 2) nervous, 3) restless or fidgety, 4) hopeless, 5) that everything was an effort, or 6) worthless?" Response codes for the six items for each person were summed to yield a point value on a 0--24-point scale. A value of 13 or more was used to define serious psychological distress.
† Estimates are based on household interviews of a sample of the civilian, noninstitutionalized U.S. population.
§ 95% confidence interval.
CDC/MMWR Commentary:
In 2007, among all adults >18 years, women were significantly more likely than men to have experienced symptoms of serious psychological distress during the past 30 days. By age group, adults aged 45--64 years were more likely than adults aged >65 years to have experienced these symptoms. Overall, approximately 3% of the U.S. adult population had experienced symptoms of serious psychological distress during the past 30 days.
CDC, MMWR, QuickStats: Percentage of Adults With Symptoms of Serious Psychological Distress,* by Age Group and Sex --- National Health Interview Survey, United States, 2007 July 11, 2008 / 57(27);748 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5727a5.htm?s_cid=mm5727a5_e
Conference Information/OBOT (Buprenorphine) Certification Training
(Buprenorphine) Certification Training will be available free of charge to IHS providers October 24 in Flagstaff, Arizona. For details please contact Dr. Tony Dekker at Anthony.dekker@ihs.gov. This is an excellent training and expands dramatically your therapeutic effectiveness with patients with narcotic dependency issues – as well as facilitating care for our more complicated chronic pain patients. A personal DEA number is required for certification and federal physicians may receive one free of charge even if not currently licensed in the state of practice. Go to http://www.usdoj.gov/dea/index.html to see the DEA page (good info on pain etc) and go to http://www.deadiversion.usdoj.gov/drugreg/index.html to register for a new DEA. It is self explanatory and takes less than 5 minutes to apply.
Heads Up – Be thinking “Primary Care Based Behavioral Health”. Based on programs developed at Group Health and expanding rapidly in the VA and DOD systems this is a radical revision of the BH role in primary care and offers demonstrated benefits for patients and providers. A site with good material is http://www.mahec.net/ic/. Be patient – it loads slowly but is well worth the wait. Ask your local BH staff “When are you planning on implementing this?” to get things started.
Breastfeeding - Suzan Murphy
The Case of the Dwindling Milk Supply
Common scenario - mom and baby come for a well child check up. Mom says she has returned to work and is still breastfeeding. But her breast milk supply seems to slowing down. The following are questions to consider asking:
What kind of pump?
Hospital grade electric pumps work well. They are double sided. They cost $600+ to purchase and are usually rented instead for > $40 per month. There are also pumps that are available at many department stores for ~$250. It is intended to be a single user pump. According to the manufacturer, replacing the attachments will not reduce the risk of possible cross contamination. Many WIC programs offer single-user type pumps for clients to keep or will loan hospital grade pumps. Occasionally insurance companies will reimburse all/part of pump cost.
Anecdotal comments suggest that while other less expensive pumps may work for some moms – they do not have the general success rate of hospital grade pumps. Mothers describe ineffective output and nipple damage.
Please note - nipple damage can happen with any pump. The problem can be caused by attachments that don’t fit and/or overzealous pumping.
If a family is interested in a pump, encourage the family to contact WIC, local breastfeeding coalitions, La Leche League, their health insurance company, and their employer. Some businesses provide pump sites for employees. Encourage families to look for pumps that are designed for daily use and have been clinical tested for maintaining milk supply. Assure them that pumping/expressing is not supposed to hurt.
How often is the mom pumping/expressing?
If the baby is under 6 months, the ideal routine is pumping every 3 hours, double sided for 20 or more minutes. If the mom is able to incorporate consistent pump times into her daily routine, it will be easier to maintain supply.
If that is not possible to pump on a routine schedule, encourage the mom to pump when she can. Pumping every 4 hours, like at a lunch break will help reduce risk of plugged ducts and mastitis.
Sometimes a baby will reverse his/her long sleep pattern, so the long sleep is while mom is gone. When mom comes home, the majority of feedings happen then. The milk supply could be slowing during work time because of the baby’s pattern shift.
An anecdotal tip is to try pumping marathons - after work, pump 20 minutes, rest 20 minutes, repeat twice for a total of 3 sets. Try this for 2-3 days. It often returns the milk supply.
Other ideas -
There are medications reported to increase supply. They include metoclopramide (Reglan), cisapride (Propulsid), fenugreek, and other herbs. Unfortunately, there is no consensus regarding their utility or dosage. For more information, please see resources such as Thomas Hale’s Medications and Mothers’ Milk, UpToDate (on the ihs.gov web page), and the NIH lactation and medication search engine at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT. Also consider checking with your local NICU for what they suggest for preemie moms who are pumping.
Did the mom start birth control pills? They can disrupt milk supply, especially those with estrogen.
Several studies link regular pacifier use with decreased milk supply. Less (use of pacifiers) could be more (milk).
If the baby’s 6 month birthday is close, remind the mom that when solids start, the baby’s need for breast milk will begin slowing down.
For many different reasons, it is not always feasible for moms to pump or to pump enough... A mom may have already decided to formula feed during work time and breastfeed when they get home. With a routine work schedule, her body will adjust and produce milk when she is home.
For more ideas to supporting/advocating for breastfeeding employees, check with local breastfeeding coalitions. Helpful web resources include I.H.S. Lactation in the Workplace policy at www.ihs.gov/MedicalPrograms/MCH/M/bf.cfm and the United States Breastfeeding Committee web page at www.usbreastfeeding.org.CCC Corner Digest
Nicely laid out hard copy - A compact digest of last month’s CCC Corner
If you want a copy of the CCC Digest mailed to you each month, please contact nmurphy@scf.cc
Domestic Violence - Denise Grenier, Tucson / Rachel Locker, Warm Springs
SBIRT Program to Screen for Substance Abuse, Domestic Violence in Pregnant Women in Louisiana
A program designed to increase healthy outcomes for newborn babies is coming to Lafayette and New Orleans, Louisiana. The Healthy Babies Initiative screens pregnant women for tobacco use, alcohol use, drug use, domestic violence and depression and assists expectant mothers in getting treatment.
“This initiative focuses on critical issues that need to be addressed in order to pursue the vision that all children are born to lead healthy, productive lives,” said DHH Secretary Alan Levine. “The expansion of this program is an important step toward healthier outcomes for our state’s most vulnerable citizens.”
The program, also called SBIRT, operates through a system of Screening, Brief Intervention, Referral and Treatment measures. The women are screened within medical settings by a physician, nurse or clinician who identifies women at-risk for substance abuse or other related problems through a questionnaire process. The system provides for brief intervention or brief treatment, and refers those identified as needing more extensive services to a specialist.
http://www.dhh.louisiana.gov/news.asp?Detail=1361
More information from SAMHSA about SBIRT
http://sbirt.samhsa.gov/about.htm
Elder Care News - Bruce Finke, Elder Care Initiative
No Upper Age Limit for Benefit from Screening Mammography for Women without Significant Comorbidities
PURPOSE: Screening mammography guidelines for patients age 80 years and older are variable. We determined the effect of mammography use on stage at breast cancer diagnosis and survival among women of this age range.
PATIENTS AND METHODS: We used the linked Surveillance, Epidemiology, and End Results-Medicare database to evaluate 12,358 women >or= 80 years of age diagnosed with breast cancer between 1996 and 2002. Patients were grouped according to number of mammograms during the 60 months before diagnosis: nonusers (0 mammograms), irregular users (one to two mammograms), and regular users (three or more mammograms). Effects of mammography on disease stage (I to IIa v IIb to IV) and survival were determined by logistic regression and Cox proportional hazards analyses.
RESULTS: Percentages of women with nonuse, irregular use, and regular use of mammography during the 5 years preceding diagnosis were 49%, 29%, and 22%, respectively. On multivariate analysis, patients were 0.37 times less likely to present with late-stage cancer for each mammogram obtained (odds ratio, 0.63; 95% CI, 0.63 to 0.67). Breast cancer-specific 5-year survival among nonusers was 82%, that among irregular users was 88%, and that among regular users was 94%. However, survival from causes other than breast cancer was also associated with mammography use, suggesting a bias for healthier patients to undergo mammography. CONCLUSION: Regular mammography among women >or= 80 years of age was associated with earlier disease stage, although improved survival remains difficult to demonstrate. Health care providers should consider discussing the potential benefits of screening mammography with their older patients, particularly for those without significant comorbidity.
Badgwell BD , et al. Mammography before diagnosis among women age 80 years and older with breast cancer. J Clin Oncol. 2008 May 20;26(15):2482-8. Epub 2008 Apr 21. http://www.ncbi.nlm.nih.gov/pubmed/18427152
FDA notifies healthcare professionals that both conventional and atypical antipsychotics are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis.
http://www.fda.gov/cder/drug/InfoSheets/HCP/antipsychotics_conventional.htm
Family Planning
Cochrane Database - Interventions for Emergency Contraception
BACKGROUND: Emergency contraception is using a drug or copper intrauterine device (Cu-IUD) to prevent pregnancy shortly after unprotected intercourse. Several interventions are available for emergency contraception. Information on the comparative efficacy, safety and convenience of these methods is crucial for reproductive health care providers and the women they serve. OBJECTIVES: To determine which emergency contraceptive method following unprotected intercourse is the most effective, safe and convenient to prevent pregnancy.
SEARCH STRATEGY: The search included the Cochrane Controlled Trials Register, Popline, MEDLINE, PubMed, Biosis/Embase, Chinese biomedical databases and UNDP/UNFPA/WHO/World Bank Special Programme on Human Reproduction ( HRP) emergency contraception database (December 2006). Content experts and pharmaceutical companies were contacted.
SELECTION CRITERIA: Randomised controlled trials and controlled clinical trials including women attending services for emergency contraception following a single act of unprotected intercourse were eligible.
DATA COLLECTION AND ANALYSIS: Data on outcomes and trial characteristics were extracted in duplicate and independently by two reviewers. Quality assessment was also done by two reviewers independently. Meta-analysis results are expressed as relative risk (RR) using a fixed-effects model with 95% confidence interval (CI). In the presence of statistically significant heterogeneity a random-effect model was applied.
MAIN RESULTS: Eighty-one trials with 45,842 women were included. Most trials were conducted in China (70/81). There were more pregnancies with levonorgestrel compared to mid-dose (25-50 mg) (15 trials, RR: 2.01; 95% CI: 1.27 to 3.17) or low-dose mifepristone (<25 mg) (9 trials, RR: 1.43; 95% CI: 1.02 to 2.01). Low-dose mifepristone was less effective than mid-dose (20 trials, RR:0.67; 95% CI: 0.49 to 0.92), but this effect was no longer statistically significant when only high quality trials were considered (6 trials, RR: 0.75; 95% CI: 0.50 to 1.10). Single dose levonorgestrel (1.5 mg) administration seemed to have similar effectiveness as the standard 12 hours apart split-dose (0.75 mg twice) (2 trials, 3830 women; RR: 0.77, 95% CI: 0.45 to 1.30). Levonorgestrel was more effective than the Yuzpe regimen in preventing pregnancy (2 trials, RR: 0.51; 95% CI: 0.31 to 0.83). CDB-2914 (a second-generation progesterone receptor modulator) may be as effective as levonorgestrel (1 trial, 1549 women; RR:1.89; 95% CI: 0.75 to 4.64) but the confidence interval is wide and the result compatible with higher or lower effectiveness. Delay in the onset of subsequent menses was the main unwanted effect of mifepristone and seemed to be dose-related.
AUTHORS' CONCLUSIONS: Mifepristone middle dose (25-50 mg) was superior to other hormonal regimens. Mifepristone low dose (<25 mg) could be more effective than levonorgestrel 0.75 mg (two doses) but this was not conclusive. Levonorgestrel proved more effective than the Yuzpe regimen. The copper IUD was another effective emergency contraceptive that can provide ongoing contraception.
Cheng L , Gülmezoglu AM, Piaggio G, Ezcurra E, Van Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008 Apr 16;(2):CD001324. http://www.ncbi.nlm.nih.gov/pubmed/18425871
Intermediate-term glucose tolerance in women with a history of gestational diabetes: natural history and potential associations with breastfeeding and contraception
OBJECTIVE: This study investigated the natural history of glucose tolerance by using modern definitions in women after delivery of a pregnancy complicated by gestational diabetes. The association between deterioration of glucose metabolism and contraceptive methods was also studied.
STUDY DESIGN: Retrospective chart review of 592 indigent, primarily Latina women who had been diagnosed with gestational diabetes, monitored for up to 24 months' postpartum. RESULTS: At the first postpartum visit, 230 women (40.2%) had prediabetes or diabetes. Within the first 12 months, 26.4% experienced deterioration. Of the 89 women monitored for 12-24 months, another 38.5% had prediabetes (n = 13) or diabetes (n = 11) develop. About 22% of women by using only nonhormonal contraception experienced worsening of their glucose status, whereas 35% of combined hormonal contraceptive users and 34% of progestin-only users worsened. CONCLUSION: Gestational diabetes is a sentinel event signaling the need for frequent testing postpartum.
Nelson AL , Le MH, Musherraf Z, Vanberckelaer A. Intermediate-term glucose tolerance in women with a history of gestational diabetes: natural history and potential associations with breastfeeding and contraception. Am J Obstet Gynecol. 2008 Jun;198(6):699.e1-7; discussion 699.e7-8. Epub 2008 Apr 25. http://www.ncbi.nlm.nih.gov/pubmed/18439553Featured Website
The “JOINT COMMISSION” (formerly JCAHO)
The Joint Commission has released the 2009 standards on-line; press release follows:
The Joint Commission’s revised standards, rationales and elements of performance for 2009 are now available online. The standards will take effect January 1, 2009 and have been placed online to give all health care organizations time to become familiar with the new language, ordering and numbering.
The changes are part of the Standards Improvement Initiative ( SII), launched in 2006 as part of The Joint Commission’s ongoing quality improvement efforts. SII focuses on clarifying standards language, ensuring that standards are program-specific, deleting redundant and nonessential standards, and consolidating similar standards. While no new requirements were added, chapter overviews, standards, introductions, rationales, and elements of performance were designed for ease of use. In the standards reorganization, requirements were split or consolidated. Standards have been renumbered and reordered to allow electronic sorting and to allow the addition of new requirements in the future.
“The Standards Improvement Initiative represents The Joint Commission’s continuous commitment to make the standards clear, relevant, and applicable in the specific health care setting in which they are used,” says Mark R. Chassin, M.D., M.P.P., M.P.H., president, The Joint Commission. “These changes will better guide health care organizations in providing patients with the best care possible.”
A history tracking report is available online to help organizations see what changes occurred from previous to revised standards. The history tracking allows users to see what happened to each standard, its new number, and how it changed.
The Joint Commission sought extensive input from accredited and non-accredited health care organizations, advisory groups, payers, purchasers, consumers, governmental agencies, Joint Commission surveyors and other experts. Online surveys, interviews, meetings, and focus groups were all utilized to gather comments and suggestions. The Joint Commission will engage in extensive education efforts and discussions in the coming months to assist organizations in understanding the changes.
Other important aspects of the Standards Improvement Initiative include:
- Phase I of the SII focused on the accreditation programs for hospitals, critical access hospitals, ambulatory care, office-based surgery, and home care organizations;
- SII ’s Phase II for behavioral health care, laboratory and long term care accreditation programs began in 2008 and the standards changes will take effect in January 2010. The Accreditation Participation Requirements, Life Safety Code, and Leadership chapters for these programs are online;
- Changes in the scoring and decision process will take place January 1, 2009 for all accreditation and certification programs;
- Single-user license electronic E-ditions of the manuals will be provided for the first time;
- Color-coded tabs in print manuals distinguish standards and requirements from accreditation policies and procedures;
- Accreditation program-specific language used in all manuals;
- With E-ditions, ability to sort relevant standards and elements of performance applicable to the services provided by an individual organization;
- Links from certain EPs to associated requirements in other chapters; and
- Standards and EPs related to a focused area of improvement placed in relevant chapters.
Additional details about the revisions are available on the Standards Improvement Initiative web page.
Main JC Website:
http://www.jointcommission.org/
View the press release at:
http://www.jointcommission.org/NewsRoom/NewsReleases/nr_sii_gen.htm
Direct access to Standards page:
http://www.jointcommission.org/Standards/SII/default.htm
Joint Commission Issue Sentinel Event Alert on Behaviors that Undermine a Culture of Safety
Intimidating and disruptive behaviors can foster medical errors, contribute to poor patient satisfaction and to preventable adverse outcomes, increase the cost of care, and cause qualified clinicians, administrators and managers to seek new positions in more professional environments. Safety and quality of patient care is dependent on teamwork, communication, and a collaborative work environment. To assure quality and to promote a culture of safety, health care organizations must address the problem of behaviors that threaten the performance of the health care team…
Read the full Sentinel Event Alert at: http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_40.htmFrequently Asked Questions
Q. How to Strengthen Your Patient’s Pelvic Floor Muscles?
A. The Women’s Health web page offers many resources to help your patient improve her symptoms of pelvic prolapse, urinary incontinence, and pelvic pain.
http://www.ihs.gov/MedicalPrograms/MCH/W/MatPT.cfm#top
Indian Child Health Notes - Steve Holve, Pediatrics Chief Clinical Consultant
“It doesn’t matter if the cat is black or white as long as it catches mice.”
Deng Hsaio P’ing
This a page for sharing “what works” as seen in the published literature as well as what is done at sites that care for American Indian/Alaskan Native children. If you have any suggestions, comments or questions please contact Steve Holve, MD, Chief Clinical Consultant in Pediatrics at steve.holve@tchealth.org
IHS Child Health Notes
August 2008
Quote of the month
“ The beginning is the most important part of any work .”
Plato
Introduction
This spring and summer most IHS clinics have had requests for blood lead level testing for children entering Head Start. This is an attempt to explain why blood level testing is mandatory for Medicaid eligible AI/AN children even if the risk of elevated lead levels is likely small in our patients.
Blood Lead Screening in American Indian/Alaska Native Children
In March of 2008 the Office of Head Start issued an Information Memorandum that reaffirmed the requirement within the Head Start Program Performance Standards for all children entering the program to be screened for lead poisoning as per the schedule of well child care utilized by the EPSDT program.(1). It appeared that many Head Start programs that serve American Indian/Alaska Native (AI/AN) children were encountering difficulty in obtaining documentation of the required screening blood lead levels (BLLs) performed at 12 and 24 months of age. The Health Director of the IHS Head Start Program asked the Indian Health Service for guidance on this issue. This request prompted a review of current standards for screening BLLs in children < 6 years old.
Lead is a potent neurotoxin. At higher levels (>70ug/dL) lead poisoning can cause encephalopathy and death. Such extreme lead toxicity is now rare as primary prevention efforts in the past few decades mandated the removal of lead from gasoline and consumer products. Lead exposure from paint in older buildings remains a persistent source of exposure to lead. Recently there has been concern over lead toxicity in toys imported from China. Unfortunately, even small amounts of lead can have irreversible effects on brain development in young children. It is estimated that every 10ug/dL increment in BLLs can decrease IQ scores by 3 to 7 points (2). At present, the accepted definition for elevated BLLs is >10ug/dL. However, there is recent research that BLLs even below 10ug/dL may be deleterious (3). Since BLLs <25ug/dL are clinically undetectable only screening can detect these cases.
Who should be screened for lead exposure? Data show that lead poisoning is overwhelmingly a disease of young children in poverty. In the NHANES III survey in 1994 the overall prevalence of elevated BLLs (>10ug/dL) in 1- 5 year olds was 4.4%. However, the prevalence of elevated BLLs was only 0.9% in upper income children while the rate was as high as 21% in poor children who lived in older homes. Further analysis showed that 10% of children enrolled in Medicaid had elevated BLLs. Medicaid enrollees also accounted for 60% of the total children with BLL >10ug/dL and 83% of the children with BLLs>20ug/dL (4).
Many AI/AN children potentially would fall into the high- risk group because of widespread poverty in many AI/AN communities. However, few AI/AN communities have commercial homes built before the 1950s, which would tend to diminish the likelihood of exposure to lead paint.
The current prevalence of elevated BLLs in AI/AN children is unclear. A review of Pubmed for the past 20 years with the search terms, “American Indian”, “children” and “lead” disclosed only one article on mining exposures in Oklahoma. The Indian Health Service has unpublished data from the mid 1990s on lead levels in 7of 12 Areas. The percentage of children screened in each area varied widely and not all tribal groups were included. However, in this limited data set the overall rate of elevated BLLs after confirmatory testing was < 1% which was lower than the national rate.
Given the low prevalence of lead poisoning in AI/AN children tested, it was agreed that targeted screening of AI/AN children at high risk for lead exposure was a better strategy than universal testing. Universal testing was also discouraged by the United States Preventive Services Task Force (5). No lead screening or targeted lead screening became the standard in most clinics but was never explicitly made a policy for the Indian Health Service. A summary of the costs and benefits of lead screening in AIA/AN children was published in The IHS Primary Care Provider in 1994 (6).
An informal survey of Indian Health Service/Tribal/Urban (I/T/U) clinics suggests that most practitioners have assumed that lead exposure is not a problem in their communities. This lack of lead screening is hardly unique for I/T/U clinics. Despite the demonstrated elevated risk in poor children only 20% of Medicaid enrollees nationwide were screened for BLLs in a recent evaluation by the Office of the Inspector General (7).
However, a review of federal standards indicates BLL testing is a mandated service for all children eligible for Medicaid and Head Start. The Center for Medicaid and Medicare Services (CMS) requires that all Medicaid-eligible children receive a screening blood test at 12 months and 24 months of age (8). Children between the ages of 36 to 72 months must also have a BLL test if lead screening has not been done previously. Head Start is also required to follow the standard of care set forth by CMS. CMS made this decision based on the demonstrated risk of elevated BLL in Medicaid enrollees compared to the general population.
Data show that up to 80% of AI/AN children < 6 years of age in reservation communities are eligible for Medicaid and Head Start. With this high percentage of Medicaid enrollees it is likely more efficient to screen all AI/AN children < 6 years old for elevated BLLs. The risk of elevated BLLs in AI/AN children is likely low, but there is no recent, comprehensive data, and there is no data that includes all tribal groups.
Given the high incidence of iron deficiency anemia in AI/AN children many I/T/U clinics already screen for anemia with a complete blood count (CBC) at 9 months and 18 months. Testing at 9 months of age is preferable to testing at12 months as this will result in the earlier detection of iron deficiency anemia earlier treatment. It is also known that iron deficiency promotes lead absorption and that repletion of iron stores will diminish absorption of lead (9 & 10). Therefore, timely treatment of iron deficiency is one of the best preventive measures to minimize lead poisoning. BLLs tend to peak at age 24 months so this is the optimal time for the follow-up test to be done. To minimize the number of blood draws CBC and lead testing could be combined in the following schedule:
9-12 months: draw a CBC and lead level
24 months: draw a CBC and lead level
36-72 months – draw a lead level if not done previously
Management of elevated BLLs (>10ug/dL) is beyond the scope of this report. Follow-up should be based on the guidelines published in the AAP Policy statement lead poisoning published in 2005 (2).
Lead testing will cost I/T/U sites money. This test is usually performed in a reference lab and a BLL usually costs about $15. Medicaid pays a global fee for outpatient visits to most I/T/U clinics so the cost of the test is not recoverable. However, BLL is a CMS mandate: Failure to perform a mandated test could jeopardize Medicaid reimbursement. Money may need to be reprogrammed to I/T/U laboratory budgets to cover this cost.
Lastly, each clinic will need to track BLLs in their patient population over the next year. This will let us answer the question of which, if any, AI/AN children are at risk for lead poisoning. Tribal groups with low lead levels may be able to request a waiver of BLL testing from CMS in the future. As important, if some tribal groups are found to be at elevated risk, appropriate environmental investigation and amelioration can begin.
References
- Lead Screening ACF-IM-HS-08-07. Administration for Children and Families. U.S. Department of Health and Human Services. Issued 3/20/08. www.acf.hhs.gov/programs/ohs/policy/im2008/acfimhs_08_07.html
- Lead Exposure in Children: Prevention, Detection and Management. American Academy of Pediatrics Policy Statement. Pediatrics: 2005 Oct;116(4):1036-1046.
- Canfield RL, Henderson CRjr, Cory-Slechta DA, Cox C, Jusko TA, Lamphear BP. Intellectual Impairment in children with blood concentrations below 10ug per deciliter. N Eng J Med 2003:348:1517-1526.
- Recommendations for blood lead screening of young children enrolled in Medicaid: Targeting a group at high risk. MMWR December 08, 2000 / 49(RR14);1-13.
- USPSTF Recommendations for Lead Screening. Pediatrics. 2006;118:e2514-2518.
- Batholomew M Vanderwagen C. Lead screening of Native American children: Targeted or Screening? The IHS Primary Care Provider 1999 August 24(8):125-126.
- Lead Poisoning: Federal Health Care Programs are not Effectively Reaching At-Risk Children. U.S. General Accounting Office, January 1999.
- EPSDT Benefits: Accessed on June 12, 2008 http://www.cms.hhs.gov/MedicaidEarlyPeriodicScrn/02_Benefits.asp.
- Barton JC, Conrad ME, Nuby S, Harrison L Effects of iron on the absorption and retention of lead. J Lab Clin Med. 1978 Oct;92(4):536-47.
- A Bradman, B Eskenazi, P Sutton, M Athanasoulis, and L R Goldman Iron deficiency associated with higher blood lead in children living in contaminated environments. Environ Health Perspect. 2001 October; 109(10): 1079–1084.
Infectious Disease Updates.
Steve Holve, MD
Pentacel® : New May not be Better for American Indian/Alaska Native Infants
A new combination vaccine, Pentacel® (DTaP-IPV- Hib [PRP-T]), was recently licensed for use in the United States. This vaccine is not ideal for American Indian/Alaska Native (AI/AN) infants served in federal, tribal and urban Indian clinics.
Pentacel® is a vaccine that protects against five serious infections; diphtheria, tetanus, pertussis, polio, and haemophilus influenzae type b (Hib). It is the first combination vaccine in the United States that includes both poliovirus and haemophilus antigens. The vaccine is administered to children at 2, 4, 6, and 15-18 months of age.
There are currently several Hib vaccines available which vary in the protein used to make the vaccine more immunogenic. In Pentacel®, the Hib component of the vaccine is a purified polyribosylribitol phosphate (PRP) capsular polysaccharide covalently bound to tetanus protein (PRP-T). The current Hib vaccine recommended for AI/AN infants has a meningococcal outer membrane protein (OMP) bound to the PRP molecule and is designated as a PRP-OMP Hib vaccine .
The Redbook of the American Academy of Pediatrics recommends that for AI/AN infants the first dose of a Hib conjugate vaccine should contain PRP-OMP. For AI/AN infants “the administration of a PRP-OMP-containing vaccine leads to more rapid seroconversion to protective concentrations of antibody within the first 6 months of life, and failure of use has been associated with excess cases of Hib disease in young infants in this population”. (1)
In 1996 Alaska switched from a PRP-OMP Hib vaccine to combination HbOC-DTP vaccine (Tetramune®). During 1996-1997, 17 Hib cases occurred in Alaska Native children <5 years of age, increasing the rate of Hib disease from 19 to 91 cases per 100,000 per year. 8 of the cases occurred in partially vaccinated children who had received 1 or 2 doses of HbOC. Since 2001, Alaska has adopted a schedule using PRP-OMP alone, and the subsequent rate of Hib disease in Alaska Native infants decreased to 5.4 per 100,000 per year.(2)
Physicians, pharmacists and immunization coordinators who work for federal, tribal and urban Indian clinics need to be aware of this change and insure that their state’s VFC program continues to supply a PRP-OMP Hib vaccine to their AI/AN patients.
1. 2006 Report of the Committee on Infectious Diseases. American Academy of Pediatrics; Elk Gove, IL page 89.
2. Singleton et al., The Alaska Haemophilus influenzae type b experience: Lessons in controlling a vaccine-preventable disease. Pediatrics 2006;118:421-429.
Addendum:
Questions have arisen over whether this recommendation applies to all AI/AN children. The above update was written for an audience that works in federal, tribal and urban Indian clinics. Private clinics near reservation communities may serve significant numbers of AI/AN patients and may wish to have a PRP-OMP containing Hib vaccine available. The risk of invasive Hib disease for AI/AN infants who do not live on or near reservation communities is unknown.
This issue of increased risk for invasive Hib disease was addressed in the MMWR in December 2007 due to a decrease in the supply of PRP-OMP Hib vaccine due to production difficulties. The pertinent summary of the risk for AI/AN infants is below. Each practitioner should make a decision based on their assessment of risk of invasive Hib for their patients. The full posting with references is available at:
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5650a4.htm
American Indian/Alaska Native (AI/AN) children also are at increased risk for Hib disease, particularly in the first 6 months of life. Before the use of Hib conjugate vaccines, the incidence of Hib disease among young AI/AN children in AI/AN communities was approximately 10 times higher than among children of comparable age in the general population. Compared with PRP-TT conjugate vaccines, the administration of PRP-OMP vaccines leads to a more rapid seroconversion to protective antibody concentrations within the first 6 months of life. Failure to use PRP-OMP vaccines for the first dose is associated with excess cases of Hib disease in AI/AN infants living in communities where Hib transmission is ongoing and exposure to colonized persons is likely. Although PRP-OMP and PRP-TT vaccines are equally effective after completion of the primary series, availability of more than one Hib vaccine in a clinic could lead to administration of the wrong vaccine for the first dose in these populations. For these reasons, CDC recommends that providers who currently use PRP-OMP--containing Hib vaccines (PedvaxHIB and Comvax) to serve predominantly AI/AN children in AI/AN communities continue to stock and use only PRP-OMP-- containing Hib vaccines not affected by the recall and vaccinate according to the routinely recommended schedules, including the 12--15 month booster dose. In its vaccine stockpile, CDC has PRP-OMP--containing Hib vaccines not affected by the recall and will prioritize distribution of available PRP-OMP vaccines for use in AI/AN communities. AI/AN children not in AI/AN communities or who already receive PRP-TT conjugate vaccines should continue to be vaccinated with available vaccines according to the routinely recommended schedules, including the 12--15 month booster dose.
Recent literature on American Indian/Alaskan Native Health
Michael L. Bartholomew, MD
Brim SN, Rudd RA, Funk RH, Callahan DB. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008 Jul;122(1):e217-22.
Asthma continues to be a prevalent childhood disease in the United States. Recent studies estimate that 8.9% of US children ages 0 to 17 years of age have asthma 1. Asthma prevalence among Asian Americans, specifically subgroups of Asian Americans, and American Indians and Alaska Natives (AI/AN) has largely been unknown.
Anecdotal reports of the 1960’s and 1970’s suggested that asthma in American Indians was rare 2. In the 1990s, Clark et al showed that asthma was more prevalent than once believed when he documented the asthma burden in Jemez Indian childhood population 3. Between 2004-2005, the prevalence of asthma in AI/AN children was estimated to be 9.9% 1.
Many recent studies defining asthma prevalence rates among children have yielded varied results in regards to age, race, socioeconomic and ethnic backgrounds. Due to small sample sizes in large scale studies, the national prevalence of diseases has thus far been difficult to determine for certain ethnic groups or subgroups.
This study investigates the prevalence of current asthma, lifetime asthma, and asthma attacks among subpopulations (Chinese, Filipino, and Asian Indian) of Asian American and AI/AN children ages 2 to 17 years and place of birth by analyzing aggregate data from the 2001-2005 National Health Interview Surveys. The prevalence of lifetime and current asthma and the prevalence of 12-month asthma attacks were calculated from responses to questions posed during the survey. Logistic regression models were used to determine the association of current asthma prevalence with race and place of birth by controlling for certain confounding demographic variables.
Estimates of current asthma prevalence according to race and place of birth ranged from 4.4% for Asian Indian children to 13.3% in black children. AI/AN children had an asthma prevalence of 13.0%, while Filipino and Chinese children had a prevalence of 10.7% and 5.1%, respectively. White children had a current asthma prevalence of 8.4%.
Lifetime asthma prevalence estimates among races showed a similar trend. The lifetime prevalence among the races ranged from 9% in Chinese children to 18.1% for black children. The lifetime asthma prevalence estimates for AI/AN children was 18 % and for Asian Indian and Filipino was 9.4% and 15.7% respectively. A high prevalence of current and lifetime asthma was noted in children born in the United States than in children born outside the United States (Current asthma: 9.4% vs. 4.3%; Lifetime asthma: 13.6% vs. 7.3%). The prevalence of asthma attack in the past 12 months did not vary among races or place of birth.
After controlling for place of birth, gender, age, ethnicity, region, household income, and health insurance coverage, AI/AN children are 1.82 times likely to report having asthma than their White counterparts. Black children are 1.57 times likely while Filipino children are 1.64 times likely. Additionally, children born inside the United States were twice as likely to report having asthma as children born outside the United States.
This study is not without limitations. The authors cite small sample sizes of the study populations to be problematic, requiring aggregate data of 5 years to provide limited statistical power. There is also the potential for selection bias since the survey was administered in either the English or Spanish language. Lastly, additional risk factors (BMI and environmental tobacco smoke exposure) for asthma were not analyzed due to lack of inclusion in the survey model. Despite these limitations, the authors concluded that the results support previous assertions that certain ethnicities including black, AI/AN, and Filipino children as well as those born in the United States tend to have a disproportionately higher prevalence of asthma.
Reference:
- Akinbami L; Centers for Disease Control and Prevention, National Center for Health Statistics. The state of childhood asthma, United States, 1980-2005. Adv Data. 2006;(381):1-24.
- Galloway JM, Goldberg BW, Alpert JS. 1999. Primary Care of Native American Patients: Diagnosis, Therapy, and Epidemiology. Boston: Butterworth-Heinemann
- Clark D, Gollub R, Green WF, Harvey N, Murphy SJ, Samet JM. Asthma in Jemez Pueblo schoolchildren. Am J Respir Crit Care Med. 1995 May;151(5):1625-7.
Information Technology
iCare Population Management GUI version 1.1 Released
The Indian Health Service Office of Information Technology (OIT) and the iCare Development team are pleased to announce the release of iCare Population Management GUI version 1.1.
A release notice from OIT Software Quality Assurance (SQA) has been sent to all Area CIOs/ ISCs and RPMS site managers. Because iCare v1.1 provides the ability to display performance data from the newest version of the RPMS Clinical Reporting System ( CRS), users may need access to this new version as soon as possible. Software installation is required for both the RPMS server and all end-user workstations; iCare v1.0 client software will not work with v1.1 server software.
The beta sites for this version of iCare were:
- Red Lake
- United American Indian Involvement Urban Site in Los Angeles, CA
- Parker Indian Hospital
iCare v1.1 is available for download at http://www.ihs.gov/Cio/RPMS/index.cfm?module=home&option=softwarechoice
The following end user enhancements are included:
- iCare will now automatically display national performance data based on the most recent version of RPMS Clinical Reporting System ( CRS) loaded on your facility’s server; this should be CRS 2008 ( BGP v8.0). That is, an iCare patch will not be required to maintain synchronization with new versions of CRS.
- Individual Health Maintenance Reminders can now be displayed for an entire panel in the grid View, not just on an individual Patient Record. The Reminders grid can be sorted, filtered and customized by users to display any specific reminders they want to see.
- An Aggregated Health Maintenance Reminders view is additionally provided as a performance overview, similar to the Aggregated National Performance view. The Aggregate view provides the percent of eligible patients in the panel who are overdue or on schedule for each reminder.
- The Patient Record has expanded to display most Patient Care Component ( PCC) data: Elder Care; Exams; Health Factors; Immunizations; Lab; Measurement; Medication; Patient Education; Procedures; Radiology; Skin Test. These views are located under the new PCC tab; users can select display timeframes and can sort by any data column.
- An existing case management function to enter PCC historical event data has been enabled.
- The Referral Tab on the Patient Record displays data from the Referred Care Information System (RCIS).
- The Diagnostic Tag display on the Patient Record has been expanded to show additional data. Including a link to the visit(s) containing the specific data that “qualified” the patient for the tag.
- The Panel Definition Search options have been enhanced to include filters for the visit date range by minimum number of visits; past appointments; and Community Taxonomies.
All training for iCare is done remotely and is web based. Specific information about these sessions and how to register for them can be found on the IHS iCare Website: http://www.ihs.gov/CIO/ca/icare/index.asp.
Additionally, several of these sessions have been recorded so that users can access them at any time. They are available to you from the WebEx website:
https://ihs-hhs.webex.com/mw0305l/mywebex/default.do?siteurl=ihs-hhs. Type iCare in the search bar and make your selection of which recorded session you want to access.
IMPORTANT POINTS:
Users will not be able to access the new version of iCare unless their workstation (client) is updated with v1.1 software. Depending on your facility’s abilities to automate user workstation updates, Site Managers may need to manually update software on workstations.
Site Managers are encouraged to deploy the server software on Friday evening so that background processes can run over the weekend and be ready for users at the beginning of the week.
Existing iCare v1.0 panels created by and/or shared with current iCare users will transfer to iCare version 1.1.
For user support please contact the RPMS help desk at RPMSHelp@ihs.gov or call (888) 830-7280.International Health Update - Claire Wendland, Madison, WI
Cervical cancer screening where resources are scarce
Every year, over a quarter million women worldwide die of cervical cancer. Cervical cytology has made a huge impact in the wealthier parts of the world, but cytology programs require substantial infrastructure. If your region (or even your country) doesn’t have a cytologist, or has no reliable means of transporting Pap tests to a central location or tracking women down to follow up positive results, cytology makes a poor screening tool. It’s not surprising, then, that today third-world women are disproportionately affected by cervical cancer. Researchers have been looking for simple, effective, and affordable screening tests, and two recent studies take us a step further toward this goal.
Arbyn and colleagues report a new meta-analysis of eleven large screening studies in India and in Francophone Africa. The studies, sponsored by the Gates Foundation, enrolled more than 58000 women. Each woman was screened with two or more of the following methods: VIA (visual inspection with acetic acid); VILI (visual inspection with Lugol's iodine solution); VIAM (visual inspection with acetic acid and a magnifying loupe); Pap (standard Papanicolaou screening); or HC2 (HPV high-risk probe of the Hybrid Capture-2 assay). No matter whether screening was positive or negative, colposcopy was immediately done on all women. Negative colposcopy was accepted as a negative result for purposes of analysis. Positive colposcopy required colposcopic biopsies which were then interpreted by a pathologist. The authors then compared sensitivity and specificity of all of these methods for detecting biopsy-proven CIN2 or worse – with some surprising results.
The visual inspection methods, especially VILI, performed very well. The authors were surprised at the poor performance of the HPV test and speculate that samples may have deteriorated due to high temperatures at many of the study sites.
The study has two significant weaknesses. First, colposcopy and colposcopically directed biopsies tend to identify -- and perhaps more importantly, to miss -- the same lesions as the VIA or VILI methods. Thus its use as the "gold standard" in this study probably led to overestimation of the visual methods as screening tests. (Cone biopsy or hysterectomy specimens would be better gold standards methodologically, but impossible to justify ethically.) Second, other studies have showed much greater variability in the quality of visual methods for screening. The good performance in this group probably reflects their rigorous training. All screeners had gone through an intensive 5-day course and had been proctored for 150-200 exams before they began enrolling patients, which certainly represents more formal training than I had as an obstetrician-gynecologist performing screening for cervical cancer!
In real-world situations, then, visual methods have a good (12-13%) positive predictive value and an acceptable (well over 99%) negative predictive value. They’re affordable, quick, and easy. So what sort of difference might a very simple visual screening program make? Another large study shows the savings in women’s lives can be substantial. Investigators working in Tamil Nadu randomized panchayaths or municipal units in one administrative district to "standard care" -- meaning no cervical cancer screening -- or an intervention in which study nurses did a single round of VIA screening. Women who were VIA-positive immediately had colposcopy. When colposcopy suggested precancerous lesions (low-grade or high-grade) directed biopsies were taken and cryotherapy or LEEP done immediately. Follow-up of the over 70,000 women in this district used an intention-to-treat model and lasted seven years from the initiation of the study. Cervical cancer incidence, cervical cancer mortality, and overall mortality rates were equal between the two groups the first year of analysis, but there have been widening gaps every year since. Despite the bolus of patients diagnosed with cervical cancer in the intervention group at screening, by the time of this report the intervention group demonstrated a significant 25% reduction in cervical cancer incidence, and a 35% reduction in cervical cancer mortality compared with the control group.
Simpler screening methods aren’t perfect, but they turn out to be pretty good. Now it’s time to figure out how to get them implemented in the poorer parts of the world.
Sankaranarayanan R et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet 370:398-406, 2007
http://www.ncbi.nlm.nih.gov/pubmed/17679017
Arbyn M et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. International Journal of Cancer 123 (1): 153-60, 2008
http://www.ncbi.nlm.nih.gov/pubmed/18404671
MCH Alert
Randomized Controlled Trial Investigates the Safety of Dental Treatment in Pregnant Women
"In this population, periodontal treatment and EDT [essential dental treatment], administered at 13 to 21 weeks' gestation, did not significantly increase the risk of any adverse outcome evaluated," state the authors of an article published in the June 2008 issue of the Journal of the American Dental Association. Collectively, recent findings suggest that use of oral health services during pregnancy may be driven more by attitudes than by economics or convenience. Attitudes and behaviors may be influenced by fear of harm to the pregnant woman or fetus, fear of litigation, or patient safety concerns. The article describes safety outcomes related to the provision of oral health care in pregnant women.
Data for the study were drawn from a multi-center randomized controlled clinical trial (the Obstetrics and Periodontal Therapy [ OPT] Trial) conducted to determine whether periodontal therapy in pregnant women reduces the risk of preterm delivery. The study sample included women recruited from obstetrics clinics serving minority and other underserved groups who are at an elevated risk of experiencing preterm birth. All women had periodontitis. A total of 413 women were randomly assigned to receive scaling and root planing at a time before 21 weeks' gestation.
The 410 women in the control group were monitored during their pregnancy and treated with scaling and root planing after delivery. Women in both groups were also evaluated for EDT needs (defined as the presence of moderate-to-severe caries or fractured or abscessed teeth). The analysis examined associations between adverse pregnancy outcomes (spontaneous abortion or fetal death, pregnancy ending before 37 weeks' gestation, fetal or congenital anomaly) and EDT, anesthetic use during nonsurgical periodontal treatment, and combinations of EDT and periodontal treatment.
The authors found that
- EDT administered at 13 to 21 weeks' gestation was not associated with an increased risk of experiencing serious medical adverse events, preterm (less than 37 weeks' gestation) deliveries, spontaneous abortions or fetal deaths, or fetal anomalies.
- Use of topical and local anesthetics for scaling and root planing was not associated with an increased risk of experiencing these adverse events and outcomes.
"Several ongoing randomized controlled intervention trials should help to define more precisely the risk of adverse pregnancy outcomes associated with nonsurgical periodontal procedures, including the risk of spontaneous abortion and stillbirth," state the authors. "Nevertheless," the authors conclude, "our study provides evidence that dental care providers can safely meet the preventive and routine treatment needs of their pregnant patients."
Michalowicz BS, DiAngelis AJ, Novak MJ, et al. 2008. Examining the safety of dental treatment in pregnant women. The Journal of the American Dental Association 139(6):685-695. Abstract available at http://jada.ada.org/cgi/content/short/139/6/685.
More information is available from the following MCH Library resource:
Oral Health and Pregnant Women, Infants, Children, and Adolescents at http://www.mchlibrary.info/KnowledgePaths/kp_oralhealth.html
Article Evaluates Impact of a Prenatal Substance Abuse Treatment Program on Perinatal Outcomes
"Early Start's replicable model of integrating substance abuse treatment with prenatal care is cost-effective and significantly decreases negative birth outcomes as well as maternal morbidity," state the authors of an article published in the June 26, 2008, issue of the Journal of Perinatology. Substance abuse during pregnancy is a serious problem in the United States; it results in considerable adverse effects for women and their infants. Kaiser Early Start is a coordinated prenatal substance abuse treatment program that is part of Kaiser Permanente Northern California's (KPNC's) comprehensive prenatal program. The purpose of the study described in this article was to provide a comprehensive evaluation of the effect of Early Start on maternal and neonatal outcomes.
The study cohort included 49,985 female KPNG members who completed Early Start prenatal substance abuse screening questionnaires between January 1, 1999, and June 30, 2003. Fetal and neonatal outcomes analyzed included intrauterine fetal demise (IUFD), neonatal-assisted ventilation, low birthweight, preterm delivery, completed weeks of gestation, neonatal intensive care unit admission, infant re-hospitalization within 30 days of discharge from birth hospitalization, and infant emergency department visits within 180 days of discharge from birth hospitalization. Maternal outcomes analyzed were Cesarean delivery, preterm labor, and placental abruption. The authors defined four study groups. Group 1: screened, assessed positive for substance abuse, and treated by Early Start ( SAT); Group 2: screened, assessed positive, and not treated (SA); Group 3: screened only and identified as substance abusers (S), and Group 4: controls (C)
(women with no evidence of substance abuse during pregnancy).
The authors found that:
- Compared with controls, SAT women had similar or higher rates on most risk factors (weekly/daily use of alcohol, methamphetamines, THC [marijuana], cocaine, heroin, and cigarettes both before and after pregnancy), but lower rates than S women. For the most part, the rates of SA women were between SAT and S rates.
- In multivariate analyses, the S group had significantly worse outcomes than the SAT group in the following areas: preterm delivery (odds ratio [OR] = 2.1, 1-3-3.2), placental abruption (OR = 6.8, 3.0-15.5), and IUFD (OR = 16.2, 6.0-43.8).
The authors conclude that "The results of this study reflect the importance of widespread implementation of this model of care as a national standard."
Goler NC , Armstrong MA, Taillac CJ, et al. 2008. Substance abuse treatment linked with prenatal visits improves perinatal outcomes: A new standard. Journal of Perinatology [published online ahead of print on June 26, 2008]. http://www.nature.com/jp/journal/vaop/ncurrent/abs/jp200870a.html .
Preconception and Pregnancy: Knowledge path at: http://www.mchlibrary.info/KnowledgePaths/kp_pregnancy.html
Annual Study Released on Women’s Health Outcomes in U.S. Hospitals
The Fifth Annual HealthGrades Women's Health Outcomes in American Hospitals Study identifies outcomes for maternity care and in-hospital treatment of cardiovascular disease ( CVD) in women using data from the period 2004-2006 in 17 states. The report provides maternal complication rates for vaginal, clinically indicated Cesarean section (C-section), and patient-choice (non-clinically indicated) C-section deliveries. The report also includes neonatal mortality rates for all hospitals evaluated. Risk-adjusted analysis of in-hospital treatment identifies mortality rates in CVD and stroke among women. In addition, the report identifies top-performing hospitals. The report is available at:
http://www.healthgrades.com/media/DMS/pdf/HealthGradesWomensHealthStudy2008.pdf
HRSA Report Studies Safety Net Health Centers
Health Centers: America’s Primary Care Safety Net describes the history of safety net health center programs; safety net health centers' role in providing a medical home; the model of care used, and how connections are sustained between primary care associations, offices, and other partners. The report, produced by the Health Resources and Services Administration’s Bureau of Primary Health Care, also discusses the unprecedented growth of the health center program during the period 2002-2007 and outlines issues related to the program's future in the following areas: work force, health information technology, emergency management, quality, and performance measurement. Program successes are discussed, as well. The report is available at:
ftp://ftp.hrsa.gov/bphc/HRSA_HealthCenterProgramReport.pdf
MCH Headlines - Judy Thierry HQE
Three Maternal Risk Factors Associated with Elevated Risk of Postneonatal Mortality among Alaska Native Population
OBJECTIVE: Compared to non-Natives in Alaska, the Alaska Native population has a postneonatal mortality rate 2.3 times higher (95% CI 1.9, 2.7). The objective of the study was to identify variables that account for this elevated risk.
METHODS: The dataset used included birth and death certificate records for all Alaska-resident live births and infant deaths occurring during 1992-2004. Race was defined as Alaska Native or non-Native. The association between race and postneonatal mortality was examined using univariate, stratified and regression analyses. Variables were considered confounding if they resulted in a change of at least 10% in the odds ratio between race and postneonatal mortality when added to a bivariate model, or when removed from a multivariate model.
RESULTS: In stratified analysis, race remained associated with postneonatal mortality within most categories of marital status, maternal education, maternal age, prenatal tobacco or alcohol use, prenatal care utilization, parity and residence. The odds ratio between race and postneonatal mortality was reduced to 1.3 (95% CI 1.0, 1.6) by controlling for education, a composite variable of marital status and the presence of father's name on the birth certificate, and prenatal tobacco or alcohol use.
CONCLUSIONS: A small number of potentially modifiable factors explain most of the postneonatal mortality disparity between Alaska Natives and non-Natives, leaving a relatively small increase in risk. These findings suggest that by targeting Alaska Native women who display these characteristics, the postneonatal mortality gap may be reduced.
Blabey MH , Gessner BD. Three Maternal Risk Factors Associated with Elevated Risk of Postneonatal Mortality Among Alaska Native Population. Matern Child Health J. 2008 Apr 4. [Epub ahead of print] http://www.ncbi.nlm.nih.gov/pubmed/18389352
Submitted by Vanessa Hiratsuka, MPH; Tobacco Prevention and Control Program, Section of Chronic Disease Prevention and Health Promotion State of Alaska, Division of Public Health
MCH Editorial Comment:
Reducing the post-neonatal mortality gap by addressing a finite number of modifiable risk factors is as relevant in 2008 as it was in 1992. As the authors note it is well known that a variety of social and economic factors are at play in perinatal health disparities. By adapting analytic techniques first used by Baker in California and a model by Greenland, the Alaska team subjected “selected” variables (dependent variable) to their model observing the effect on the odds ratio (OR) between race (independent variable) and post neonatal mortality (also a dependent variable). Similarly during a “deletion cycle” variables were removed, the OR for race observed and then “re-entered” before another variable was deleted and an OR observed. Criteria for confounding variables were those who changed the OR by < or > 10%. Ample tables and a well referenced discussion support the author’s hypothesis focusing on elevated rates of alcohol and tobacco use, lower education levels (not completing high school) and “a greater frequency of a father’s name missing on the birth certificate among unmarried mothers” as three key variables for intervention.
Quality assurance issues of chart data abstraction and maternal interview during the birth registration process should be considered. The advantage of school health as a point of care for a segment of this vulnerable population, while not suggested, seems a reasonable and an essential focus. Attention to children with special needs and special education needs falls into this category as well. Continued services during pregnancy and postpartum for school age Alaska Natives and basic reproductive health services for adolescents (male and female) is an important investment. Intimate partner tolerance for physical and emotional abuse and risky behaviors including alcohol and tobacco use converge in adolescence or at even younger ages, pressing for increased mental health and social services located in their learning environments. Tobacco cessation programs especially for low SES women are being given more national attention. Lastly attention to education around building healthy intimate partner relationships and diffusing stress may be an indirect way to increasing the frequency of entering a fathers name on a birth certificate – just a thought.
What Every Parent Needs to Know About ... Inhalant Abuse
One out of every five teens in America has used inhalants to get high - and inhalant abuse can begin at a very young age. The Partnership for a Drug-Free America created this special section to help parents seeking specific information, guidance and resources about inhalants.
http://www.drugfree.org/Parent/Resources/Parents_Guide_to_Inhalants_Prevention
Pediatric Environmental Health Toolkit Available for Download
The Toolkit is a combination of easy-to-use reference guides for health providers and user friendly health education materials on preventing exposures to toxic chemicals and other substances that affect infant and child health. The materials are visually appealing, practical and easy to use. The Toolkit is endorsed by the American Academy of Pediatrics (AAP).
http://www.psr.org/site/PageServer?pagename=pediatric_toolkit
Preventing Alcohol, Tobacco, and Other Substance-exposed Pregnancies: A Community
Affair; A Symposium sponsored by the National Institute on Alcohol Abuse
and Alcoholism September 23-24, 2008, Rockville MD
A symposium to explore best approaches to getting the message out about the potential
harm caused by risky drinking and by other substance use during the childbearing
years and, in particular, the importance of abstaining from any and all drinking
and smoking during pregnancy. This symposium will be a starting point in
a national effort to change the common perception that the use of alcohol and
tobacco during pregnancy is safe.
Medical Mystery Tour
Fetal Heart Monitoring
Everyone knows how to read a fetal heart rate (FHR) tracing, right?
Well let’s make sure we are describing fetal heart rate (FHR) tracing with the same terms.
1.) A complete description of a fetal heart rate (FHR) tracing requires a qualitative and quantitative description of beat-to-beat variability
True
False
2.) According to standardized National Institute of Child Health and Human Development (NICHD) definitions, the normal FHR range is 110-160 beats per minute
True
False
3) According to standardized NICHD definitions, an amplitude range of between 6 and 25 beats per minute is termed ‘marked variability’
True
False
4) Before 32 weeks, a fetal heart rate acceleration is defined as a visually apparent increase in rate at least 10 beats per minute above the baseline lasting between 10 seconds and 2 minutes
True
False
5) According to standardized NICHD terminology, a variable FHR deceleration reaches its nadir more than 30 seconds after its onset
True
False
6) A prolonged FHR deceleration lasts between 2 and 10 minutes
True
False
7) Fetal heart rate accelerations and moderate variability are highly predictive of the absence of metabolic academia
True
False
For a discussion of the answers to the above questions, please tune into the September CCC Corner.
In the meantime, if you want to learn more about the results of the National Institute of Child Health and Human Development Research Planning Workshop, go here
Electronic fetal heart rate monitoring: research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop.
Am J Obstet Gynecol. 1997 Dec; 177( 6): 1385-90.
http://www.ncbi.nlm.nih.gov/pubmed/9423739
Medscape*
A sample of recent free CME offerings on Medscape:
NAMS Updates Guidelines on Postmenopausal Hormone Therapy
http://www.medscape.com/viewarticle/576970
Postmenopausal Systemic Hormone Therapy: Reaching Consensus at Last on Benefit
and Risk
http://www.medscape.com/viewprogram/14834
Brief Video May Help Reduce Incidence of Sexually Transmitted Infections
http://www.medscape.com/viewarticle/576760
Initial Diagnosis of Erosive Esophagitis: A Case Study Approach for Primary
Care
http://www.medscape.com/viewprogram/14856
Comprehensive Thrombosis Guidelines Published
http://www.medscape.com/viewarticle/576895
Dietary Fructose Consumption among US Children and Adults: The Third National
Health and Nutrition Examination Survey
http://www.medscape.com/viewprogram/14898
Weight-Loss Interventions Effective in Patients with Type 2 Diabetes
http://www.medscape.com/viewarticle/577210
Ask the Experts topics in Women's Health and OB/GYN Index, by specialty,
Medscape
http://www.medscape.com/pages/editorial/public/ate/index-womenshealth
OB GYN & Women's Health Clinical Discussion Board Index, Medscape
http://boards.medscape.com/forums?14@@.ee6e57b
Clinical Discussion Board Index, Medscape
Hundreds of ongoing
clinical discussions available
http://boards.medscape.com/forums?14@@.ee6e57b
Free CME: MedScape CME Index by specialty
http://www.medscape.com/cmecenterdirectory/Default
*NB: Medscape is free to all, but registration is required. It can be accessed from anywhere with Internet access. You just need to create a personal username and password.
Menopause Management
Gallbladder Disease Risk Lower with Transdermal vs. Oral Hormone Therapy
OBJECTIVE: To determine whether transdermal compared with oral use of hormone replacement therapy reduces the risk of gallbladder disease in postmenopausal women.
DESIGN: Prospective cohort study (Million Women Study).
SETTING: Women registered with the National Health Service (NHS) in England and Scotland.
PARTICIPANTS: 1 001 391 postmenopausal women (mean age 56) recruited between 1996 and 2001 from NHS breast screening centres and followed by record linkage to routinely collected NHS hospital admission data for gallbladder disease.
MAIN OUTCOME MEASURES: Adjusted relative risk and standardised incidence rates of hospital admission for gallbladder disease or cholecystectomy according to use of hormone replacement therapy.
RESULTS: During follow-up 19 889 women were admitted for gallbladder disease; 17 190 (86%) had a cholecystectomy. Compared with never users of hormone replacement therapy, current users were more likely to be admitted for gallbladder disease (relative risk 1.64, 95% confidence interval 1.58 to 1.69) but risks were substantially lower with transdermal therapy than with oral therapy (relative risk 1.17, 1.10 to 1.24 v 1.74, 1.68 to 1.80; heterogeneity P<0.001). Among women using oral therapy, equine oestrogens were associated with a slightly greater risk of gallbladder disease than estradiol (relative risk 1.79, 1.72 to 1.87 v 1.62, 1.54 to 1.70; heterogeneity P<0.001) and higher doses of oestrogen increased the risk more than lower doses: for equine oestrogens >0.625 mg, 1.91 (1.78 to 2.04) v 0.625 mg, 1.76 (1.68 to 1.84); heterogeneity P=0.02; estradiol >1 mg, 1.68 (1.59 to 1.77) v 1 mg, 1.44 (1.31 to 1.59); heterogeneity P=0.003. The risk of gallbladder disease decreased with time since stopping therapy (trend P=0.004). Results were similar taking cholecystectomy as the outcome. Standardised hospital admission rates per 100 women over five years for cholecystectomy were 1.1 in never users, 1.3 with transdermal therapy, and 2.0 with oral therapy.
CONCLUSION Gallbladder disease is common in postmenopausal women and use of hormone replacement therapy increases the risk. Use of transdermal therapy rather than oral therapy over a five year period could avoid one cholecystectomy in every 140 users.
Bette Liu, Valerie Beral, Angela Balkwill, Jane Green, Siân Sweetland, Gillian Reeves, for the Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study BMJ 2008; 337:a386 http://www.bmj.com/cgi/content/full/337/jul10_2/a386
Midwives Corner - Lisa Allee, CNM, 4 Corners Regional Health Center, Red Mesa, AZ
Including the Non-rational in Midwifery
Parratt and Fahy present a fascinating picture of the art of midwifery. They describe how midwives can practice using embodied knowledge that goes beyond the scientific, rational model of care and how women who are attended by midwives who use this broader-based knowledge can in turn tap into their own ways of knowing and the combination provides for superior care and experiences than care based on only rational, scientific information. Before reading this article please be sure to notice that the word is non-rational and not irrational. The authors very nicely point out that in the rational-dominated way of thinking that clings to dichotomies, the opposite of rational is irrational and since we have all been well initiated into the rational Western scientific way of thinking, our minds will tend to go directly to this dichotomy and see non-rational as irrational. Not so. The non-rational is our embodied knowledge, our inner knowing, that we gain from our lived experiences and other sources, which are hard to articulate. Actually, the non-rational overall is very difficult to articulate into language as it is felt and known in ways that defy words. However, Parratt and Fahy do well in explaining the importance and benefits of including the non-rational as the art of practicing midwifery. They use the issue of safety as one example, pointing out the importance and validity of approaching each situation as unique and drawing on both the rational and the non-rational sources of information from both the midwife and the women in order to respond to any situation at hand in the best way possible. They also do a wonderful job of pointing out that there is truly a spectrum to consider in the overall issue of safety—that the rational-thinking-only dichotomy of safe and unsafe as the only possibilities is not realistic or helpful. This article affirms to us all that using our whole being and honoring each woman’s whole being in each pregnancy, labor, and birth is the essence of the art and science of the midwifery model of care and the reason that midwifery care is so powerful, successful, satisfying, empowering, and down right excellent!
Please, if you cannot get to the full-text article via these links, feel free to contact me and I will send you the article in electronic or hard copy form—just let me know! Email: lisa.allee@ihs.gov
Parratt JA , Fahy KM, Including the nonrational is sensible midwifery. Women Birth. 2008 Mar;21(1):37-42. Epub 2008 Feb 20
PubMed link: http://www.ncbi.nlm.nih.gov/pubmed/18243836
Science Direct full article link
Navajo News - John Balintona, Shiprock
Survey of Full-Scope Navajo OB Sites
Below is a survey of the sites in the Navajo Area that provide full-scope obstetric services. The survey can be used as a guide for fellow practitioners and referring sites as to the capabilities of the various OB sites.
| Chinle | FTDEF | GIMC | Shiprock | TCRHCC | |
| Model of Care | |||||
| OB First Call | CNM | CNM | CNM | CNM,FM | CNM |
| OB Second Call | OB | OB | OB | OB | OB |
| Nursery | Level I | Level I | Level II | Level II | Level I |
| Separate Service OB/FM/CNM | NO | NO | NO | YES | NO |
| High Risk Clinic | YES | YES | YES | YES | NO |
| Diabetes Care Team | YES | NO | NO | YES | NO |
| In-office U/S | YES | YES | YES | YES | NO |
| Antenatal Testing Unit | YES | NO | YES | NO | YES |
| Epidural Service | NO | YES | YES | NO | YES |
| VBAC Capabilities | |||||
| 24 Hour Service | YES | YES | YES | YES | YES |
| Risk Stratification System | Individualized Counseling | Individualized Counseling | Individualized Counseling | Individualized Counseling | YES |
| Consent Form | YES | YES | YES | YES | YES |
| Preterm Limit Weeks EGA | 36 | 36 | 35 | 35 | 32-34 |
| Amniocentesis | |||||
| Fetal Lung Maturity | YES | NO | NO | YES | YES |
| Genetics | NO | NO | NO | YES | YES |
Nurses Corner - Sandra Haldane, HQE
Screening, Brief Intervention, and Referral to Treatment (SBIRT) Endorsed by National Emergency Nurses Association
As described by SAMHSA, the Initiative involves implementation of a system within community and/or medical settings—including physician offices, hospitals, educational institutions, and mental health centers—that screens for and identifies individuals with or at-risk for substance use-related problems. Screening determines the severity of substance use and identifies the appropriate level of intervention. The system provides for brief intervention or brief treatment within the community setting or motivates and refers those identified as needing more extensive services than provided in the community setting to a specialist setting for assessment, diagnosis, and appropriate treatment.
WHY IS SBIRT SO IMPORTANT?
Hospitals that have implemented a SBIRT program have seen:
- Reduced ED visit recidivism.
- Improved patient safety in the community.
- Decreased risk for injuries (e.g., falls, ATV, motor vehicle crashes).
- Reduced risk of harm to others due to alcohol use problems (e.g., domestic violence).
- Reduced alcohol-medication adverse events.
- Increased intervention impact on ED patients and the community.
- Reduced health care costs.
- Adoption of a universal screening policy makes reimbursement more likely because alcohol use problems are not tied to the medical event.
- Estimates indicate that approximately 12% of patients will require a brief intervention. Almost 50% of patients will be eliminated at triage by the first screening question.
WHY SHOULD I BECOME A SBIRT CHAMPION?
- Increased recognition as an ED nurse leader and pioneer. ENA is the only nursing specialty organization that has adopted SBIRT.
- Increased recognition as a trusted professional.
- Increased opportunity for you to provide prevention efforts in your ED and community.
- Once the SBIRT technique is learned, the technique can be applied to other risky behaviors.
- Increased ED staff satisfaction.
- Increased opportunity to climb the clinical ladder.
- Opportunity to obtain CECHs.
http://www.ena.org/ipinstitute/SBIRT/Benefits.asp
Office of Women's Health, CDC
Impact of Periodic Follow-Up Testing Among Urban American Indian Women with Impaired Fasting Glucose
INTRODUCTION: Impaired fasting glucose (IFG) often progresses to type 2 diabetes. Given the severity and prevalence of this disease, primary prevention is important. Intensive lifestyle counseling interventions have delayed or prevented the onset of type 2 diabetes, but it is not known whether less intensive, more easily replicable efforts can also be effective.
METHODS: In a lifestyle intervention study designed to reduce risks for type 2 diabetes, 200 American Indian women without diabetes, aged 18 to 40 years, were recruited from an urban community without regard to weight or IFG and block-randomized into intervention and control groups on the basis of fasting blood glucose (FBG). Dietary and physical activity behaviors were reported, and clinical metabolic, fitness, and body composition measures were taken at baseline and at periodic follow-up through 18 months. American Indian facilitators used a group-discussion format during the first 6 months to deliver a culturally influenced educational intervention on healthy eating, physical activity, social support, and goal setting. We analyzed a subset of young American Indian women with IFG at baseline (n = 42), selected from both the intervention and control groups.
RESULTS: Among the women with IFG, mean FBG significantly decreased from baseline to follow-up (P < .001) and converted to normal (<5.6 mmol/L or <100 mg/dL) in 62.0% of the 30 women who completed the 18-month follow-up, irrespective of participation in the group educational sessions. Other improved metabolic values included significant decreases in mean fasting blood total cholesterol and low-density lipoprotein cholesterol levels. The women reported significant overall mean decreases in intake of total energy, saturated fat, total fat, total sugar, sweetened beverages, proportion of sweet foods in the diet, and hours of TV watching.
CONCLUSION: Volunteers with IFG in this study benefited from learning their FBG values and reporting their dietary patterns; they made dietary changes and improved their FBG and lipid profiles. If confirmed in larger samples, these results support periodic dietary and body composition assessment, as well as glucose monitoring among women with IFG.
Allen P, Thompson JL, Herman CJ, Qualls C, Helitzer DL, Whyte AN, et al. Impact of periodic follow-up testing among urban American Indian women with impaired fasting glucose. Prev Chronic Dis 2008;5(3). http://www.cdc.gov/pcd/issues/2008/jul/07_0078.htm
Oklahoma Perspective Greggory Woitte – Hastings Indian Medical Center
Meet Dr. George Chiarchiaro, Oklahoma MCH Coordinator
Dr. George Chiarchiaro attended the University of California at Long Beach and graduated from Georgetown University School of Dentistry in 1978. He spent a brief time in the private practice of dentistry and entered the Indian Health Service in 1980. After serving four years as dental chief, clinical director, and health systems administrator on the Rocky Boys Reservation, Dr. Chiarchiaro transferred to Gallup Indian Medical Center to enter a Dental Advanced General Practice Residency. In 1997 Dr. Chiarchiaro received his Master of Health Administration from the University of Oklahoma Health Sciences Center.
George Chiarchiaro, DDS, MHA
Consultant for Dental, Medical Imaging, and MCH
Division of Health Care Systems
Oklahoma City Area IHS
Five Corporate Plaza,
3625 NW 56th Street,
Oklahoma City, OK 73112
Phone: (405) 951-3818
Fax: (405) 951-3916
Cell: (405) 204-7664
Oklahoma MCH Coordinator Website
http://www.ihs.gov/MedicalPrograms/MCH/F/MCHC07.cfm
Osteoporosis
National Osteoporosis Foundation Releases New Clinical Recommendations for Low Bone Mass and Osteoporosis Incorporating Absolute Fracture Risk
The National
Osteoporosis Foundation (NOF) is releasing its new Clinician’s
Guide to Prevention and Treatment of Osteoporosis representing a major breakthrough
in the way healthcare providers evaluate and treat people with low bone mass
or osteoporosis and the risk of fractures. NOF’s new Clinician’s
Guide introduces guidelines beyond Caucasian postmenopausal women to include
African-American, Asian, Latina and other postmenopausal women, and addresses
men age 50 and older for the first time.
Osteoporosis is a major public health problem that has both a medical and economic
impact in the U.S. Fractures caused by either osteoporosis or low bone mass can
lead to chronic pain, disability and even death, as well as psychological symptoms,
including depression. Each year broken bones due to low bone mass or osteoporosis
cause over 432,000 hospital admissions, almost 2.5 million medical office visits
and about 180,000 nursing home admissions.
“NOF’s new Clinician’s Guide dramatically alters the approach
to assessing fracture risk and treatment,” said Bess Dawson-Hughes, M.D.,
chair of the Clinician’s Guide Development Committee and past president
of NOF. “The Guide provides evidenced-based recommendations to help healthcare
providers better identify people at high risk for developing osteoporosis and
fractures and assures that those at highest risk are recommended for treatment
to lower that risk.”
The new Clinician’s Guide applies the recently released algorithm on absolute
fracture risk called FRAX® by the World Health Organization (WHO). FRAX® is
also referred to as a 10-year fracture risk model and 10-year fracture probability.
This algorithm estimates the likelihood of a person to break a bone due to low
bone mass or osteoporosis over a period of 10 years.
Absolute fracture risk methodology provides a markedly improved method to assure
that people with the highest fracture risk get treated. Those at highest risk
include postmenopausal women and older men with a diagnosis of osteoporosis,
based on a BMD test T-score of -2.5 or lower, or those with a clinical diagnosis
based on having sustained a hip or spine fracture. In addition, absolute fracture
risk calculations help to resolve many of the questions about management for
people with low bone mass, also called osteopenia. These are people with a T-score
between -1.0 and -2.5 on their bone mineral density (BMD) test.
The WHO algorithm takes into account not only bone mineral density (BMD) at the
hip but also nine specific clinical risk factors for osteoporosis and related
fractures. NOF has adapted this algorithm for the U.S. and incorporates not only
fracture outcome and mortality data from U.S. women and men, but also cost effectiveness
analysis to determine when it is cost effective to treat a person with an osteoporosis
medication to prevent a fracture.
“In developing the new Clinician’s Guide, NOF is providing healthcare
professionals in the U.S. with the newest advances for diagnosing and managing
osteoporosis,” said Ethel Siris, M.D., president of the National Osteoporosis
Foundation. “To be able to better identify and treat those patients at
risk for osteoporosis and costly fractures will have a positive impact on the
medical, emotional and economic burden that osteoporosis bears on this country.”
In the near future, some central DXA (dual-energy x-ray absorptiometry) machines
that test the bone mineral density of the hip and spine should be able to provide
a report that gives information on a person’s absolute fracture risk by
incorporating the NOF application of the WHO algorithm into the bone density
machine’s computer. Alternatively, clinicians can also enter a patient’s
bone mineral density hip T-score and other risk factor information in a simple
web-based version of the algorithm in the doctor’s office to obtain absolute
fracture risk in seconds. The information about absolute fracture risk will help
both healthcare providers and patients decide whether treatment with an osteoporosis
medication is needed.
The new Clinician’s Guide also provides recommendations for clinicians
on when to do bone mineral density testing, clinical evaluation, risk factors
for falls and universal recommendations for the prevention of osteoporosis. NOF
summarizes the universal recommendations in its 5 Steps to Bone Health. These
5 Steps advise people to:
1. Get the daily recommended amounts of calcium and
vitamin D.
2. Engage in regular weight-bearing and muscle-strengthening exercise.
3. Avoid smoking and excessive alcohol.
4. Talk to your healthcare provider about bone health.
5. Have a bone density test and take medication when appropriate.
The new Clinician’s Guide recommends that adults over age 50 get 1,200
mg of calcium and 800-1,000 IU of vitamin D3 daily. Vitamin D3 is the form of
vitamin D that best supports bone health. It is also called cholecalciferol.
The Clinician’s Guide was developed by an expert committee of NOF in collaboration
with a multi-specialty council of medical experts in the field of bone health
convened by NOF. The Clinician’s Guide provides recommendations that are
intended to serve as a reference for clinical decision making with individual
patients. The recommendations are not intended to be rigid standards, limits
or rules and should not be interpreted as quality standards. Earlier versions
of the updated Clinician’s Guide to Prevention and Treatment of Osteoporosis
were called the Physician’s Guide to Prevention and Treatment of Osteoporosis.
Established in 1984, the National Osteoporosis Foundation is the nation’s
leading voluntary health organization solely dedicated to osteoporosis and bone
health. Our mission is to prevent osteoporosis, to promote lifelong bone health,
to help improve the lives of those affected by osteoporosis and related fractures,
and to find a cure. For more information on osteoporosis and bone health, contact
NOF online at www.nof.org or by telephone (800) 223-9994.
Patient Information
New Tables for Patient Education; The Risk of Death by Age, Sex, and Smoking Status in the United States: Putting Health Risks in Context
BACKGROUND: To make sense of the disease risks they face, people need basic facts about the magnitude of a particular risk and how one risk compares with other risks. Unfortunately, this fundamental information is not readily available to patients or physicians. We created simple one-page charts that present the 10-year chance of dying from various causes according to age, sex, and smoking status. Methods We used the National Center for Health Statistics Multiple Cause of Death Public Use File for 2004 and data from the 2004 US Census to calculate age- and sex-specific death rates for various causes of death. We then combined data on smoking prevalence (from the National Health Interview Survey) and the relative risks of death from various causes for smokers vs. never smokers (from the American Cancer Society's Cancer Prevention Study-II) to determine age-, sex-, and smoking-specific death rates. Finally, we accumulated these risks for various starting ages in a series of 10-year life tables. The charts present the 10-year risks of dying from heart disease; stroke; lung, colon, breast, cervical, ovarian, and prostate cancer; pneumonia; influenza; AIDS; chronic obstructive pulmonary disease; accidents; and all causes. Results At all ages, the 10-year risk of death from all causes combined is higher for men than women. The effect of smoking on the chance of dying is similar to the effect of adding 5 to 10 years of age: for example, a 55-year-old man who smokes has about the same 10-year risk of death from all causes as a 65-year-old man who never smoked (i.e., 178 vs. 176 of 1000 men, respectively). For men who never smoked, heart disease death represents the single largest cause of death from age 50 on and the chance of dying from heart disease exceeds the chances of dying from lung, colon, and prostate cancers combined at every age. For men who currently smoke, the chance of dying from lung cancer is of the same order of magnitude as the chance dying from heart disease and after age 50 it is about 10 times greater than the chance of dying from prostate or colon cancer. For women who have never smoked, the magnitudes of the 10-year risks of death from breast cancer and heart disease are similar until age 60; from this age on, heart disease represents the single largest cause of death. For women who currently smoke, the chance of dying from heart disease or lung cancer exceeds the chance of dying from breast cancer from age 40 on (and does so by at least a factor of 5 after age 55).
CONCLUSION: The availability of simple charts with consistent data presentations of important causes of death may facilitate discussion about disease risk between physicians and their patients and help highlight the dangers of smoking.
Woloshin S , Schwartz LM, Welch HG. The Risk of Death by Age, Sex, and Smoking Status in the United States: Putting Health Risks in Context. J Natl Cancer Inst. 2008 Jun 18;100(12):845-53. Epub 2008 Jun 10. http://www.ncbi.nlm.nih.gov/pubmed/18544745
Abstract also available at: http://jnci.oxfordjournals.org/cgi/content/abstract/100/12/845
Editorial: Michael J. Thun, Lindsay M. Hannan, Michael Stefanek. Risky Business: Tools to Improve Risk Communication in a Doctor’s Office. J Natl Cancer Inst. 2008 Jun 18;100(12):830-1. Epub 2008 Jun 10. http://www.ncbi.nlm.nih.gov/pubmed/18544738
Free full text: http://jnci.oxfordjournals.org/cgi/reprint/100/12/830
Perinatology Picks - George Gilson, Maternal Fetal Medicine, ANMC
Protein/Creatinine ratio in preeclampsia: a systematic review
OBJECTIVE: To estimate the accuracy of the protein/creatinine ratio in predicting 300 mg of protein in 24-hour urine collection in pregnant patients with suspected preeclampsia.
DATA SOURCES: Articles were identified through electronic databases (MEDLINE, CINHAL, and Cochrane) using the terms "preeclampsia," "protein/creatinine ratio," and "diagnosis," during the period January 1966 to October 2007. The relevant citations were hand searched.
METHODS OF STUDY SELECTION: Included studies evaluated patients for suspected preeclampsia with a 24-hour urine sample and a protein/creatinine ratio. Only English-language articles were included. Studies including patients with only chronic illness such as chronic hypertension, diabetes mellitus, or renal impairment were excluded. Using the Quality Assessment of Diagnostic Accuracy Studies questionnaire, we created group 1 satisfying all the required criteria and group 2 not satisfying all of it. Two researchers independently extracted the accuracy data. A graph comparing six receiver operating characteristic curves was plotted.
TABULATION, INTEGRATION, AND RESULTS: Twenty-one studies were identified, but only seven met our inclusion criteria (1,717 total patients). Group 1, with three studies, had 510 patients. The studies evaluated different cut points for positivity of protein/creatinine ratio from 130 mg/g to 700 mg/g. For protein/creatinine ratio 130-150 mg/g, sensitivity ranged from 90-99%, and specificity ranged from 33-65%; for protein/creatinine ratio 300 mg/g, sensitivity ranged from 81-98% and specificity ranged from 52-99%; for protein/creatinine ratio 600-700 mg/g, sensitivity ranged from 85-87%, and specificity ranged from 96-97%.
CONCLUSION: Random protein/creatinine ratio determinations are helpful primarily when they are below 130-150 mg/g, in that 300 mg or more proteinuria is unlikely below this threshold. Midrange protein/creatinine ratio (300 mg/g) has poor sensitivity and specificity, requiring a full 24-hour urine for accurate results. Higher thresholds have not been adequately studied.
Papanna R, Mann LK, Kouides RW, Glantz JC. Protein/Creatinine ratio in preeclampsia: a systematic review. Obstet Gynecol. 2008 Jul;112(1):135-44. http://www.ncbi.nlm.nih.gov/pubmed/18591319
Delivery mode and severe intraventricular hemorrhage in single, very
low birth weight, vertex infants
OBJECTIVE: To investigate the association between delivery mode and grade 3-4
intraventricular hemorrhage in singleton, vertex presenting, very low birth weight
(VLBW) (1,500 g or less) liveborn infants.
METHODS: The Israel National VLBW Infant Database includes perinatal and neonatal data on greater than 99% of all VLBW newborns. A total of 4,658 singleton vertex-presenting infants born at 24-34 weeks were included (1995-2004). Infants with lethal congenital malformations, delivery room deaths, and home deliveries were excluded. Our population-based observational study evaluated the effect of delivery mode and confounding variables on severe intraventricular hemorrhage using univariable and multivariable logistic regression analyses.
RESULTS: The rate of severe intraventricular hemorrhage was 10.4%. Cesarean delivery rate was 54.3%. The rate of severe intraventricular hemorrhage was 7.7% for infants delivered by cesarean compared with 13.6% in vaginal delivery (P<.001). However, analysis according to gestational age showed that the rate of severe intraventricular hemorrhage was similar in cesarean and vaginal delivery in all gestational age groups. In the multivariable model, cesarean delivery had no effect on the odds for severe intraventricular hemorrhage (odds ratio [OR] 0.98, 95% confidence interval [CI] 0.77-1.24). Other factors independently associated with severe intraventricular hemorrhage included gestational age (OR 0.71, 95% CI 0.68-0.75 for each week increase), maternal hypertensive disorder (OR 0.43, 95% CI 0.30-0.61), no antenatal steroids (OR 2.70, 95% CI 2.12-3.45), 1-minute Apgar score 0-3 (OR 1.72, 95% CI 1.33-2.21), delivery room resuscitation (OR 2.16, 95% CI 1.65-2.83), and non-Jewish ethnicity (OR 1.28, 95% CI 1.03-1.59).
CONCLUSION: In this population-based study, the odds for severe intraventricular hemorrhage were not influenced by mode of delivery in vertex-presenting singleton VLBW infants after controlling for gestational age. LEVEL OF EVIDENCE: II.
Riskin A , Riskin-Mashiah S , Bader D , Kugelman A , Lerner-Geva L , Boyko V , Reichman B . Delivery mode and severe intraventricular hemorrhage in single, very low birth weight, vertex infants. Obstet Gynecol. 2008 Jul;112(1):21-8. http://www.ncbi.nlm.nih.gov/pubmed/18591303
Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring
We report cases of unexpected adverse fetal outcome from monitored labors in which the fetal heart rate tracing was interpreted as reassuring. In these cases, portions from another signal source, usually maternal, were imperceptibly substituted into the fetal tracing in a way that masked the evidence of fetal compromise.
Neilson DR Jr , Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008 Jun;198(6):717-24. Epub 2008 Apr 2. http://www.ncbi.nlm.nih.gov/pubmed/18377859
CCC Editorial Comment: This article is well-illustrated with images of tracings where the maternal heart rate (or that of the other twin) was displayed in lieu of targeted fetal heart rate tracing in labor. This is a rare problem but one for which maternity care providers must maintain vigilance. The use of a fetal scalp electrode should reveal the actual fetal heart rate.
Fetomaternal hemorrhage during external cephalic version
OBJECTIVE: To estimate the frequency and volume of fetomaternal hemorrhage during external cephalic version for term breech singleton fetuses and to identify risk factors involved with this complication.
METHODS: A prospective observational study was performed including all patients undergoing a trial of external cephalic version for a breech presentation of at least 36 weeks of gestation between 1987 and 2001 in our center. A search for fetal erythrocytes using the standard Kleihauer-Betke test was obtained before and after each external cephalic version. The frequency and volume of fetomaternal hemorrhage were calculated. Putative risk factors for fetomaternal hemorrhage were evaluated by chi(2) test and Mann-Whitney U test.
RESULTS: A Kleihauer-Betke test result was available before and after 1,311 trials of external cephalic version. The Kleihauer-Betke test was positive in 67 (5.1%) before the procedure. Of the 1,244 women with a negative Kleihauer-Betke test before external cephalic version, 30 (2.4%) had a positive Kleihauer-Betke test after the procedure. Ten (0.8%) had an estimated fetomaternal hemorrhage greater than 1 mL, and one (0.08%) had an estimated fetomaternal hemorrhage greater than 30 mL. The risk of fetomaternal hemorrhage was not influenced by parity, gestational age, body mass index, number of attempts at version, placental location, or amniotic fluid index.
CONCLUSION: The risk of detectable fetomaternal hemorrhage during external cephalic version was 2.4%, with fetomaternal hemorrhage more than 30 mL in less than 0.1% of cases. These data suggest that the performance of a Kleihauer-Betke test is unwarranted in uneventful external cephalic version and that in Rh-negative women, no further Rh immune globulin is necessary other than the routine 300-microgram dose at 28 weeks of gestation and postpartum. LEVEL OF EVIDENCE: II.
Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008 Jul;112(1):79-84.
http://www.ncbi.nlm.nih.gov/pubmed/18591311
Primary Care Discussion Forum - Ann Bullock, Cherokee, NC
How to subscribe / unsubscribe to the Primary Care Discussion Forum?
Subscribe to the Primary Care listserv
http://www.ihs.gov/cio/listserver/index.cfm?module=list&option=list&num=46&startrow=51
Unsubscribe from the Primary Care listserv
http://www.ihs.gov/cio/listserver/index.cfm?module=list&option=list&num=46&startrow=51
Questions on how to subscribe, contact ANNBULL@nc-cherokee.com
STD Corner - Lori de Ravello, National IHS STD Program
Frequency of Adolescent Self-Cutting As a Predictor of HIV Risk
OBJECTIVE: A wide range exists in the frequency of adolescent self-cutting behavior; however, the implications of this variability are relatively unexplored. Although evidence suggesting a relationship between self-harm and sexual risk behaviors has been identified, little is known regarding the relationship between frequency of self-cutting and sexual risk. The present study aimed to test the hypothesis that adolescents who repeatedly self-cut would report more HIV risk behaviors and riskier attitudes than those who had engaged in infrequent self-injury.
METHOD: Adolescents (11-18 years; mean age, 15 years) from intensive psychiatric treatment programs with a history of self-cutting (N = 105, 53% female) completed measures of self-cutting, sexual risk behaviors, and risk attitudes.
RESULTS: Frequent self-cutting (more than three times, lifetime) was associated with being sexually active, using condoms inconsistently, and sharing cutting instruments. Frequent self-cutters were significantly more likely to be female and nonwhite, and report low self-restraint. They also showed a trend toward being more likely to have a history of sexual abuse. CONCLUSIONS: This study found important differences in self-cutters based on frequency of cutting. Adolescent self-cutting may be a spectrum of behavior that ranges from habitual, repeated behavior contrasted with infrequent, experimental, socially motivated cutting. The associations between frequent cutting, sexual risk, and low self-restraint suggest that common underlying mechanisms may determine these patterns.
Brown LK, Houck CD, Grossman CI, Lescano CM, Frenkel JL. Frequency of Adolescent Self-Cutting As a Predictor of HIV Risk. J Dev Behav Pediatr. 2008 May 23. [Epub ahead of print]
http://www.ncbi.nlm.nih.gov/pubmed/18520618
Barbara Stillwater, Alaska State Diabetes Program
Why young adults hold the key to assessing the obesity epidemic in children
Despite significant increases in prevalence rates of childhood obesity in the United States during the past 2 decades, rates of type 2 diabetes mellitus among children at the population level have not followed a similar trajectory as those in adults. In this review, hypotheses for the contrasting findings in children compared with adults are explored, as are possible links between the trends in childhood obesity rates and increases in type 2 diabetes among young adults in the United States. This review concludes with observations about the profound policy implications from current patterns of type 2 diabetes among youth and particularly young adults and a proposed research agenda regarding childhood obesity and type 2 diabetes risk over the life course.
Lee JM . Why young adults hold the key to assessing the obesity epidemic in children. Arch Pediatr Adolesc Med. 2008 Jul;162(7):682-7. http://www.ncbi.nlm.nih.gov/pubmed/18606940
Women's Health Headlines, Carolyn Aoyama, HQE
New Research Indicates That Significant Numbers Of Children As Young As 11 Are Engaging In Sexual Activity And That Dating Violence And Abuse Are Part Of Their Relationships
A new survey reports today that a surprising number of young adolescents are experiencing significant levels of dating violence and abuse. One in five children between the ages of 13 and 14 (20%) say their friends are victims of dating violence and nearly half of all tweens in relationships say they know friends who are verbally abused. Alarmingly, 40% of the youngest tweens, those between the ages of 11 and 12, report that their friends are victims of verbal abuse in relationships and nearly 1 in 10 (9%) say their friends have had sex.
Liz Claiborne Inc. and loveisrespect.org, National Teen Dating Abuse Helpline, commissioned the survey on Tween and Teen dating relationships that was conducted by Teenage Research Unlimited ( TRU) to explore how relationships among young adolescents are fueling high levels of dating violence and abuse.
This very interesting study of “tween” dating violence and abuse highlights the need to start DV/SA screening at around age 11. View the results of the study at:
http://www.loveisnotabuse.com/pdf/Tween%20and%20Teen%20Dating%20Abuse%20Survey.pdf
The “Love is Not Abuse” website can be found at: http://www.loveisnotabuse.com/
Family Violence Prevention Fund; National Conference on Health and Domestic Violence
New Orleans , Louisiana ; October 8-10, 2009
The Family Violence Prevention Fund’s Fifth National Conference on Health and Domestic Violence will be held October 8-10, 2009 at the Sheraton New Orleans Hotel. The National Conference provides valuable professional education on the latest research and innovative health prevention and clinical responses to domestic violence. Co-chaired by 35 organizations, including the American Medical Association (AMA), the American Academy of Nursing ( ANA), and the National Network to End Domestic Violence (NNEDV), the gathering has been hailed as “the best violence related conference” in the country and is expected to draw more than 1,000 participants from around the world.
Specialized half and full-day pre-conference institutes will be held on October 8th followed by the two-day conference, October 9-10, 2009. The Conference includes an exhibit hall for vendors.
For more information, contact Anna Marjavi at anna@endabuse.org, phone (415) 252-8900 or visit http://www.endabuse.org/health/conference/ .
Save the dates
Sexual Assault Nurse Examiner/Forensic Examiner (SANE/ SAFE) Training Course
- August 18-22 , 2008
- Oklahoma City , Oklahoma
- 40 hour didactic portion of SANE/ SAFE training
- For additional information contact Lisa Palucci, lisa.palucci@ihs.gov, at the IHS Clinical Support Center
Postgraduate Course on Obstetric, Neonatal and Gynecologic Care
- September 14-18, 2008
- Salt Lake City , Utah
- Comprehensive Women’s Health Update for Nurses, Advanced Practice Nurses, and Physicians
- NRP offered as pre-conference session
- Contact Yvonne Malloy, ymalloy@acog.org, for more information
2008 Indian Health Information Management Conference, “Managing Health Information Technology to Improve Performance and Outcomes”
- December 15 – 19, 2008 ; Phoenix, Arizona
- Information at: http://www.ihs.gov/cio/ihimc/
First International Meeting on Indigenous Women’s Health/Third International Meeting on Indigenous Child Health Conference; Many Voices into One Song
- Women’s Health March 4-6, 2009
- Child Health March 6-8, 2009
- Albuquerque , NM
- Joint conference of Women’s Health and Children’s Health Providers from Canada and the United States
What's new on the ITU MCH web pages?
About Todd Baughman, Deputy CCC, Physician Assistants
There are several upcoming Conferences
and Online CME/CEU resources, etc….
and the latest Perinatology Corners (free online CME from IHS)
…or just take a look at the What’s New page
Did you miss something in the last OB/GYN Chief Clinical Consultant Corner?
The July 2008 OB/GYN CCC Corner is available.
Hot Topics ‹ Previous
OB/GYN
Dr. Neil Murphy is the Obstetrics and Gynecology Chief Clinical Consultant (OB/GYN C.C.C.). Dr. Murphy is very interested in establishing a dialogue and/or networking with anyone involved in women's health or maternal child health, especially as it applies to Native or indigenous peoples around the world. Please don't hesitate to contact him by e-mail or phone at 907-729-3154.

