For over two decades, NIH has required researchers to include women, members of racial and ethnic minority groups, and children in their work absent an acceptable scientific or ethical rationale for their exclusion. Now, for the first time, selected inclusion data on sex/gender and race/ethnicity are publicly available disaggregated for various research, condition, and disease areas.
This reporting step continues our move towards enhancing transparency and accountability of the research we support. As part of implementing the 21st Century Cures Act and responding to recommendations from the Government Accountability Office, public reporting by NIH Research, Condition, and Disease Classification (RCDC) category helps ensure that women and minorities are appropriately included in biomedical research across a diverse array of diseases and conditions. At a recent meeting of the NIH Advisory Committee on Research on Women’s Health (go to 02:45), where this announcement was made, we also reported that in Fiscal Year (FY) 2018 over 52% of participants in NIH-supported clinical research were women, while about 29% of participants were members of racial minority groups, and 9% were ethnic minorities.
On the new NIH RCDC Inclusion Statistics Report webpage, you can see and download information for 269 of the 281 RCDC spending categories. Please note that inclusion data are not available for the remaining categories as they do not follow the standard RCDC categorization process (see this NIH Open Mike blog for more).
On the page, drop-down menus allow data to be easily filtered by the NIH Institute or Center. The menus allow you to exclude studies that focus on a single sex/gender, race, or ethnicity. Check out the FAQs for more information on how to interpret the data. And, similar to information available on other RePORT tools, data can be exported for offline analyses.
Let’s now look at some inaugural FY 2018 data. As shown in the screenshot displayed in Figure 1, RCDC categories are listed with inclusion data available on total counts for all participants and broken down by female and male. Median proportions are reported to give a sense of what a typical NIH study looks like (since averages are easily skewed by outliers).
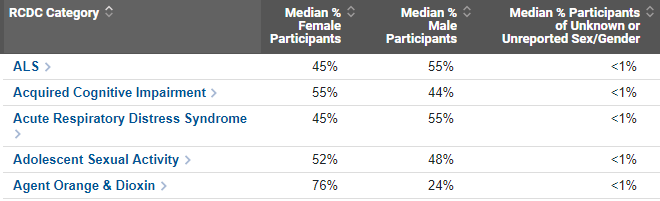
By clicking on a single RCDC spending category, like amyotrophic lateral sclerosis (ALS) for example, NIH Institute and Center level inclusion data are presented, along with additional data on the number and proportion of participants in each category (Figure 2). Clicking on a specific NIH Institute or Center allows you to review all information on sex/gender, race, and ethnicity on one screen.

Focusing on the first NIH IC listed, as an example, we see that National Institute on Aging research included 451 participants in projects associated with the ALS category in FY 2018. 242 (54%) of participants were female, with a median proportion of 52% female participants. 208 participants were male (46%), with a median proportion of 47% male participants. Less than 11 participants (<1%) reported unknown sex/gender, thus exact counts and proportions are not displayed to protect participant privacy. Additional data on race and ethnicity of participants are available by using the drop-down menu on the page or clicking on the Institute name.
As part of overall RCDC reporting, the availability of inclusion data on research participants is another important step in increasing transparency of NIH-supported clinical research. It also helps us understand the generalizability of NIH research across populations. In future years, NIH plans to add data on age at enrollment of participants and allow users to view trends over time. We look forward to increased understanding of the distribution of participants in our research to ensure the knowledge gained from NIH research is applicable to those populations with the condition or disease under study.
We would like to thank staff within the NIH Office of Research on Women’s Health and the Office of Extramural Research for their work on this activity.