September 14, 2016

The ICT rule updates standards issued under Section 508 of the Rehabilitation Act that apply to electronic and information technology procured by the federal government, including computer hardware and software, websites, multimedia such as video, phone systems, and copiers. It also refreshes guidelines for telecommunications products and services required to be accessible under Section 255 of the Communications Act.
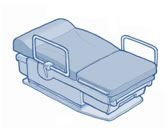 The other rule establishes new accessibility standards, the first of their kind, for medical diagnostic equipment, including examination tables and chairs, weight scales, radiological equipment, and mammography equipment. These standards, which are authorized by the Patient Protection and Affordable Care Act, provide technical requirements to ensure that people with disabilities can independently access and use such equipment. Further information on this rulemaking is available on the Board’s website.
The other rule establishes new accessibility standards, the first of their kind, for medical diagnostic equipment, including examination tables and chairs, weight scales, radiological equipment, and mammography equipment. These standards, which are authorized by the Patient Protection and Affordable Care Act, provide technical requirements to ensure that people with disabilities can independently access and use such equipment. Further information on this rulemaking is available on the Board’s website.
Each of the rules will be published on the Board’s website and in the Federal Register once cleared by OMB. To receive updates on the progress of these rules, subscribe to Board news.



