 |

ALTTO Trial Seeks to Define Best Therapy for HER2 Breast Cancer
A major clinical trial to determine the best treatment for early-stage breast cancer testing positive for the HER2 protein is now enrolling women in North America, researchers from the Mayo Clinic and NCI announced last week at the NCI Science Writers' Seminar in New York City. The international trial, known as the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (ALTTO) study, will recruit 8,000 women with HER2-positive breast cancer in 50 countries across 6 continents and may set the standard of care for women with this aggressive type of breast cancer.
About 20 to 25 percent of breast cancers are studded with the HER2 protein, also known as human epidermal growth factor receptor 2. HER2 is involved in transmitting growth signals from external growth factors to a cell's nucleus. In some types of cancer, this growth signaling pathway leads to increased tumor cell proliferation, suppression of programmed cell death (apoptosis), enhanced motility, and increased tumor angiogenesis. HER2-positive breast cancer tends to be more aggressive, less responsive to standard treatments, and more likely to recur than breast cancer that does not contain HER2.
Read more 


Cancer Risk Persists After Ending Hormone Therapy
The increased risk of breast cancer associated with combination hormone therapy (estrogen plus progestin) may not go away once the hormones are stopped. More than two years after discontinuing hormones, women who had used the treatment for 5 years still had a higher risk of breast cancer than women who never used the hormones, according to an update from the Women's Health Initiative (WHI).
The results, in the March 5 Journal of the American Medical Association, confirm the main finding of the WHI's estrogen-plus-progestin trial - that the health risks of this treatment for menopausal symptoms such as hot flashes outweigh the benefits. The trial was halted in 2002 largely because women using hormones had an increased risk of breast cancer and experienced no clear health benefits. (A WHI trial of estrogen-alone ther-apy did not show increased cancer risks.)
Read more 
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

ALTTO Trial Seeks to Define Best Therapy for HER2 Breast Cancer
A major clinical trial to determine the best treatment for early-stage breast cancer testing positive for the HER2 protein is now enrolling women in North America, researchers from the Mayo Clinic and NCI announced last week at the NCI Science Writers' Seminar in New York City. The international trial, known as the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (ALTTO) study, will recruit 8,000 women with HER2-positive breast cancer in 50 countries across 6 continents and may set the standard of care for women with this aggressive type of breast cancer.
About 20 to 25 percent of breast cancers are studded with the HER2 protein, also known as human epidermal growth factor receptor 2. HER2 is involved in transmitting growth signals from external growth factors to a cell's nucleus. In some types of cancer, this growth signaling pathway leads to increased tumor cell proliferation, suppression of programmed cell death (apoptosis), enhanced motility, and increased tumor angiogenesis. HER2-positive breast cancer tends to be more aggressive, less responsive to standard treatments, and more likely to recur than breast cancer that does not contain HER2.
Patients with HER2-positive breast cancer may benefit from treatments that target this protein, and two targeted drugs, trastuzumab (Herceptin) and lapatinib (Tykerb), have been approved by the Food and Drug Administration to treat patients with HER2-positive tumors. While both of these agents target HER2, they do so in different ways. Trastuzumab, which is approved as a first-line treatment for metastatic disease or adjuvantly for early-stage breast cancer, is a monoclonal antibody that attaches to HER2-positive cancer cells and prevents the protein from receiving growth signals. It may also help a patient's immune system mount a response against the tumor. Lapatinib belongs to a class of drugs called small molecule inhibitors; its small size allows it to permeate the cancer cell and interrupt the HER2 signaling pathway from inside. Currently, lapatinib is only approved for the treatment of metastatic HER2-positve breast cancer. This study will be the first major trial of adjuvant lapatinib for early-stage breast cancer.
Approval of trastuzumab for treatment of early-stage, HER2-positive breast cancer in 2006 greatly improved outcomes for women with this disease, and researchers hope that the ALTTO trial will further extend the benefits of adjuvant therapy targeting HER2. In this trial, women who have undergone surgery to remove their tumors will be randomly assigned to receive lapatinib or trastuzumab alone, lapatinib administered after trastuzumab, or the two drugs administered together.
"The primary questions this trial is intended to answer are: Does lapatinib work better than trastuzumab or are they equivalent in terms of disease-free survival? Or might the combination of the two be better than either single drug alone?" says Dr. Edith Perez of the Mayo Clinic in Jacksonville, FL, and the North American coordinator for the trial. "We know that trastuzumab is a fantastic drug, but we're still not curing all women with this type of cancer. This trial is an important step toward curing more patients with breast cancer."
In addition to the targeted therapies being tested, all patients must have completed at least four cycles of anthracycline-based chemotherapy before randomization; patients for whom chemotherapy with a taxane is indicated will be treated with the drug paclitaxel (Taxol) along with their assigned targeted therapy.
"Adjuvant chemotherapy is very important for the treatment of HER2-positive breast cancer and is a required part of this study," says Dr. Perez.
The ALTTO trial is the first global initiative in which two large, academic breast cancer research networks covering different parts of the world - The Breast Cancer Intergroup of North America (TBCI), based in the U.S., and the Breast International Group (BIG) in Brussels, Belgium - have jointly developed a study in which all care and data collection are standardized, regardless of where patients are treated.
"We hope that this model of international collaboration is one which we can build upon in the future," says Dr. Jo Anne Zujewski, a senior investigator in the Clinical Investigations Branch in NCI's Cancer Therapy Evaluation Program.
—Daryl McGrath
|
 |
 |
Cancer Risk Persists After Ending Hormone Therapy
The increased risk of breast cancer associated with combination hormone therapy (estrogen plus progestin) may not go away once the hormones are stopped. More than two years after discontinuing hormones, women who had used the treatment for 5 years still had a higher risk of breast cancer than women who never used the hormones, according to an update from the Women's Health Initiative (WHI).
The results, in the March 5 Journal of the American Medical Association, confirm the main finding of the WHI's estrogen-plus-progestin trial - that the health risks of this treatment for menopausal symptoms such as hot flashes outweigh the benefits. The trial was halted in 2002 largely because women using hormones had an increased risk of breast cancer and experienced no clear health benefits. (A WHI trial of estrogen-alone therapy did not show increased cancer risks.)
To update the findings, Dr. Gerardo Heiss of the University of North Carolina, Chapel Hill, and his colleagues tracked 95 percent of the original 16,608 trial participants for an additional 2.4 years. In the post-treatment period, women who had stopped taking hormones were 27 percent more likely to develop breast cancer than women who did not take hormones. However, this finding was not statistically significant (79 women in the post-treatment group developed breast cancer compared with 60 in the placebo group).
The risk of any type of cancer was 24 percent higher in women who had stopped taking hormones, and this was statistically significant (281 women in the post-treatment group developed cancer compared with 218 in the placebo group). The reasons for the additional cancers are not yet clear, but the researchers stress the importance of carefully monitoring women for any long-term effects of hormone use in the years ahead.
"When we started the WHI, the prevailing belief was that menopausal hormones were good for women and that we could prevent many major age-related diseases with them," said Dr. Marcia Stefanick of Stanford University, who chaired the WHI steering committee during the trial.
"But we learned during the trial that the risks of combined estrogen-and-progestin therapy, which included increases in breast cancer, heart attacks, stroke, and serious blood clots, clearly outweighed the benefits of fewer fractures and colorectal cancers," Dr. Stefanick continued. "And we now know that the risk of cancer, and in particular, breast cancer, continues years after stopping the hormones, whereas, none of the benefits persist."
The good news is that the cardiovascular risks for the most part disappear after stopping the combined hormone therapy. Still, many experts recommend that women avoid combined hormone therapy or minimize their exposure. Another WHI follow-up study recently showed that combined hormone therapy leads to abnormal mammograms and compromises the ability of mammograms and breast biopsies to detect cancers.
Together, the two reports highlight complementary downsides of this therapy. "Not only does the breast cancer risk not disappear once hormone therapy is stopped, but the sensitivity of mammograms to detect cancer is not as good and women may continue to have abnormal mammograms for at least a year," said Dr. Leslie Ford of NCI's Division of Cancer Prevention and the institute's WHI liaison.
Hormone Therapy Interferes with Breast Cancer Detection
Two tools used to detect breast cancer - mammograms and breast biopsies - are less effective in women who use combined hormone therapy (estrogen plus progestin) than in women who do not. And the diminished ability to find cancers may persist for at least a year after women have discontinued therapy, according to a follow-up study of participants in the Women's Health Initiative (WHI).
It has been known that breast cancer is often detected at a later stage in women who use hormones to treat menopausal symptoms than in other women. This study also shows that women who use combined hormone therapy have more mammograms with abnormalities and more breast biopsies than women who do not use hormones.
Dr. Rowan Chlebowski of Harbor-UCLA Medical Center and his colleagues followed nearly 16,000 women in the WHI estrogen-plus-progestin trial for an extra year after the study ended. During this year, women who had used hormones during the study continued to have more mammogram abnormalities than women who had not. This suggests that discontinuing hormone therapy for a short interval before mammography may not affect either mammogram findings or breast cancer diagnoses, the researchers note in the February 25 Archives of Internal Medicine.
Women considering even short-term combined hormone therapy should factor the adverse effects on breast cancer detection into their risk-benefit analyses, the researchers conclude. During the original WHI estrogen-plus-progestin trial, women used either hormones or placebo for 5.6 years. The study was cut short in 2002 after the increased health risks associated with combined hormone therapy became evident.
A second WHI follow-up study, released this week, reports that the increased risk of breast cancer in women using combined hormone therapy persists for up to 3 years after treatment ends.
Study Confirms Clot, Mortality Risks with Anemia Drugs
A meta-analysis of clinical trials testing anti-anemia drugs in cancer patients has confirmed findings from earlier studies demonstrating increased risk of blood clots and death.
The findings, published in the February 27 Journal of the American Medical Association, come less than a month before the Food and Drug Administration's (FDA) Oncologic Drugs Advisory Committee (ODAC) will meet for the third time in 4 years to discuss the safety of the drugs, known as erythropoiesis-stimulating agents (ESAs).
Overall, the analysis found a 57 percent increased risk of venous thromboembolism (VTE) and a 10 percent increased mortality risk in patients treated with ESAs. The review included 51 phase III trials with survival information and 38 trials with data on VTEs.
"Our findings, in conjunction with basic science studies, raise the concern that the drug may be stimulating cancer and shortening cancer patients' survival," said the study's lead author, Dr. Charles L. Bennett from Northwestern University Feinberg School of Medicine.
The review - which involved trials with patients with anemia caused by cancer, as well as chemotherapy- or radiotherapy-related anemia - included data from eight recent phase III trials in patients with a variety of cancer types. With one exception, the recent trials were all designed to achieve hemoglobin levels in the 13 to 15 g/dl range, what have been referred to as "beyond anemia correction" studies.
The FDA, which issued two public health advisories on ESA use in 2007, is engaged in an ongoing safety review of the drugs.
Sentinel Node Dissection Safe in Early Vulvar Cancer Treatment
An international group of researchers led by the University Medical Center Groningen in the Netherlands has shown that sentinel lymph node dissection (SLND) to detect microscopic metastases in women with early-stage cancer of the vulva is safe compared with the standard treatment of inguinofemoral lymphadenectomy, which involves removing extensive lymph nodes in the groin and upper leg and can have long-lasting side effects.
The investigators enrolled 403 women into their study, all of whom underwent resection of their primary tumor and SLND. Of these, 127 had metastatic cells in the examined nodes, and underwent full inguinofemoral lymphadenectomy. The other 276 women did not have evidence of metastases on SLND, and did not undergo additional surgery.
All women returned for follow-up visits at least every 2 months for the first 2 years after surgery. Only four women were lost to follow-up; for the rest, the investigators compared short- and long-term complications and incidences of groin recurrences of the cancer between those who did and did not undergo inguinofemoral lymphadenectomy.
Women who only underwent SLND had significantly fewer short- and long-term complications - including difficulty with wound healing, lymphedema, and persistent infections - than women who underwent inguinofemoral lymphadenectomy. The overall rate of groin recurrence in women who only underwent SLND was 3 percent, which the authors deemed comparable to a calculated historical rate of 2 percent.
"The low groin recurrence rate...and excellent disease-specific survival rate of 97 percent at 3 years in sentinel node-negative patients suggest that the sentinel node procedure is a safe alternative to inguinofemoral lymphadenectomy for selected vulvar cancer patients," concluded the authors. However, they stress, extensive clinical experience with SLND for both the surgeon and the multidisciplinary team supporting the procedure is essential for replicating these positive results.
Smoking Threatens Pregnant Women in Developing Countries
A survey of nearly 8,000 pregnant women from 9 developing nations in Latin America, Asia, and Africa indicates that "rising tobacco use in the developing world threatens to impede or reverse hard-won global health gains." The multicenter, cross-sectional survey examined pregnant women's tobacco use, secondhand smoke (SHS) exposure, and attitudes toward women's tobacco use. Results were published online February 28 in the American Journal of Public Health.
The authors conclude that cigarette smoking during pregnancy is a current or emerging problem in the five Latin American countries surveyed. In Uruguay, 78.3 percent of pregnant women surveyed had ever tried cigarettes and two-thirds of those went on to become regular smokers, a third of whom continued to smoke while pregnant. Argentina - and to a lesser extent Ecuador, Brazil, and Guatemala - had similar patterns. If women's smoking were to become more culturally acceptable, say the authors, these other sites could expect much higher levels of smoking by pregnant women.
Pakistan had the most homes where smoking was allowed: 92 percent of those surveyed. Consequently, half of Pakistani pregnant women and half of their young children are frequently or always exposed to SHS. The use of other tobacco products, like smokeless tobacco and snuff, was highest in Orissa, India (34 percent).
The study was conducted by an international team of investigators, including researchers from two NIH institutes - NCI and the National Institute of Child Health and Human Development (NICHD). The researchers conducted the study at nine sites in the Global Network for Women's and Children's Health Research, operated by NICHD, with funding from the Bill and Melinda Gates Foundation. The Department of Health and Human Services' Office on Women's Health also provided study support.
The findings "highlight the urgent need to implement evidence-based interventions to prevent and control tobacco use among pregnant women" in these countries, writes lead author Dr. Michele Bloch of NCI's Tobacco Control Research Branch and colleagues.
|
 |
 |

Keeping Recent Mortality Figures in Perspective
The headlines varied, but when the Cancer Facts & Figures 2008 data were released late last month, the dominant message that many TV, print, and electronic news stories carried was that the decline in the number of actual cancer deaths had come to an end.
After back-to-back drops in the number of actual cancer deaths, the most recent data show an increase of more than 5,400 deaths from 2004 to 2005. But the larger story - a true success story - is that cancer mortality rates are continuing to decline. From the time the decline began in the early 1990s, in fact, cancer death rates for men have decreased more than 18 percent, while the rate for women has seen a more modest 10.5 percent decrease.
According to American Cancer Society estimates, that's more than half a million lives saved from death due to cancer - nearly equivalent to the population of Portland, Oregon.
The increase in the number of cancer deaths was no surprise. Not only has the U.S. population continued to swell, but it's also aging: More than 20 percent of the population is 55 or older, an age group that represents more than three of every four cancer diagnoses. So, even though fewer people as a proportion of the whole population are dying from cancer, the actual number of deaths still increased.
The new report also provides some important insights into the cancers and populations where some of our most significant challenges lie.
Estimates are that lung cancer, for example, will account for more than a quarter of all cancer deaths among women in 2008. Clearly this is an area where we must do better. Disparities in care and outcomes persist. The cancer death rate among African American men, for example, is 37 percent higher than it is among white men.
Hopefully the cancer and public health communities can work with policymakers to develop and implement the necessary changes to address these disparities - something NCI is already spearheading with important initiatives like the NCI Community Cancer Centers Program and the Community Networks Program.
Looking to the future, recent advances and promising research suggest that we can maintain the cancer death rate decline, and perhaps even accelerate it.
Last year, for instance, we saw significant advances in the treatment of some of the most difficult-to-treat cancers, including small-cell lung cancer, liver cancer, and kidney cancer. And while the introduction of HPV vaccines stands to greatly reduce cervical cancer incidence in the future, recent evidence demonstrating an association between HPV and head and neck cancers also suggests widespread vaccination could have unforeseen benefits.
We are also pursuing new avenues of cancer prevention research, including identifying biomarkers of cancer risk, such as hypermethylation patterns, while continuing to pursue promising new chemoprevention agents, such as DFMO for the prevention of colon polyps, which are often precursors to colon cancer.
And as we continue to learn more about cancer stem cells, also known as tumor-initiating cells, we are gaining a better understanding of their resistance to treatment and how to target these cells. We are also learning to effectively mine molecular clues from patients' tumors to guide treatment. One such example is the NCI-funded TAILORx trial, which is testing whether a 21-gene assay can guide the use of adjuvant chemotherapy for certain breast cancer patients.
Collectively, these examples represent an extensive investment in our attack on the collection of malignancies we call cancer. Progress will be swift in some areas, slower in others, and of course will be heavily reliant on the budgets under which NCI operates.
But, as the continued decline in death rates demonstrates, the foundation for progress has been laid. NCI is committed to finding ways to strengthen that foundation through judicious management, strategic collaboration, and wise investment in the best science possible.
Dr. John E. Niederhuber
Director, National Cancer Institute
|
 |
 |
 |

Tiny Nodules, Big Dilemma
This article is the second in a two-part series on thyroid cancer. Part one focused on thyroid cancer's rapidly increasing incidence. Part two discusses the treatment of small thyroid tumors.
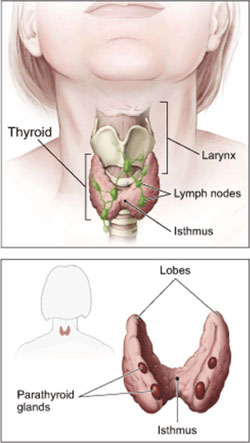 Phrases such as "controversial issue" or "a matter of great debate" litter the published literature about the initial treatment of patients with small tumors of the most common type of thyroid cancer, papillary thyroid cancer (PTC). Of particular interest is the most appropriate treatment for tumors 1 centimeter or smaller in size. Phrases such as "controversial issue" or "a matter of great debate" litter the published literature about the initial treatment of patients with small tumors of the most common type of thyroid cancer, papillary thyroid cancer (PTC). Of particular interest is the most appropriate treatment for tumors 1 centimeter or smaller in size.
The primary disagreement is whether just the wing, or lobe, of the thyroid in which the tumor resides should be removed, known as a lobectomy, or whether all or most of the thyroid gland should be removed, a total or near-total thyroidectomy. And the debate doesn't stop there. Experts disagree about whether patients who undergo the more aggressive procedure also require a treatment known as radioactive iodine (RAI) ablation.
These are important questions, because as the incidence of thyroid cancer continues to rise steadily, it's these small, malignant thyroid nodules that are driving it. By one estimate, small tumors may soon account for 90 percent of all PTCs treated in the United States, many of which will be smaller than 1 centimeter.
The Mortality Remains the Same
If clinical practice is any indication, much of the debate has largely been settled in favor of total or near-total thyroidectomy. One recent study, in fact, found that 90 percent of all PTC patients underwent the procedure, compared to approximately 70 percent two decades earlier.
Typically, cancer treatment patterns change when a given treatment generates notable improvements in survival or fewer side effects. But the mortality rate from PTC has remained flat, and extremely low, for the last four decades.
In any discussion about treatment, one thing seems abundantly clear: Observing the smallest tumors - what in prostate cancer is often called "watchful waiting" - isn't an acceptable option from the perspective of most patients.
"If they have cancer, my patients want it out," says Dr. Carmen Solorzano, chief of the Division of Endocrine Surgery at the University of Miami Miller School of Medicine.
And that's where the disagreements begin.
In patients with the smallest tumors confined to one lobe, argues Dr. Ashok R. Shaha, a professor of surgery at Memorial Sloan-Kettering Cancer Center, "there is no rationale for taking out the whole thyroid. You are punishing the patient, more or less, for their whole life."
The punishment, Dr. Shaha says, is that patients who undergo total thyroidectomy have to take thyroid hormone replacement for the remainder of their lives and require regular doctor visits to ensure the dosage is appropriate.
However, some endocrinologists also put patients treated with lobectomy on thyroid hormone medication, at least for a period of time.
Total thyroidectomy also is associated with other side effects, Dr. Shaha says, including hypoparathyroidism, in which the parathyroid gland can't produce enough calcium and phosphorous, and permanent damage to the laryngeal nerve.
In the hands of a good endocrine surgeon, counters Dr. Keith Heller, chief of endocrine surgery at New York University Medical Center, these risks are minimal.
In addition, Dr. Heller says, sometimes after a lobectomy, the final pathology report shows multifocal cancer, vascular invasion, or extension beyond the thyroid. If a lobectomy is done instead of removing the entire thyroid, a second operation might be recommended anyway, he says. Although he acknowledges that, in patients considered low risk, total thyroidectomy offers no survival advantage, Dr. Heller believes more aggressive treatment of tumors 1 centimeter or smaller is preferable, for just these situations. He's not alone.
"The problem is that these very small cancers, most of them have a good outcome, but not all of them," says Dr. Ernest Mazzaferri, chair of medicine at The Ohio State University, a long-standing proponent of more aggressive treatment.
It's not always as simple as just a single, small malignant nodule, he continues. The tumor may be invading outside of the thyroid "capsule." Or perhaps there is a family history of thyroid cancer or exposure to radiation. All suggest a tumor with the potential, albeit limited, to become aggressive.
These patients "don't always do so well," Dr. Mazzaferri says. "They can die from pulmonary or brain metastases. A small number, 1 percent, will die. None of us want to miss those."
To Irradiate or Not to Irradiate
Many patients who undergo a total or near-total thyroidectomy for small tumors also receive RAI. This long-used treatment, typically taking place 1 to 2 months after thyroidectomy, was an early precursor to targeted therapies: Thyroid cells excel at concentrating iodine, so the radioactive iodine migrates to any thyroid cancer cells that evaded the surgeon's scalpel and destroys them.
But the use of RAI in patients with smaller tumors is not without its critics.
Dr. Ian Hay, a heralded endocrinologist at the Mayo Clinic, and others have performed retrospective studies showing no significant difference in recurrence rate or cancer-specific mortality in low-risk patients who received RAI and those who did not.
Using RAI in these patients, Dr. Shaha believes, "is just unnecessary extra treatment."
Dr. Mazzaferri, however, has been outspoken in his support for RAI, even in patients whom some endocrinologists would consider low-risk.
"There are 11 different staging systems, and that, in and of itself, tells you part of the problem," he says. "We miss a lot of patients who have recurrent disease or who are in trouble by assigning some number to them that says you're low risk and we're not going to treat you any further."
In most cases, he argues, RAI is now a one-time treatment, and treatments have been modified so that smaller and smaller doses of radiation are used.
Because most patients with thyroid cancer live for decades after diagnosis, any sort of prospective clinical trial to compare treatment regimens is unlikely to happen. It would be extremely expensive, requiring thousands of patients and decades to complete.
There is, however, optimism that molecular markers can be found to identify patients with small tumors who should receive more aggressive therapy.
Many in the endocrinology community are hanging their hopes on a specific marker, a mutation in the BRAF gene. Several studies have shown an association between this mutation and outcomes such as treatment failure and recurrence.
The research into this mutation, Dr. Mazzaferri admits, is still in the developmental stage. But, he says, "I think down the road it will be a big player in helping us sort things out."
—Carmen Phillips
|
 |
 |

Bevacizumab Approved for Metastatic Breast Cancer
The Food and Drug Administration (FDA) has approved bevacizumab (Avastin), in combination with paclitaxel chemotherapy, for the treatment of metastatic HER2-negative breast cancer in women who have not previously received chemotherapy.
The drug was given "accelerated approval,' which makes it available to women with this deadly disease based on limited clinical trial results. Full approval is contingent on additional positive results from ongoing clinical trials.
Accelerated approval was based on a phase III study in which bevacizumab plus paclitaxel chemotherapy resulted in a 52-percent reduction in the risk of disease progression or death compared with those treated with paclitaxel alone and a doubling in progression-free survival, according to the drug's maker, Genentech.
The drug has not yet been shown to improve overall survival in this disease. An FDA advisory panel recommended last December against making bevacizumab available for breast cancer in a 5-4 vote. Some experts were concerned that the risk of serious side effects outweighed the potential benefit of the drug's ability to slow the spread of tumors.
Bevacizumab is designed to fight cancer by blocking the growth of blood vessels (angiogenesis) that supply tumors with nutrients. The drug is also approved for the treatment of colorectal and lung cancers.
|
 |
 |

Continuing Bevacizumab Therapy for Metastatic Colorectal Cancer
Name of the Trial
Phase III Randomized Study of Irinotecan Hydrochloride-Based Chemotherapy and Cetuximab with versus without Bevacizumab in Patients with Metastatic Colorectal Cancer that Progressed on First-Line Therapy (SWOG-S0600). See the protocol summary at http://cancer.gov/clinicaltrials/SWOG-S0600.
 Principal Investigators
Principal Investigators
Dr. Philip Gold and Dr. Anthony Shields, SWOG; Dr. Axel Grothey, North Central Cancer Treatment Group; Dr. Leonard Saltz, CALGB; Dr. Steven Cohen, ECOG; and Dr. Scott Berry, NCIC-Clinical Trials Group
Why This Trial Is Important
Patients with metastatic colorectal cancer are often treated with the drugs oxaliplatin and bevacizumab in combination with other drugs. If the cancer progresses while the patient is being treated with these agents, second-line therapy with different drugs may be initiated.
Bevacizumab, a monoclonal antibody that inhibits the formation of new blood vessels tumors need for continued growth, has been shown to help extend the survival of patients with advanced or metastatic colorectal cancer when used as part of initial, or first-line, therapy. It is unknown, however, whether continuing bevacizumab will help improve survival when used in second-line therapy to treat patients whose cancer has progressed despite first-line treatment with bevacizumab and chemotherapy.
In this trial, patients with metastatic colorectal cancer that has progressed on first-line therapy containing oxaliplatin and bevacizumab will be treated with the drugs irinotecan and cetuximab. The patients will also be randomly assigned to receive either a low dose of bevacizumab, a high dose of bevacizumab, or no additional bevacizumab.
"The goal of this trial is to determine whether there is a benefit to continuing to treat patients with bevacizumab if they have progressed on a regimen containing it," said Dr. Gold.
"Bevacizumab may make tumors more susceptible to chemotherapy by normalizing the tumor vasculature, and emerging data suggest that bevacizumab and cetuximab may produce a synergistic effect," Dr. Gold added. "So, there is a good rationale to see if continuing bevacizumab in combination with cetuximab will help improve outcomes for patients, perhaps helping them live longer."
For More Information
See the lists of entry criteria and trial contact information at http://cancer.gov/clinicaltrials/SWOG-S0600 or call NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |
 |
 |
Special Issue on Childhood Cancers |
 |
|
Don't miss our March 18 special issue of the NCI Cancer Bulletin, which will focus on childhood cancers. The issue will feature articles about NCI's intramural and extramural research efforts in pediatric cancers and will provide a list of resources for additional information.
NCI Cancer Bulletin special issues are some of the most popular among our readership. Past special issues have focused on cancer survivorship, cancer prevention, and NCI-Frederick.
|
|
NIH Director to Testify Before Appropriations Subcommittee
NIH Director Dr. Elias Zerhouni is scheduled to testify before the House Appropriations Subcommittee on Labor-HHS-Education on March 5. Appearing with Dr. Zerhouni will be the heads of the Centers for Disease Control and Prevention, Substance Abuse and Mental Health Services Administration, and the Agency for Healthcare Research and Quality. The focus of the hearing will be on programmatic outcomes and the linkages between each agency's programs.
BSA Meeting Held
NCI's Board of Scientific Advisors (BSA) met March 3-4 on the NIH campus in Bethesda, MD. The public portions of the meeting can be viewed at http://videocast.nih.gov/PastEvents.asp.
| |
 |
 |

Lombardi Comprehensive Cancer Center
Director: Dr. Louis M. Weiner • Research Building, Suite E501, 3970 Reservoir Road NW, Washington, DC 20057 • Phone: 202-697-2110 • Web site: http://lombardi.georgetown.edu/
Background
Established in 1970, the Lombardi Comprehensive Cancer Center is named in honor of legendary football coach Vince Lombardi, who was treated for cancer at Georgetown University Hospital. What began as a small clinic treating cancer patients is now a state-of-the-art cancer center housing more than 240,000 square feet of clinic and research space. As Lombardi has grown, its mission has remained constant - to provide the most advanced treatments available, to train future cancer specialists, and to cure cancer in its many forms.
Lombardi was first designated as an NCI Comprehensive Cancer Center in 1974 in conjunction with Howard University, and the designation was renewed for Lombardi as a single-site center in 1990. It remains the only comprehensive cancer center in the Washington, DC, metropolitan area. It receives $97 million in grant funding, has nearly 200 full-time faculty members, and 220 ongoing clinical trials. Today, Lombardi presses forward under the visionary leadership of its director, Dr. Louis M. Weiner, who was recruited in October 2007.
Research Activities
Lombardi treats virtually every type of cancer. The cancer center boasts six established research programs, including: the Nina Hyde Center for Breast Cancer Research, ranked sixth in the world for breast cancer publications by ESI Thompson Scientific; Cancer Control; Cancer Genetics and Epidemiology; Growth Regulation of Cancer; Molecular Targets and Developmental Therapeutics; and Radiation Biology and DNA Repair. A developing Pediatric Cancer Research Program brings together faculty from Lombardi and Children's National Medical Center to develop novel therapies for pediatric cancers.
Patient Care Specialties
Lombardi offers a full range of services focused on enhancing the quality of life of its patients and their families, including social work support, nutritional counseling, palliative care and symptom management, support groups, and an arts and humanities program that is considered a leader in its field.
Other Notable Programs
Lombardi is expanding its community outreach. In 2006, Lombardi helped develop the first cancer control plan for the District of Columbia, which has the highest incidence of cancer in the United States. Lombardi's Capital Breast Care Center provides free mammograms to underserved women in the Washington area. And, in 2000, Georgetown University formed a partnership with MedStar Health, one of the largest not-for-profit networks in the mid-Atlantic region, giving patients throughout the region expanded access to cancer care and clinical trials from Lombardi.
Education and training at the Lombardi Comprehensive Cancer Center spans all levels of learning. The cancer center has four master's programs (in biostatistics, health physics, tumor biology, and a joint program in cancer genetics, epidemiology, and control), as well as a Ph.D. and combined M.D./Ph.D. program in tumor biology. Additionally, three postdoctoral training programs are offered at Lombardi: a medical genetics fellowship, clinical fellowships in hematology/oncology and palliative care, and research fellowships in tumor biology.
|
 |
|