CFSAN 2004 Program Priorities
Report Card
Letter from Center Director
Attachment (summary chart and graph)
Final Report - Accomplished (summary list)
Report Card Table of Contents
Dear Colleague, FDA Foods Community:
I am pleased to provide you with the end-of-year report on our 2004 program
priority accomplishments for FDA's foods program. We had an ambitious plan and
we have met our goal of completing at least 90% of our "A" List goals. I am proud
of the Center's success rate. These results reflect a continued commitment to the
management strategy of focusing our resources on where we provide the most benefit
to American consumers and the continued dedication of the CFSAN workforce.
I would like to highlight a few program areas that have seen significant accomplishments
this fiscal year. First, I have placed a renewed focus on the Nutrition program and we
are starting to see the results of those efforts. In May, we completed consumer research
on qualified health claim messages in the labeling of foods and dietary supplements. This
research is aimed at ensuring the most effective wording of the qualified health claim
to ensure the messages are not misleading to consumers. The Obesity Working Group completed
its Working Group Report and Recommendations, Calories Count, in February 2004
and we have begun to implement the recommendations of the report. We are working with
a third-party facilitator to develop options for providing nutrition information at the
point of purchase in a restaurant setting and to develop approaches, including partnerships
for educating consumers, particularly adolescents about obesity. Our overarching goal is to
make available more and better information about foods and dietary supplements, to help
American consumers prevent diseases and improve their health by making sound dietary decisions.
I am also delighted that we have taken positive steps forward in the area of produce safety.
Produce is recognized as an important component of a healthy diet and it can play an important
role in weight management as well. Because most produce is grown in a natural environment,
it is vulnerable to contamination with pathogens. In September, we released our plan to
minimize the incidence of foodborne illness associated with the consumption of fresh produce.
The plan extends to all parts of the food chain from farm through retail or consumer
preparation and consumption.
Let me address our commitment to protecting consumers from misleading claims and unsafe
dietary supplements. This year, we published a final regulation declaring dietary supplements
containing ephedrine alkaloids adulterated under the Federal Food, Drug and Cosmetic Act.
Dietary supplements containing ephedrine alkaloids present an unreasonable risk of illness
or injury and the sale of these products is now prohibited. Active steps also have been taken
through the issuance of warning letters to cease the distribution of products sold as dietary
supplements that contain androstenedione. We believe these products may increase the risk of
serious health problems because they are converted in the body to testosterone which is an
androgenic and anabolic steroid. We responded to 47 notifications for dietary supplements
containing new dietary ingredients. The notifications were reviewed for science-based
evidence of safety. We identified deficiencies in the information submitted or had safety
concerns with 31 of the notifications. We are committed to working with all of our stakeholders
and are seeking public comment on the type, quantity, and quality of evidence manufacturers
should provide in a new dietary ingredient notification.
Finally, let me mention our efforts related to Food Defense formally referred to as Food Security.
Following publication of the interim final rules for the Registration of Food Facilities and the
Prior Notice of Imported Foods, we have worked diligently to implement the information technology
systems for these regulations. We have worked closely with our stakeholders to ensure these systems
are functional and are user-friendly. The systems have been operational since December 2003. We
have worked closely with Customs and Border Protection on the development of the prior notice systems
and successfully implemented a phased in approach to enforcement.
In closing, I would like to express my appreciation for the support I have received from our many
stakeholders. Your reviews and perspectives are invaluable to me in establishing our program priorities.
Working together we can improve public health. I look forward to working with you and continuing the
tradition of building predictability, transparency and accountability into FDA's foods program.
Sincerely,
Robert E. Brackett, Ph.D.
Director
Center for Food Safety and Applied Nutrition
Enclosures
Attachment
Program Priorities in FDA's Foods Program
1999 - 2004
| | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 |
|---|
| Total "A" List Goals | 83 | 108 | 127 | 118 | 150 | 168 |
|---|
| (Includes those added at mid-year) | (5) | (11) | (17) | (18) | (10) | (8) |
| Mid-Year Completions | 14 | 23 | 53 | 43 | 59 | 71 |
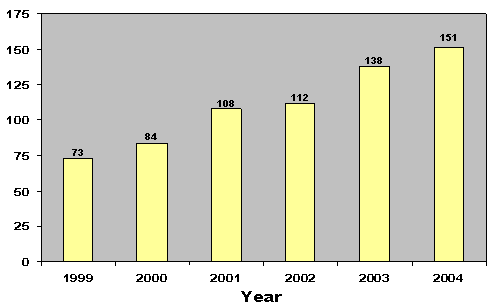
| End-of-Year Completions | 73 | 84 | 108 | 112 | 138 | 151 |
|---|
| Percent "A" List Completions | 88% | 78%* | 85% | 95% | 92% | 93% |
| * In order to align our workplan with the federal budget cycle,
we condensed the 2000 workplan into three-fourths of the year. Accordingly, our goal was to complete
75% of the "A" List activities. |
Number of "A" List Items Completed
1999-2004
FY 2004 Program Priority Status Report
Final Report
Accomplished
Table of Contents
-
- Food Security: Implementing New Legislation
- Food Security: Emergency Preparedness
- Domestic Inspections
- Imports Foreign Inspections
- Seafood Safety
- Fruits and Vegetables
- Egg Safety
- Listeria
- Chemical Contaminants, Pesticides and Other Hazards
- Transmissable Spongiform Encephalopathies (TSEs)
- Game Meats
- Food Allergens
- Dairy Safety
- Education
-
- Nutrition, Health Claims and Labeling
- Dietary Supplements
-
- Food and Color Additives: Premarket Review
- Cosmetics
-
- Science Base
- International
- Food Biotechnology
- Internal Processes
- Focused, Economic-based Regulations
- EEO/Diversity Initiatives
- Management Initiatives
Highlights
Assuring Food Safety and Security
- Published final regulation for "Administrative Detention" under the Bioterrorism Act.
- Conducted outreach and training on the proposed and interim final rules.
- Issued a revised Compliance Policy Guide (CPG) providing guidance to FDA's and
the Customs and Border Protection's (CBP) enforcement of the prior notice of imported foods regulation.
- Published guidance documents on preventive measures for the food, dairy and cosmetics
industries, importers, and retail establishments.
- Conducted more than 70,926 field examinations of imported food shipments entering U.S. ports
of entry (goal was 60,000).
- Produced an unclassified version of the CFSAN Operational Risk Management (ORM) Vulnerability Assessment.
- Initiated collaborative research to characterize the ability of non-traditional vegetative pathogens to survive
and grow in a variety of foods.
- Providing guidance for FDA and Custom and Border Protection's (CBP) enforcement of the prior notice of
imported foods regulation.
Improving Nutrition and Dietary Supplement Safety
- Issued "Calories Count" Report containing short and long-term recommendations for dealing with
the obesity epidemic.
- Completed consumer research on qualified health claim messages in the labeling
of foods and dietary supplements.
- Promoted Trans Fat Education and Outreach.
- Published final rule prohibiting the sale of dietary supplements containing ephedrine alkaloids.
- Published draft Guidance on Substantiation for Dietary Supplement Claims.
- Issued warning letters asking companies to cease distribution of products sold as dietary supplements
that contain androstenedione.
Food Safety and other Program Activities
- Completed the review of 47 notifications for dietary supplements containing new dietary ingredients.
- Conducted more than 7,244 domestic inspections of firms that produce "high risk" foods (goal was 6,840).
- Issued a joint consumer advisory with the Environmental Protection Agency about mercury in fish ad shellfish.
- Published a proposed rule to require shell egg producers to implement measures to prevent
Salmonella Enteritidis (SE) from contaminating eggs on the farm.
- Continued outreach to industry and consumers on food allergens.
- Completed the safety evaluation of food and color additive petitions in a timely manner.
- Completed review of biotechnology final consultations, GRAS notifications and food contact substance
notifications within statutory timeframe.
- Implemented electronic submission capability (eLACF) for low acid canned food registrations.
- Completed all batch certification of color additives in a timely way.
- Served as major participant in international Codex activities: Attended one Codex Commission
Session meeting, a Trilateral U.S./Canada/Mexican Committee meeting; and provided the U.S. delegate for ten
cross-cutting Codex Committees/Task Forces.
- Published a guidance related to the processing of juice.
- Published the 2004 Produce Safety Action Plan to further minimize foodborne illness associated with
the consumption of fresh produce.
- Published an interim final rule to prohibit the use of certain cattle material to address the
potential risk of BSE inhuman food, including dietary supplements and cosmetics.
Part I: Assuring Food Safety and Security
Food Security: Implementing New Legislation
- Development
of the Registration of Food Facilities Final Rule: The Food
and Drug Administration issued on October 10, 2003, an interim final
regulation (IFR) on Registration of Food Facilities under the Bioterrorism
Act of
2002. In developing the final regulation, the FDA held a series of domestic
and international outreach meetings with the public, government officials
and certain manufacturers, processors and transporters of foods to discuss
the final regulation. Also during the development of the interim final
rule, FDA allowed two opportunities for public input; the registration
IFR was open for comment for 75 days following its publication and FDA
reopened the comment period for an additional 30 days in March 2004, to
ensure that those commenting on the IFR had the benefit of FDA's outreach
and educational efforts and had experience with the information systems,
timeframes, and data elements of the registration program. The Food
Facility Registration final regulation is positioned for publication
in FY 2005.
- Development
of the Prior Notice of Imported Foods Final Rule: The Food and
Drug Administration issued on October 10, 2003, an interim final regulation
(IFR) on Prior Notice of Imported Foods under the Bioterrorism Act
of
2002. In developing the final regulation, the FDA held a series of domestic
and international outreach meetings with the public, government officials
and certain manufacturers, processors and transporters of foods to discuss
the final regulation. Also during the development of the interim final
rule, FDA allowed two opportunities for public input; the Prior Notice
IFR was open for comments for 75 days following its publication and FDA
reopened the comment period for an additional 30 days in March 2004,
to ensure that those commenting on the IFR had the benefit of FDA's outreach
and educational efforts and had experience with the information systems,
timeframes, and data elements of the prior notice system. The Prior
Notice of Imported Foods final regulation is positioned for publication
in FY 2005.
- Publication of the Administration Detention Final Rule: On
June 4, 2004, published in the Federal Register (69 FR 31659)
a final regulation entitled: "Administrative Detention of Food for Human or Animal Consumption Under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002." This final rule implements the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (the Bioterrorism Act), which authorizes the use of administrative detention and requires regulations establishing procedures for instituting on an expedited basis certain enforcement actions against perishable food subject to a detention order. This rule was effective July 6, 2004.
Federal Register (69 FR 31659)
- Food
Terrorism Risk Assessment: On October 10, 2003, announced in the Federal
Register (68 FR 59078) the availability of a risk assessment
for food terrorism and other food safety concerns. Development of this
risk assessment is one of a number of steps the Agency is taking to improve
its ability to prevent, prepare for, and respond to an incident of food
sabotage.
Risk Assessment for Food Terrorism and Other Food Safety Concerns
- Outreach
for the Bioterrorism Rules: On October, 28, 2003, CFSAN/FDA held
a public meeting via satellite downlink to discuss the final regulations
implementing two sections in Title III of the Bioterrorism Act regarding
Registration of Food Facilities (docket No. 2002N-0276) and Prior Notice
of Imported Food Shipments (docket No. 2002N-0278). The purpose for the
meeting was to provide information on the rules to the public and to provide
a comprehensive picture of the rules and provide the public an opportunity
to ask questions of clarification.
Videos of the October 28, 2003 Satellite Broadcast
(also available in French and Spanish)
On April 1, 2004, CFSAN completed a nine-city domestic
outreach effort for small businesses and other stakeholders on the two
interim final rules. From April 21-29,
2004, FDA met with over 1,000 government
officials, manufacturers, processors, packers, holders, and transporters
of foods in a series of outreach meetings on the Food Facility Registration
and Prior Notice Regulations. The FDA team conducted international meetings
in Beijing, China; Seoul, Korea; Tokyo, Japan; and Bangkok, Thailand. Press briefings
and television interviews were conducted during the international tour. The
highly successful tour was organized in coordination with the American
Embassy in each of the countries visited and in Bangkok, co-sponsored
by the National Food Processors Association (Asia Branch). On May
4, 2004, CFSAN staff provided a brief
overview of the Bioterrorism (BT) Regulations to the Republic of Armenia
delegation at the CFSAN Headquarters in College Park , MD.
- Food Safety and Security Guidance for the Retail Sector: On
December 17, 2003, published in the Federal Register (68 FR 70278)
a notice announcing the availability of a guidance document related to
food security entitled "Retail Food Stores and Food Service Establishments:
Food Security Preventive Measures Guidance." This guidance identifies the kinds of preventive measures that operators
may take to minimize the risk that food under their control will be subject to tampering or other malicious,
criminal, or terrorist actions.
Guidance for Industry - Retail Food Stores and Food Service Establishments:
Food Security Preventive Measures Guidance
- Food Safety and Security Guidance for Cosmetics: On December 17, 2003, published in the Federal Register (68 FR 70278)
a notice announcing the availability of a guidance document related to
cosmetics security entitled "Cosmetics Processors and Transporters: Cosmetics
Security Preventive Measures Guidance." This guidance is designed as an aid to operators of cosmetics establishments
(e.g., firms that process, store, repack, relabel, distribute, or transport cosmetics or cosmetics ingredients).
It identifies the kinds of preventive measures that operators may take to minimize the risk that cosmetics under
their control will be subject to tampering or other malicious, criminal, or terrorist actions.
Guidance for Industry - Cosmetic Processors and Transporters Cosmetics
Security Preventive Measures Guidance
- Registration
of Food Facilities Small Entities Compliance Guide: On December
12, 2003, published in the Federal Register (69 FR 69408)
a notice announcing the availability of a small entity compliance guide
(SECG) for the Registration of Food Facilities IFR. This SECG restates,
in simplified format and language, FDA's current requirements for registration
of food facilities.
Small Entity Compliance Guide -
What You Need to Know About
Registration of Food Facilities (also available in French and Spanish)
- Registration
of Food Facilities Compliance Policy Guide: On December 19,
2003, published in the Federal Register (68 FR 70817) a
notice announcing the availability of a CPG Sec. 110.300 entitled "Registration
of Food Facilities Under the Public Health Security and Bioterrorism Preparedness
and Response Act of 2002." This CPG provides written guidance to FDA staff
on enforcement of section 305 of the Bioterrorism Act and the Agency's
implementing regulation, which require, beginning on December 12, 2003,
registration with FDA for all domestic and foreign facilities that manufacture/process,
pack, or hold food for human or animal consumption in the United States.
Compliance Policy Guide, Guidance for FDA Staff: Registration of Food Facilities Under
the Public Health Security and Bioterrorism Preparedness and Response Act of 2002
- Registration of Food Facilities IFR - Q&A Guidance Document: On
December 4, 2003, issued guidance to industry entitled "Questions and
Answers Regarding the Interim Final Rule on Registration of Food Facilities." This
guidance responds to various questions raised about section 305 of the Bioterrorism
Act and the Agency's implementing regulation, which require facilities
that manufacture/process, pack, or hold food for consumption in the U.S. to
register with FDA by December 12, 2003.
Guidance for Industry - Questions and Answers Regarding Registration of Food Facilities
- Registration of Food Facilities IFR -- Q&A Guidance Document: On
January 12, 2004, announced in the Federal Register (69 FR 1675) the availability of the
revised guidance entitled: Questions and Answers
Regarding the Interim Final Rule on Registration of Food Facilities (Edition 2). As noted in the first notice
in response to requests for clarification of the registration interim final
rule by providing guidance in a question and answer format.
Guidance for Industry - Questions and Answers Regarding Registration of
Food Facilities (Edition 2)
- Registration of Food Facilities IFR
- Q&A Guidance Document: On February 17, 2004, announced in the Federal
Register (69 FR 7347) the availability of a revised guidance
entitled: "Questions and Answers Regarding the Interim Final Rule on
Registration of Food Facilities (Edition 3)." This guidance continues to respond
to various questions raised about section 305 of the Bioterrorism Act.
Guidance for Industry - Questions and Answers Regarding Registration of
Food Facilities (Edition 3)
- Registration of Food Facilities IFR
- Q&A Guidance Document: On August 6, 2004, announced in the Federal
Register (69 FR 47765) the availability of a revised guidance
entitled: "Questions and Answers Regarding the Interim Final Rule on
Registration of Food Facilities (Edition 4)." As noted in previous notices announcing
the availability of guidance for food facility registration, FDA continues
to respond to requests for clarification of the registration interim final
rule by providing guidance in a question-and-answer format.
Guidance for Industry - Questions and Answers Regarding Registration of
Food Facilities (Edition 4)
- Prior Notice of Imported
Foods Small Entities Compliance Guide: On December 12, 2003, published
in the Federal Register (69 FR 69408) a notice announcing
the availability of a SECG for the IFR on Prior Notice of Imported Foods. This
SECG restates, in simplified format and language, FDA's current requirements
for Prior Notice of Imported Foods.
Small Entity Compliance Guide
- What You Need to Know About
Prior Notice of Imported Food Shipments (also available in French
and
Spanish)
- Prior Notice of Imported Foods Compliance Policy Guide: On December 15, 2003, published in the Federal
Register (68 FR 69708) a notice announcing the availability
of a Compliance Policy Guide (CPG) Sec 110.310 entitled: "Prior Notice
of Imported Food Under the Public Health Security and Bioterrorism Preparedness
and Response Act of 2002." This CPG provides written guidance to FDA staff
and the U.S. Customs and Border Protection (CBP) staff on enforcement of
section 307 of the Bioterrorism Act and the Agency's implementing regulations,
which require, beginning on December 12, 2003, prior notice for all food
imported or offered for import into the United States.
Compliance Policy Guide, Guidance for FDA and CBP Staff: Prior Notice of Imported Food
Under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (also available in French and Spanish)
- Prior Notice of Imported
Foods IFR -- Q&A Guidance Document: On December 16, 2003, announced
in the Federal Register (68 FR 69957) a notice of availability
of the Guidance for Industry: Questions and Answers on the Interim Final
Rule on Prior Notice of Imported Food.
Guidance for Industry - Prior Notice of
Imported Food Questions and Answers
- Joint FDA/CBP Plan for
Prior Notice Timeframes: On April 14, 2004, the FDA and the U.S. Bureau
of Customs and Border Protection (CBP) announced in the Federal Register (69
FR 19765) the availability of the plan entitled "Joint FDA-CBP Plan
for Increasing Integration and Assessing the Coordination of Prior Notice
Timeframes." The plan, which includes an assessment schedule, describes
the process by which FDA and CBP intend to increase integration and examine
whether to amend the timeframe requirements in FDA's prior notice IFR to
have the same advanced notice timeframes for arrivals by land via road
or rail, or arrival via air that are currently in CBP's advance electronic
information rule.
Joint FDA-CBP Plan for Increasing Integration and Assessing
the Coordination of Prior Notice Timeframes
Joint FDA-CBP Plan for Increasing Integration and
Assessing the Coordination of Prior Notice Timeframes (REVISED August 2004)
- Prior Notice of Imported
Foods IFR -- Q&A Guidance Document: On May 3, 2004, announced in
the Federal Register (69 FR 24070) the availability of a
revised guidance entitled: "Questions and Answers Regarding the Interim
Final Rule on Prior Notice of Imported Foods (Edition 2)." This
guidance responds to various questions raised about section 307 of the
Bioterrorism Act and the Agency's implementing regulation which require
the submission to FDA of prior notice of food, including animal feed, that
is imported or offered for import into the U.S.
Guidance for Industry - Prior Notice of Imported Food Questions and Answers (Edition 2)
- Revised Prior Notice of
Imported Foods Compliance Policy Guide: On June 29, 2004, announced
in the Federal Register (69 FR 38906) the availability of
a revised Compliance Policy Guide (CPC) regarding Prior Notice of Imported
Foods under the Bioterrorism Act. The original CPG which was published
in the Federal Register of December 15, 2003 (68 FR 69708),
provides written guidance to FDA's and the Customs and Border Protection's
(CBPs) staff on enforcement of section 307 of the Bioterrorism Act. This
CPG has been revised to provide additional guidance to FDA and CBP staff
regarding how to address food that is imported or offered for import for
noncommercial purposes with a noncommercial shipper. The revised CPG also
reflects a change in the date of Stage III enforcement guidance for the
interim final rule for Prior Notice of Imported Foods from May 13, 2004,
to June 4, 2004.
Compliance Policy Guide, Guidance for FDA and CBP Staff: Prior Notice of Imported Food Under the
Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (REVISED June 2004)
- Revised Prior Notice of
Imported Foods Compliance Policy Guide: On August 16, 2004, announced
in the Federal Register (69 FR 50389) the availability
of a revised joint FDA/Customs and Border Protection (CBP) Compliance Policy
Guide regarding Prior Notice of Imported Foods. This document describes
certain changes to the Joint FDA/CBP plan for Increasing Integration and
Assessing the Coordination of Prior Notice Timeframes that was announced
in the Federal Register of April 14, 2004. Since the prior notice
interim final rule (IFR) became effective in December 2003, FDA and CBP
have been reviewing the data quality of prior notice submissions. This
review has revealed practical implementation problems with certain data
elements, such as registration number, bill of lading number, and ultimate
consignee. In part, these problems result from a lack of standardization. The
problems also arose due to the practical difficulties faced by submitters
in obtaining required information in complex commercial settings. Therefore,
the CPG was revised to address these practical implementation problems.
Compliance Policy Guide, Guidance for FDA and CBP Staff: Prior Notice of Imported Food Under the
Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (REVISED August 2004)
- Prior Notice Contingency
Plan for Systems Outages: On August 16, 2004, announce the availability
in the Federal Register (69 FR 50388) Guidance for Industry:
Prior Notice of Imported Food Contingency Plan for System Outages. The
contingency plan provides guidance on submitting prior notice of imported
food during system outages affecting the applicable FDA and Customs and
Border Protection (CBP) program systems.
Guidance for Industry - Prior Notice of Imported Food Contingency Plan for System Outages
- IT System to Support FDA's
Prior Notice Requirements: The FDA's Office Regulatory Affairs (ORA)
completed the initial implementation of the IT systems to support the prior
notification requirements for all imported food shipments as a requirement
of the Public Health Security and Bioterrorism Preparedness and Response
Act of 2002 (Bioterrorism Act). On December 12, 2003, ORA modified its
OASIS system and implemented the Prior Notice System Interface (PNSI) to
support the prior notice provisions of the Bioterrorism Act. Between December
12, 2003, and October 30, 2004, four additional software releases were
implemented to support the enforcement phases of the provision.
- IT System to Support the
Registration of Food Facilities: As part of the new "FDA Industry
Systems (FIS)," FDA implemented the "Food Facility Registration Module
(FFRM)" for the electronic registration of food facilities. This responds
to a requirement of the Bioterrorism Act that all domestic and foreign
facilities that manufacture, process, pack, or hold food for human or animal
consumption in the United States to register with the FDA.
Food Security: Emergency Preparedness
- Food Emergency Response
Network (FERN): CFSAN initiated, in conjunction with FDA's Office
of Regulatory Affairs, a multi year effort to support the development
of the Food Emergency Response Network (FERN), with progress this year
critical
to the establishment of the laboratory network. As such, CFSAN has
been involved in multiple activities including:
- Serving
as the lead for the proficiency program subcommittee, serving
as the operational laboratory for microbiological proficiency samples,
and supporting
the activities
of the ORA Forensics laboratory for chemical proficiency samples.
- Posted
interim methods for priority chemical and microbiological agents
on both the FERN and the CDC Laboratory Response Network (LRN)
websites.
- Provided
training to ORA, USDA, FERN, and LRN labs on detection of priority
microbiological agents in a BSL-3 environment and on general
food sampling protocols
- Initiated
with AOAC a review of the criteria for the validation of microbiological
methods.
- Supported
the development of the organizational structure of FERN including
active participation in the Steering Committee and all subcommittees.
- Established
a "FERN store" for the stockpiling and distribution of kits and specialized
reagent to the FERN labs.
- Continued
to identify and address infrastructure, training, and procedural
needs for increased preparedness of CFSAN labs including acquisition
of key equipment
for microbiological and chemical agent detection.
- Completed
all requirements for the use of select agents within three of
the CFSAN labs, including inspection of laboratories by CDC.
- Two
additional CFSAN laboratories (NCFST and College Park) into the
LRN.
- Food Threat Assessment
Evaluations: FDA produced a "For Official Use Only" (FOUO)
version of its classified Operational Risk Management (ORM) vulnerability
assessment. CFSAN briefed FDA management, field management, state commissioners
of health and agriculture and numerous food trade associations on the content
of the document.
FDA utilized a new vulnerability assessment tool to further refine our initial vulnerability assessments. These assessments were completed utilizing the CARVER targeting tool. CARVER is an offensive targeting tool contrasted with the Operational Risk Management vulnerability assessment (ORM) which is a defensive vulnerability assessment tool.
- Intramural and extramural
research to determine threat agent characteristics in foods and dose response
for humans: CFSAN's Office of Plants and Dairy Foods (OPDF) has initiated
a collaborative project with the National Center for Food Safety and
Technology (NCFST) entitled "Survival and Growth of Non-traditional Pathogens
in Food." This project will characterize the ability of non-traditional
vegetative pathogens (or pathogen surrogates) to survive and grow in
a variety of foods using classical and high-throughput techniques. Both
liquid
(dairy, fruit juice) and solid (produce) food systems will be tested.
- Establishment of Prevention
Measures: In an effort to establish prevention measure shields
for foods identified as a high security concern, CFSAN continued to
acquire
and communicate scientific information to the appropriate sectors in
the following areas:
- CFSAN
conducted numerous briefings with food industry representatives
and State Agriculture and Health Commissioners on its initial food
security assessment
efforts.
- Awarded a contract to
the Institute of Food Technologists to develop and conduct
threat assessment training for medium and small food producers
nationwide
that will lead
to improved security of food production facilities and processes.
- Partnered
with select industries to provide technical assistance in conducting CARVER threat
assessments for food commodities identified as higher concern.
- Intramural and extramural
research on prevention strategies: Three activities were initiated:
- (1) CFSAN has initiated a collaborative project
with NCFST entitled "Thermal resistance of non-traditional microbial agents."
- (2) CFSAN is in the planning stages of two collaborative projects
with NCFST. The first project is entitled "Decontamination of Food Processing
Facilities and Equipment." The second project is entitled "Effect
of Food Processing on the Inactivation of Protein Toxins and Bacillus
anthracis Spores." CFSAN and NCFST are presently interviewing post-doctoral
candidates, who will be hired to perform these projects in the BSL-3
laboratory and pilot plant that is being built at NCFST.
- Validate and develop methods
for use in foods and prioritize according to FDA food threat assessment: In
September 2003, CFSAN awarded cooperative agreement research funds to
augment its food safety and food security research methods development
program. The
applied focus of all research categories is to enhance the ability of the
Agency to detect non-traditional microbial, chemical, and toxin adulterants
in food, dietary supplements, food additives, and cosmetics. Five new
grants totaling $2,828,759 were funded. The grants cover the full cost
of the awards, which are typically for 3 years.
The following grants
have been awarded:
- Project
1: Development of Waveguide Immunoassay for Yesinia pestis, University
of Georgia Research Foundation Inc.
- Project
2:Rapid Immunoassay Silver Amplification Test System, Cornell
University -- Department
of Food Sciences
- Project
3: Rapid Food Screening for Biological Toxins on a Microchip,
Naval Research Laboratory
- Project
4: Development of a PCR Device for Pathogen Detection,
Cornell University - Material Research Laboratory
- Project
5: Rapid Screening of Foods for Toxins by TLC-Bioluminescence,
ChromaDex Inc.
On May 5-6, 2004, CFSAN hosted its 5th FDA/CFSAN Food Safety
and Food Security Extramural Research Review Conference for all principle
investigators of these grants (and other counter-terrorism research related
grants).
- Proficiency
Test Samples: During FY 2004, CFSAN provided FDA's food testing
laboratories both chemistry and microbiological proficiency test samples
to evaluate analyst proficiency in testing for organisms of food security
and safety concerns.
- Emergency Response Exercises: CFSAN
participated in numerous emergency response exercises that included all
levels of government:
- March 2004, FDA participated in an FDA Radiological Emergency Response Plan Exercise.
- May 2004 FDA participated in an FDA Chemical/Biological Plan Emergency Response Exercise.
- Emergency Response Training: The
CFSAN Emergency Response Plan (CERP) was revised, posted on CFSAN's intranet
and distributed. In cooperation with CFSAN's Staff College, a workshop was
conducted to prepare the "CFSAN Situation Room" Staff for a 3-day course
at the Emergency Management Institute in Emmitsburg, MD and refresher Emergency
Management Training for CFSAN leadership.
- Training on the Bioterrorism
Final Rules: Training on two of the four BT Regulations, Food Facility
Registration and Prior Notice of Imports, has been completed. A worldwide
"Satellite downlink" public broadcast on the two final regulations was
held on October 28, 2003. On the 3rd and 7th of
November 2003, FDA held (1) Bioterrorism Legislations' Rules and Procedures -- Handling questions (Satellite Downlink); and (2) Implementing the Bioterrorism
Legislation's Rules and Procedures.
- Participation in IFWG: Under the initial direction of the White House Homeland Security Council’s Interagency Food Working Group (IFWG), CFSAN served
as lead for the Department of Health and Human Services (HHS) in combination
with the U.S. Department of Agriculture (USDA), the Department of Homeland
Security (DHS), the National Association of State Departments of Agriculture (NASDA),
the Association of State and Territorial Health Officials (ASTHO) and the
National
Association of County and City Health Officials (NACCHO) in forming the
Food and Agriculture Sector critical infrastructure protection organization
that
brings together state officials and industry to further strengthen homeland
security in the area of food defense.
- Bioterrorism Help Desk: October
16, 2003, FDA implemented the "FDA Industry Systems Help Desk" to respond
to general and technical questions about the Bioterrorism Act with respect
to food facility registration and prior notice of imported foods. The
Help Desk has responded over 100,000 inquiries from stakeholders on the
Bioterrorism
rules.
- FDA Counter Terrorism Research
Report to Congress: Title III, subtitle A, section 302 of the Public
Health Security and Bioterrorism Preparedness and Response Act of 2002
requires FDA to submit an annual report to Congress describing progress
made in counter terrorism research. CFSAN has completed a review of its
food security research efforts and prepared a summary of its activities
and accomplishments for FY 2004. The full report has been completed
and is currently undergoing Administration clearance.
- Laboratory Upgrade: CFSAN
initiated the upgrade of laboratory facilities at the National Center
for Food Safety and Technology (NCFST) to the BSL-3 level. The purpose
of this upgrade is to allow NCFST to conduct food processing and packaging
research
that is geared to enhancing food security.
- Continue MOD I Upgrade: In
FY 2004, the BSL3 build-out at the CFSAN's MOD-1 facility was brought
to an estimated 95% completion level. Formal inspection, certification
and other final commissioning tasks are planned for completion in early
FY 2005.
Domestic Inspections.
- Domestic
"High-Risk" Inspections: Conducted 7, 244 (106%) domestic inspections
of firms that produce "high risk" foods (through FDA's Office of Regulatory
Affairs and the states, under FDA auspices). This exceeds FDA's goal
to annually inspect 95% (6,840) of the "high risk" domestic food establishments.
- "High-Risk" Inspection
Evaluation: As of September 30, 2004,
all "High Risk" data
for FY-01, 02 & 03 was compiled and delivered to the responsible program
offices within CFSAN. The information and summary conclusions were used
in making recommendations and redirection of the program where appropriate.
Imports and Foreign Inspections
- Field Examinations
of Imported Food: In conjunction with FDA's Office of Regulatory
Affairs, completed 70,926 (118%) field examinations of imported food
shipments entering
U.S. ports of entry for release into the U.S. commerce. Our goal was to
complete 60,000 field examinations for the current fiscal year. FDA exceeded
its goal by 164 percent. This is more than a 6-fold increase from the
12,000 field import examinations conducted just three years ago in FY
2001.
- Foreign Inspections: Through
FDA's Office of Regulatory Affairs (ORA), conducted 150 inspections of
foreign food establishments with top priority to "high"risk" foods. Due to travel
restrictions based on world events and budget constraints, FDA was able to
conduct only limited foreign inspections. Many foreign inspections planned
for FY 2004 will be rescheduled in FY 2005.
- Foreign Inspections Evaluations: CFSAN
continues to receive FY2004 foreign Establishment Inspection Reports (EIR)
for evaluation, as the ORA Field Districts complete reports covering inspections
conducted during the last quarter of FY 2004. As of 10/20/04, CFSAN has
received 145 of the current 150 foreign inspections (EIRs) reported by
the field districts. Of the 145 EIRs received, the Center is currently
reviewing 33 and the remaining 112 EIRs have been reviewed and given a
final inspection classification (OAI-25, VAI-49, NAI-38).
- Private Laboratory Requirements: On April 29, 2003, published
in the Federal Register (69 FR 23460) a proposed rule on
requirements pertaining to sampling services and private laboratories used
in connection with imported food. The proposal would require samples to be properly identified, collected, and maintained. Additionally, the proposal would require laboratories to use validated or recognized analytical methods, and to submit analytical results directly to FDA. The proposal is intended to
help assure the integrity and scientific validity of data and results submitted to FDA.
Proposed Rule: Requirements Pertaining to Sampling Services and Private
Laboratories Used in Connection With Imported Food (69 FR 23460)
Seafood Safety
- Report to Congress -- Seafood
HACCP: House Report 108-193 of the Agriculture Rural Development,
Food and Drug Administration and Related Agencies 2004 Appropriations Language
directed FDA to provide a description of the HACCP temperature requirements
for imported seafood products and identify any planned or ongoing FDA research
projects on the effectiveness of new technologies to control hazards due
to improper temperature. CFSAN prepared a response
to Congress and it is currently under review by the Administration.
- Report to Congress -- Chloramphenicol
in Shrimp: House Report 108-193 of the Agriculture Rural Development,
Food and Drug Administration and Related Agencies 2004 Appropriations
Language directed FDA to report on the sampling frequency and violation
rates for
imported shrimp, and efforts to ensure that any shrimp that test positive
for chloramphenicol will not be subsequently consumed. CFSAN prepared
a response to Congress and it is currently under review by the Administration..
- Methylmercury Advice: On
March 19, 2004, FDA and the Environmental Protection Agency (EPA) announced
the joint consumer advisory entitled: "What You Need to Know about Mercury
in Fish and Shellfish." The joint advisory made several recommendations
for reducing exposure to high levels of mercury in women who may become pregnant,
pregnant women, nursing mothers, and young children. This unifies advice
from both FDA and EPA and supersedes FDA's and EPA's 2001 advisories.
What You Need to Know About Mercury in Fish
and Shellfish (also available in Spanish)
- Vibrio
vulnificus: FDA has continued to work with the Interstate Shellfish
Sanitation Conference (ISSC) to encourage the post-harvest treatment
of Gulf Coast oysters and to monitor progress toward the ISSC illness
reduction goals. FDA participates in the Vibrio Management
Committee of the ISSC and a number of working groups organized under
that committee.
The ISSC conducted a survey that demonstrates that the shellfish industry's
capacity to conduct post-harvest treatment in Gulf Coast oysters well
exceed its goal of 25%. The ISSC continued to refine the standardized
methods for validating post harvest treatment processes to facilitate
industry adoption of the processes.
Fruits and Vegetables
- Mexican government Certification
Program for Cantaloupes Intended for Export to the U.S.: FDA/CFSAN
provided technical assistance to Mexican counterparts on several iterations
of the Mexican Federal Recognition Program (FRP) that contain requirements
to be followed during the production, harvest, packing, and processing
of cantaloupe to reduce the risk of foodborne illness outbreaks associated
with the consumption of cantaloupe from Mexico. The final Mexican FRP
for cantaloupe was circulated for review by all agencies in January, 2004. Mexico
has also developed a FRP for the production green onions, based largely
on the FRP for cantaloupe.
- Juice HACCP Guidance: On
March 3, 2004, published in the Federal Register (69 FR 10051)
a notice announcing the availability of a guidance document related to
the processing of juice entitled: "Guidance for Industry: Juice HACCP Hazards
and Controls Guidance, First Edition." The guidance represents FDA's views
on potential hazards in juice products and recommends how to control such
hazards and it is designed to assist juice processors in the development
of their HACCP plans.
Guidance for Industry - Juice HACCP Hazard and Controls Guidance, First Edition
- FDA Food CGMP Regulations: CFSAN
conducted three research projects related to Food GMP modernization, a
literature search, an expert elicitation and survey of food recalls from
1999-2003. Based
in part on this research, we concluded that a modernization of the Food GMPs
(21CFR 110) was needed. On May 21, 2004, announced in the Federal Register (69
FR 29220) three public meetings intended to obtain comments about FDA's
Good Manufacturing Practices (GMPs) in manufacturing, packing, or holding
human food regulations (21 CFR part 110).
Notice of Public Meetings (69 FR 29220)
- International
Good Agriculture Practices (GAP) Outreach in Conjunction with the Joint
Institute for Food Safety and Applied Nutrition (JIFSAN): The U.S.
Food and Drug Administration (FDA) and the Joint Institute for Food
Safety and Applied Nutrition (JIFSAN) conducted a
train-the-trainer program on Good Agricultural Practices (GAPs) and
Good Manufacturing Practices (GMPs) for the production of fresh produce
in
three countries -- Guatemala, Honduras and Korea. Participants were trained in
good agricultural and manufacturing practices. The course consisted
of lectures, case studies, practical exercises, and in come cases farm
visits. Participants took part in an extensive, commodity specific,
farm exercise based on conditions specific to the region.
- Produce Safety: On
October 18, 2004, FDA posted the 2004 Produce Safety Action Plan.
Because
of the importance of fresh produce in a healthy diet and continuing outbreaks
associated with the consumption of fresh produce, FDA developed the 2004
Produce Safety Action Plan to further minimize foodborne illness associated
with the consumption of fresh produce. The 2004 Produce Safety Action
Plan addresses all major points from the farm to the table where contamination
of produce could occur. It covers fresh fruits and vegetables in their
unpeeled natural form and raw minimally processed products, i.e., fresh-cut
produce.
Egg Safety
- Egg Safety Proposed Rule: On
September 22, 2004, published in the Federal Register (69 FR 56823)
the proposed rule: "Prevention of Salmonella Enteritidis in Shell
Eggs During Production." This proposal would require shell egg producers
to implement measures to prevent Salmonella Enteritidis (SE) from
contaminating eggs on the farm.
Proposed Rule: Prevention of Salmonella Enteritidis in Shell Eggs During Production (69 FR 56823)
Listeria
- On May 24, 2004, issued in
the Federal Register (69 FR 29564) a notice announcing that
a petition has been filed that requests that the Agency establish a regulatory
limit of 100 colony forming units per gram for Listeria monocytogenes in
foods that do not support the growth of the microorganism. The Agency requested
comments on the petition. The comment period closed August 9, 2004.
Notice: Listeria Monocytogenes; Petition to
Establish a Regulatory Limit (69 FR 29564)
Chemical Contaminants, Pesticides and Other
Hazards
- Acrylamide: In March
2004, FDA released acrylamide testing results for the FY03 Total Diet Study
(approximately 700 samples) and other selected samples analyzed at CFSAN
(approximately 50 samples).
FDA is now preparing to release data from the FY04 Total
Diet Study (approximately 400 samples) and other selected samples tested
by a
contract laboratory (approximately 470 samples). These data will be useful
to FDA for determining consumer exposure to acrylamide from the diet to
assess the risk of acrylamide in foods.
Exploratory Data on Acrylamide in Food, FY 2003 Total Diet Study Results
Exploratory Data on Acrylamide in Food
- Radionuclides: On January
14, 2004, published in the Federal Register (69 FR 2146)
a notice announcing the availability of a draft CPG entitled: "Guidance Levels
for Radionuclides in Domestic and Imported Foods." The draft CPG rescinds
and replaces the current CPG Sec. 560.750 Radionuclides in Imported Foods
-- Levels of Concern (CPG 7119.14). The draft CPG provides updated guidance
levels for radionuclide activity concentration in food offered for import
and makes these same guidance levels for radionuclide activity concentration
applicable to food in domestic interstate commerce for the first time.
Compliance Policy Guide, Guidance for FDA Staff - Guidance Levels for Radionuclides in Domestic and Imported Foods
- Pesticides Monitoring: Over
8,000 food samples were collected and analyzed for pesticide residues during
the Fiscal Year 2004. FDA must maintain resource levels devoted to the sampling
and analyses of pesticide, not only to ensure that the U.S. food supply
is safe, but also to reduce dietary exposure.
- FDA's Dioxin Strategy: FDA
continued implementation of its dioxin strategy including monitoring,
method development, and identification of opportunities to reduce exposure.
Specific
accomplishments in FY2004 include the following:
- Perchlorate Analytical
Method: FDA developed an accurate and sensitive method to determine
the perchlorate anion in selected fruits and vegetables and also in
bottled water and milk using ion chromatography-tandem mass spectrometry. The
method was posted on the CFSAN website in March 2004 and it is successfully
being used by not only FDA, but other government and private laboratories.
- Chloramphenicol: In FY 2001 and early FY 2002, the European Union
and Canada reported finding chloramphenicol (CAP), a banned substance,
in honey exported from China. In response to their findings, FDA developed
new analytical methodology and began testing honey for CAP. From March
1, 2002 through December 31 2003, FDA tested 698 imported honey samples
and found 37 positive samples. During 2004, FDA also tested 13 domestic
honey samples, all of which were negative. From January 1, 2004, through
September 2004, FDA tested 108 imported honey samples and found 1 positive
sample.
- Lead in Candy: On March
25, 2004, issued letters to manufacturers, importers and distributors of
imported candy. The letter is to inform applicable parties of actions the
FDA intends to take to reduce further the potential exposure of children
to lead from candy products.
Letter to Manufacturers, Importers, and Distributors of Imported Candy
- Lead in Imported Candy: Reviewed
all FDA data on lead in imported candy from FY 2000 thru FY 2004 and
collaborated with FDA's field offices to prioritize categories of Mexican
candy for sampling.
Transmissible Spongiform Encephalopathies (TSEs)
- BSE Interim Final Rule: On July 14, 2004, published in the Federal Register (69 FR 42255)
an interim final rule: "Use of Materials Derived From Cattle in Human Food and Cosmetics prohibiting the use of certain cattle material, in human food, including dietary supplements, and cosmetics. Prohibited cattle materials include specified risk materials, small intestine of all cattle, material from nonambulatory disabled cattle,
material from cattle not inspected and passed for human consumption, and mechanically separated (MS)(Beef).
Interim Final Rule: Use of Materials Derived From
Cattle in Human Food and Cosmetics (69 FR 42255)
- Initiation
of BSE Risk Assessment: Initiated work on a BSE risk assessment. Harvard
University is in the process of modifying the BSE risk assessment model
to use the available data to accommodate FDA-regulated products.
- BSE Risk Assessment: On July 14, 2004, announced in the Federal Register (69 FR 42191)
the availability of the risk assessment regarding the potential for variant
Creutzfeldt-Jakob Disease (vCJD) in humans from exposure to cosmetics containing
cattle-derived protein infected with the bovine spongiform encephalopathy
(BSE) agent. FDA is making this document available to communicate publicly
the potential risk to public health from cosmetics made with cattle materials
that may be contaminated with the BSE agent.
An Evaluation of the Risk of Variant Creutzfeldt-Jakob Disease from Exposure to Cattle-Derived Protein Used in Cosmetics
- BSE in CFSAN-regulated
Products: On July 14, 2004, published in the Federal Register (69
FR 42287) an advance notice of proposed rulemaking (ANPRM): "Federal
Measures to Mitigate BSE Risks: considerations for Further Action."
Proposed Rule: Federal Measures to Mitigate BSE Risks: Considerations for
Further Action (69 FR 42287)
- BSE Recordkeeping Requirements: On
July 14, 2004, published in the Federal Register (69 FR 42275)
a proposed rule: Recordkeeping Requirements for Human Food and Cosmetics
Manufactured From, Processed With, or Otherwise Containing, Material From
Cattle. This is a companion rulemaking to FDA's interim final rule entitled
"Use of Materials Derived From Cattle in Human Food and Cosmetics," published
in this issue of the Federal Register.
Food Allergens
- Food Allergen Industry
Outreach: CFSAN participated in more than 21 industry, consumer and
regulatory conferences to discuss FDA's activities aimed at increasing
awareness of the presence of allergens in foods.
Dairy Safety
- Federal Import Milk Act
(FIMA) CPG: On October 29, 2004, announced in the Federal Register (69
FR 63158) the availability of the draft revised Compliance Policy
Guide: Imported Milk and Cream -- Federal Import Milk Act. This
revision of the FIMA CPG updates the policy regarding which dairy products
require permits
to enter the U.S. This updated CPG revision will benefit FDA employees
who examine dairy products presented for entry into the U.S. and foreign
dairy processors and exporters by clarifying which dairy products are
subject to the FIMA permit requirements.
Notice: Draft Revised Compliance Policy Guide "Sec. 560.400--Imported
Milk and Cream--Federal Import Milk Act (CPG 7119.05) (69 FR 63158)
Education
- Listeria and Methylmercury
Education: In our effort to train health educators to teach food safety
to pregnant women and women who may become pregnant about the risks of
methylmercury in seafood and Listeria monocytogenes in refrigerated
food, print materials and videos are completed and in distribution to
targeted audiences(s) in English only.
- Seafood Safety: In
our effort to develop and distribute seafood safety education materials,
methylmercury advisory information and fotonovelas for Vibrio vulnificus in
seafood are completed and in distribution to targeted audiences nationwide.
- Hispanic Outreach: In
FY 2004 CFSAN exhibited and distributed Spanish and English food safety
materials at seven Radio Unica health fairs held in San Francisco, CA; Miami, FL; Phoenix, AZ; and Houston,
Dallas, San Antonio, and McAllen, TX.
- Preventive Controls: On
May 28, 2004, the Institute of Food Technologists completed a contract awarded to review the preventive controls that industry
may take to reduce the risk for an intentional act of terrorism or contamination
using the US food supply for high risk and medium risk food commodity/agent
combinations.
- Methylmercury Advisory: On
December 10-11, 2003, FDA's Food Advisory Committee (FAC) met to provide
a status report and response to the FAC recommendations on methylmercury
in fish and shellfish.
Transcripts:
Food Advisory Committee Meeting Methylmercury (Volume I)
Food Advisory Committee Meeting Methylmercury (Volume II)
- Food Safety and Security Health Professionals Program: On April 7, 2004, FDA, in partnership with the U.S. Centers for Disease Control and prevention (CDC), the U.S. Department of Agriculture/Food Safety and Inspection Service (FSIS), the American Medical Association (AMA) and the American Nurses Association (ANA) issued an educational primer entitled: "Diagnosis and Management of Foodborne Illnesses: A Primer for Physicians and Other Health Care Professionals."
Part II: Improving Nutrition and Dietary Supplement Safety
Nutrition, Health Claims and Labeling
- Obesity Working Group (OWG): On
August 11, 2003, then-Commissioner Mark B. McClellan, M.D., Ph.D., established
FDA's Obesity Working Group (OWG). The OWG met eight times; received briefings
from invited experts; held one public meeting, one workshop, two roundtable
discussions; solicited comments on obesity-related issues for submission
to the Docket (Docket No. 2003N-0338); and prepared and delivered a report
with recommendations to address the obesity problem from FDA's perspective
and authorities.
- OWG Report: On March
12, 2004, the OWG publicly released its "Calories Count" report. The report
contains a series of short and long-term recommendations for dealing with
the obesity problem centered on the scientific fact that, at its most basic
level, weight control requires caloric balance.
Calories Count - FDA Obesity Working Group Report and Related Information
- Enforcement Against Misleading
Claims: FDA continued to work with the Federal Trade Commission (FTC)
to address false or misleading claims in dietary supplement products, with
a strong focus on weight loss claims. In FY2004, FDA and FTC took enforcement
action against Cortislim, a product that was heavily advertised through
infomercials, as well as radio and print advertisements and Internet websites. FDA
also issued 16 Warning Letters to firms promoting weight loss products
on the Internet with false or misleading claims. These letters were followed-up
by a field assignment to inspect those firms that did not make corrections.
- Serving Size Declaration: As part of FDA's Obesity Initiative, serving
size declaration on food products was highlighted as a priority for the
agency. CFSAN's Office of Nutritional Products, Labeling and Dietary Supplements
issued a "Dear Manufacturer" letter on March
12, 2004, to remind the food industry about the rules for determining appropriate
serving size. The "Dear Manufacturer" letter also advised the industry
that FDA intends to highlight accurate serving size as an enforcement priority.
Letter to Food Manufacturers about Accurate Serving Size Declaration on Food Products
- Nutrition Information in
Restaurants: In working with a third-party facilitator to develop options
for providing voluntary standardized nutrition information at point of
purchase in a restaurant setting, CFSAN staff is serving on the Project
Advisory Group for the contract with the Keystone Center, signed in June
2004, to conduct a national policy dialogue to address obesity issues involving
foods consumed away from home (including restaurants). Keystone has conducted interviews with a broad cross-section
of representative stakeholders on both of these issues (including representatives
from CFSAN). As
a next step Keystone will provide a report on the interviews and future
plans for convening the national policy dialogue forum.
- Adolescent Obesity: In
working with a third-party facilitator to develop approaches including partnerships
for educating consumers, particularly adolescents, about obesity, CFSAN staff
is serving on the Project Advisory Group for the contract with the Keystone
Center, signed in June 2004, to conduct a national policy dialogue to address
obesity issues involving ways to combat pediatric obesity. As of mid-September,
Keystone has conducted 45 initial interviews with a broad cross-section of
representative stakeholders on both of these issues (including representatives
from CFSAN), and has plans to conduct an additional 20-35 interviews. As
a next step Keystone will provide a report on the interviews and future plans
for convening the national policy dialogue forum.
- Qualified Health Claims: On
November 25, 2003, published in the Federal Register (68 FR 66040)
an advance notice of proposed rulemaking (ANPRM) to request comments on alternatives
for regulating qualified health claims in the labeling of conventional human
foods and dietary supplements. FDA also solicited comments on various other
issues related to health claims and on the appropriateness and nature of
dietary guidance statements on conventional food and dietary supplement labels.
ANPRM: Food Labeling; Health Claims; Dietary Guidance (69 FR 66040)
- Consumer Research on Qualified
Health Claims: On May 2004, completed consumer research to help ensure
that qualified health claim messages in the labeling of foods and dietary
supplements employ the most effective wording so that the messages are
not misleading to consumers.
- Health Claims, Dietary
Guidance: On January 27, 2004, published in the Federal Register (69
FR 3868) an extension of the comment period for the ANPRM entitled:
"Food Labeling: Health Claims; Dietary Guidance." FDA extended the comment
period for the ANPRM for 30 days, until February 25, 2004.
ANPRM: Food Labeling; Health Claims; Dietary Guidance; Extension of Comment Period (69 FR 3868)
- Health claims: Nutrient
Content Claims: On May 4, 2004, published in the Federal Register (69
FR 24541) a notice reopening for 60 days the comment period for the
proposed rule entitled: "Food Labeling: Nutrient Content Claims, General
Principles; Health Claims, General Requirements and other Specific Requirements
for Individual Health Claims. In that document, FDA proposed to amend
its existing nutrient content claims and health claims regulations to provide
additional flexibility in the use of these claims on food products.
Proposed Rule, Reopening of the Comment Period: Food Labeling: Nutrient Content Claims, General Principles; Health Claims, General Requirements
and Other Specific Requirements for Individual Health Claims (69 FR 24542)
- Nutrient Content/Health
Claims Petitions: During FY 2004 CFSAN completed the review of eight
nutrient content claim petitions/notifications and twenty-three health
claim petitions/notifications. All were completed within the statutory
timeframe.
- Infant Formula Premarket
Notifications: Completed twenty-five 90-day infant formula notifications
in FY 2004. All were completed within the mandated 90-day review
period.
- Trans Fat Education: FDA Public Affairs Specialists were provided a technical
presentation promoting trans fat education and outreach, including
a script about the new labeling requirements to facilitate accurate communication
to stakeholders. The FDA Consumer featured a cover story about trans fats
and all information, including press documents, regulations, Q&A's,
and consumer information was posted on the CFSAN Web site. These documents
also were sent to numerous CFSAN stakeholders. CFSAN also completed a
Web-based interactive article in English and Spanish and a new presentation
to accompany the consumer article.
Trans Fat Now Listed With Saturated Fat and Cholesterol on the Nutrition Facts Label
- Trans Fat Proposed
Rulemaking: On March 1 and April 19, 2004, published in the Federal
Register (69 FR 9559 and 69 FR 20838, respectively) two notices
extending the comment period for an ANPRM on Trans Fatty Acids in
Nutrition Labeling published in the Federal Register of July 11,
2003 (60 FR 41507).
ANPRM, Reopening of Comment Period: Trans Fatty Acids in Nutrition Labeling (69 FR 9559)
ANPRM, Extension of Comment Period: Trans Fatty Acids in Nutrition Labeling (69 FR 20838)
- Enforcement Activities
for Inappropriate Labeling of Conventional Foods: CFSAN approved 83 Warning Letters, 31 Import Detentions
and 1 Seizure recommendation from the FDA field district offices. In addition,
CFSAN issued three Warning Letters related to inappropriate labeling of
conventional foods.
Dietary Supplements
- 75-Day New Dietary Ingredient
Notification: During FY 2004, CFSAN filed 49 and responded to 47 notifications
for dietary supplements containing new dietary ingredients. The notifications
are reviewed for science-based evidence of safety. Letters are issued
to the notifier to acknowledge receipt and, when necessary, to identify
deficiencies and safety concerns. A total of 31 letters identified deficiencies
or safety concerns, one (1) did not fulfill the regulations found at 21
CFR 190.6, eight (8) were acknowledgements and seven (7) were not dietary
ingredients.
- 30-Day Nutrient Content/Health
Claim Notifications: FDA/CFSAN received almost 2000 submissions pursuant
to sec. 403(r) (6) of the Act and 21 CFR 101.93(a). Each submission
identified the claims being made for one or more products. FDA/CFSAN
sent out 47 letters in response these submissions that addressed one or
more issues associated with the product and/or the claims being made for
it, such as claims contained in the notifications that were outside the
scope of section 403(r) (6), of technical deficiencies of the submission,
or that products did not appear to be dietary supplements under 201(ff)
of the act.
- Substantiation Guidance: On
November 9, 2004, announced in the Federal Register the availability
of a draft guidance entitled: "Guidance for Industry: Substantiation for
Dietary Supplement Claims Made Under Section 403(r)(6) of the Federal Food,
Drug, and Cosmetic Act." The draft guidance describes the amount, type,
and quality of evidence that FDA recommends a manufacturer have to substantiate
a claim under the Act.
Guidance for Industry - Substantiation for Dietary Supplement Claims Made Under
Section 403(r)(6) of the Federal Food, Drug, and Cosmetic Act
- Ephedrine Alkaloids Final
Rule: On February 11, 2004, published in the Federal Register (69
FR 6787) a final regulation declaring dietary supplements containing
ephedrine alkaloids adulterated under the Federal Food, Drug, and Cosmetic
Act. This rule became effective on April 12, 2004.
Final Rule Declaring Dietary Supplements Containing Ephedrine Alkaloids Adulterated Because They Present
and Unreasonable Risk (69 FR 6787)
- Dietary Supplement Enforcement
Actions: Sent warning letters to 23 companies asking them to cease
distributing products sold as dietary supplements that contain androstenedione
and warning them that they could face enforcement action if they do not
take appropriate actions.
Press Release: HHS Launches Crackdown on Products Containing Andro;
FDA Warns Manufacturers to Stop Distributing Such Products
Androstenedione Warning Letters
- FDA/FTC
Actions on Unsubstantiated Claims on Dietary Supplements: On March 17, 2004, FDA announced that Seasilver USA, Inc., and Americaloe,
Inc., of Carlsbad, California, and their principals, Bela Berkes and Jason Berkes, signed a consent decree of permanent injunction in which they agreed
to stop manufacturing and distributing violative products, including
"Seasilver" - a purported cure-all liquid supplement. Seasilver USA
marketed their product "Seasilver" as a dietary supplement that the companies
promote on the Internet and in marketing materials as a treatment for
"over 650" diseases including, for example, cancer, heart disease, stroke,
diabetes, hepatitis, arthritis, depression and other diseases.
These claims cause Seasilver to be an unapproved
new drug under the Federal Food, Drug, and Cosmetic Act (FD&C Act).
Such claims also cause Seasilver to be misbranded under the FD&C Act
because it lacks adequate directions for use. This action is the culmination
of coordinated efforts by FDA and the Federal Trade Commission (FTC).
Press Release: Dietary Supplement Firms, Seasilver USA, Inc., and Americaloe, Inc., Sign Consent Decree With FDA To Stop Selling Product Claiming To Cure "Over 650" Diseases
Part III: Assuring Food & Cosmetic Safety
Food and Color Additives: Premarket Review
- Food
and Color Additive Petitions - Expedited Review: for the petition
receipt cohort of FY 2003, met goal to complete within 360 days of filing,
the safety evaluation of two of the three food additive petitions that
qualify for expedited review. A petition qualifies for expedited review
if the food additive is intended to decrease the incidence of foodborne
illnesses through its antimicrobial actions against human pathogens that
may be present in food.
- Food
and Color Additive Petitions - Non-expedited Review: for
the petition receipt cohort of FY 2003, completed within 360 days of filing,
the safety evaluation of four (80%) of five food additive petitions that
do not qualify for expedited review. This exceeds our goal of completing
at least 70% of these petitions within 360 days.
- Biotechnology
Consultations: Completed the scientific evaluation of 6 of 7 (85%)
biotechnology consultations within 180 days.
- GRAS Notifications: Completed
the scientific evaluation of 19 of 23 (83%) GRAS notifications within 180
days. CFSAN has accepted and filed 157 GRAS notifications since the initiation
of the program.
- Food Contact
Notifications: Completed the review of all Food Contact Notifications
within 120-day statutory timeframe.
- Enforcement: In
an effort to increase enforcement actions for dietary supplement-type ingredients
in conventional foods whose use is neither approved under section 409 nor
generally recognized as safe (GRAS), CFSAN sent letters to companies marketing
conventional foods containing "kava" and warning letters were sent to companies
marketing products containing "stevia."
- Prefiling Consultations: Continued to interact with stakeholders proactively by encouraging prefiling
consultations for new industry submissions for food and color additive
petitions, food contact substance premarket notifications and biotechnology
and GRAS consultations. This is evidenced by the successful completion
of 64 premarket notification consultations, 12 presubmission meetings with
companies submitting food and color additive petitions, 4 presubmission
consultation meetings with companies regarding biotechnology products and
10 presubmission consultation meetings with companies regarding GRAS notifications.
- Guidance for
Industry: Issued guidance for industry with five review templates related
to the submission of toxicology/safety data submitted in food and color
additive petitions and in food contact notifications.
Guidance for Industry - Templates for Reporting Toxicology Data
Cosmetics
- Certified Color
Additives: CFSAN continued to analyze all batches of color additives
and determine certification status (certify or refuse to certify) within
an average of 5 working days. The Federal Food, Drug, and Cosmetic
Act requires batch certification for all color additives listed in 21 CFR
part 74 and for all color additives provisionally listed in 21 CFR part
82.
- Report to Congress
– Color Certification Fees: Senate Report 108-107 of the Agriculture
Rural Development, Food and Drug Administration and Related Agencies 2004
Appropriations Language directed FDA to provide a report on the steps that
will be taken to ensure that there will not be any future excessive fluctuations
in the cost of the Color Certification Program. CFSAN prepared a response to Congress and it is currently
under review by the Administration.
- Improved Tracking and Filing: Completed the development of an electronic tracking and filing
system to improve workflow, document tracking, and ease sharing of documents
and information within the Office of Cosmetics and Colors (OCAC). Historical
and new documents are now being entered into the system.
- Naming Cosmetic
Ingredients: CFSAN's Office of Cosmetics and Colors developed a strategy
for amending 21 CFR 701.3(c)(2) to address the outdated sources for naming
cosmetic ingredients.
- Phthalates: Analytical Method: Developed an analytical method for the determination of phthalates
in cosmetic products. The method determines phthalates in cosmetic products
using high performance liquid chromatography (HPLC). A draft manuscript
has been prepared for publication of the method, entitled "Analysis of
phthalate esters in consumer cosmetic products." The manuscript will be
submitted to the Journal of Cosmetic Science.
- Phthalates – Survey: Conducted
a survey of products to determine the contribution of phthalates to human
exposure. CFSAN's Office of Cosmetics and Colors (OCAC) surveyed 48 cosmetic
products and identified 5 phthalate esters in 32 of the products. Phthalate
levels ranging from 16 ppm to 59,000 ppm were found; the highest levels
found were in nail enamels.
Part IV: Assuring Food Safety:
Crosscutting Areas
Science Base
- Food Safety
Goals: In FY 2004, CFSAN established food safety goals to address risks
associated with foodborne illnesses. CFSAN staff evaluated risk factors
in retail and food service establishments that are related to foodborne
illness. Factors that CFSAN will measure in a proposed Food Safety Program
Assessment Rating Tool (PART) Goal have been identified. Next step will
be to implement a food safety intervention strategy to track progress toward
the attainment of the public health food safety goal.
- Provide training on methods of risk analysis for foods:
CFSAN's Staff College sponsored,
in conjunction with JIFSAN, a Food Safety Risk Analysis Professional Development Training Program and a Distance Learning Modality program.
- Human Subject Protection:
CFSAN has enhanced its system of protecting human subjects
that are undergoing CFSAN related research. These enhancements were established
through both a better monitoring system in the Center as well as mandatory
training and a web based information package for investigators.
- Laboratory Quality Assurance System:
CFSAN developed a framework for re-engineering the CFSAN
Laboratory Quality Assurance System. Implementation of the new system
will be complete in FY 2005.
- Cooperative Agreement between FDA and the University of Mississippi: On September 9, 2003, issued notice of grant award to continue the five-year
cooperative research agreement between CFSAN/FDA and the University of Mississippi's National Center for Natural Products
Research.
- FY 2005 Food Survey: Planning for the 2005 Food Safety Survey began in January 2004. One of the first efforts was to invite other interested people from CFSAN and USDA’s Food Safety and Inspection Service to participate in the survey planning. Planning has been completed. The data collection phase of the survey is planned for completion by late summer 2005.
- Program Effectiveness Conference: On October 29, 2003, A white paper summarizing the results of
the: "Working Conference on Measuring the Effectiveness of Foodborne Disease
Prevention Programs was completed and presented to CFSAN Management. The
conference focused on how to develop meaningful measures that link our
regulatory and enforcement actions to public health outcomes. The white
paper evaluated mechanisms and methods to measure the effect of FDA's
programs on food related health risks. The conference, held on June 30
through July 1, 2003, was attended by public health officials from State Public
Health Departments, and the Centers for Disease Control and Prevention,
along with key staff from CFSAN and FDA's Office of Regulatory Affairs.
- Leveraging Activities: Established
leveraging activities with the Department of Homeland Security to develop
methods for strain attribution of foodborne pathogens.
International
- Codex Alimentarius Commission: Participated in the 27th Session of the Codex
Alimentarius Commission was held June 28-July 3, 2004, in Geneva, Switzerland. Key accomplishments
of this meeting of the Commission included the following:
- Adoption
of more than 25 new Codex standards including: a Code of Practice for Good
Animal Feeding; a Code of Hygienic Practice for Milk and Milk Products;
Codes of Practice for the prevention and reduction of aflatoxin contamination in peanuts and for lead
contamination in food; Guidelines for Nutrition and Health Claims; and,
Principles and Guidelines for the Exchange of Information in Food Safety
Emergency Situations;
- Approval
of fourteen new work items including: implementing guidance on the Judgment
of Equivalence of Sanitary Measures Associated with Food Inspection and
Certification; Guidelines on Risk-Based Inspection of Imported Foods (CCFICS);
and, revision of the Code of Practice for Foods for Infants and Children.
- Codex
Committee on Nutrition and Foods for Special Dietary Uses
(CCNFSDU): CFSAN provided the U.S. Delegate and Alternate Delegate
to CCNFSDU and attended the Committee's 25th Session of this
Codex Committee. CCNFSDU advanced the Codex Proposed Draft Guidelines
for Vitamin and Mineral Food Supplements to Step 5 in the 8-Step
Codex Step Procedure. Ongoing work of the Committee includes revision
to the Codex Standard for Infant Formula, revision of the Codex Standard
for Processed Cereal Based Foods for Infants and Young Children,
and the development of Recommendations on the Scientific Basis of
Health Claims. CFSAN has provided input to the development of these
and other documents under development by the Committee.
- Codex Committee
on Food Import and Export Inspection and Certification Systems (CCFICS): CFSAN
provided the U.S. Delegate to CCFICS and attended the 12th Session
of this Codex Committee. CCFICS completed work on the Codex Guidelines
for the Exchange of Information in Food Control Emergency Situations. Work
continues on the subject traceability/product tracing for which CFSAN is
providing a leadership role. New work is planned on: implementation guidance
for equivalence determinations with respect to sanitary measures; risk-based
inspection of imported foods; and revision to guidance on certification
of foods, including electronic certification. CFSAN is participating in
or is leading the work effort in all these new work areas.
- Codex Committee
on Food Hygiene (CCFH): CFSAN provided the U.S. Delegate and Co-Alternate
Delegate to CCFH and attended the 32nd Session of this Codex
Committee. The Committee completed work on the Codex Code of Hygienic
Practice for Milk and Milk Products, for which the U.S. was the lead
drafting country and CFSAN provided the U.S. lead. The Committee also completed
work on definitions for food safety objectives, performance objectives
and performance criteria, an area which establishes new international concepts
for the area of food hygiene and in which CFSAN played a leading role internationally.
The Committee has under development a number of important hygiene texts
for which CFSAN is providing the Committee lead or leading the U.S. involvement
in the Committee's working groups including: Guidelines for Validation
of Food Hygiene Control Measures; Principles and Guidelines for Microbiological
Risk Management; Guidelines for the Control of Listeria monocytogenes; and
the Code of Hygienic Practice for Eggs and Egg Products.
- Codex Committee
on Methods of Analysis and Sampling (CCMAS): CFSAN provided the U.S.
Delegate to CCMAS and attended the Committee's 25th Session.
The Committee completed work on Codex General Guidelines for Sampling and Guidelines
on Measurement of Uncertainty. The Committee initiated consideration
of the handling of methods for the detection of foods derived from modern
biotechnology and continued work on other topics including Guidelines
for Evaluating Acceptable Methods of Analysis. The Committee also carried
out its endorsement of analytical procedures appearing in Codex commodity
standards.
- Codex Committee
on Fish and Fishery Products (CCFFP): CFSAN provided the U.S. Delegate
to CCFFP and attended the Committee's 26th Session. The Committee
completed work on sections of the Code of Practice for Fish and Fishery
Products dealing with Aquaculture and Quick Frozen Coated Products;
work continues on other sections of the Code. Work also continues on a
model health certificate to accompany export shipments of fish and on various
fish standards. U.S., and particularly, CFSAN input has been significant
in all of this work.
- Codex Committee
on Food Additives and Contaminants (CCFAC) : CFSAN provided the U.S.
Delegate and Alternate delegate to CCFAC and attended the Committee's 36th Session.
The Committee completed work on Risk Analysis Principles for use
of the Committee in its work on food additives and contaminants, a document
for which the United States was the lead drafter. With respect to food
additives, the Committee continued to progress work on the General Standard
for Food Additives, endorsed additive provisions in various Codex Commodity
standards, and considered how best to handle processing aids. With respect
to contaminants, the Committee completed work on: an initial section of
the General Standard for Contaminants and Toxins in Foods; a Policy
for Exposure Assessment of Contaminants and Toxins in Foods or Food Groups; the Code
of Practice for the Prevention and Adulteration and Reduction of Aflatoxin
Contamination in Peanuts; and the Code of Practice for the Prevention
and Reduction of Lead Contamination in Foods.
- Codex Committee
on General Principles (19th (Extraordinary) (CCGP)): CFSAN
attended the 19th (Extraordinary) Session of CCGP that dealt
with recommendations resulting from the evaluation of Codex by WHO and
FAO. Significant recommendations were made relating to improved management
of the Codex standards setting process; the governance of Codex, including
the structure and function of the Codex Executive Committee and establishing
annual sessions of the Codex Alimentarius Commission; and in the operation
of Codex Committees and Task Forces.
- Codex Committee
on Food Labeling (CCFL): CFSAN provided the U.S. Delegate to CCFL and
attended the 32nd Session of the Committee. The Committee completed
work on the Codex Guidelines for the Use of Nutrition and Health Claims. Discussions
continued on approaches to the labeling of foods derived from modern biotechnology,
an area in which countries hold significantly different views and in which
the CFSAN has provided significant leadership both within the United States
and within Codex. The Committee agreed to undertake a discussion on the
appropriateness of developing guidance on advertising with the context
of Codex.
- Codex Committee
on Milk and Milk Products (CCMMP): CFSAN provided the U.S. Alternate
Delegate to CCMMP and attended the 6th Session of the Committee.
The Committee advanced a number of dairy product standards to Step 5 in
the 8 Step Codex Step Procedure (including those for cheddar cheese, whey
cheeses, and various dairy fat blends) and continued work on several other
dairy product standards. The Committee considered the subject of geographic
indicators with respect to the development of a standard for parmesan cheese,
an issue on which guidance was requested from the Codex Alimentarius Commission.
- Codex Committee
on General Principles (20th Session)(CCGP): CFSAN provided
the U.S. Alternate Delegate to the 20th Session of CCGP. The
Committee completed work on a definition for traceability/product tracing,
an area in which CFSAN has provided leadership, both domestically and internationally.
The Committee also forwarded definitions of food safety objectives, performance
objectives and performance criteria for use with respect to food hygiene
to the Commission for adoption on an interim basis. Work progressed on
the development of: proposed Working Principles for Risk Analysis for
Food Safety; a revision to the Codex Code of Ethics; guidelines
on cooperation with international intergovernmental organizations; and
principles and policies for the participation of international non-governmental
organizations and observers in Codex.
- Technical Working
Group for Food Labeling, Packaging and Standards: The Technical Working
Group (TWG) on Food Packaging, Labeling and Standards held its annual meeting
in Washington, D.C., on April 13 and 14. The group discussed a number
of important issues, such as FDA's Obesity Initiative, nutrition labeling
and claims, trans fatty acid labeling, allergen labeling and legislation,
food standards, infant formula, and food safety-related labeling statements
and claims. In addition, the meeting participants identified several
items for further follow-up as well as areas for potential harmonization. This
TWG meeting also served as a forum for developing and discussing mutual
approaches on labeling issues that were put forward at the 32nd Session
of the Codex Committee on Food Labeling, which was held on May 10-14 in
Montreal, Canada.
- Trade Negotiations: CFSAN participated in United States trade negotiations to draft the sanitary
and phytosanitary articles of the agriculture chapters of the Central American
Free Trade Agreement (CAFTA) including Morocco, Australia, Bahrain, Dominican Republic, Panama and Thailand.
Food Biotechnology
- Outreach to
Stakeholders: As part of CFSAN's effort to enhance the information
provided to the public in FDA's evaluation of foods derived from bioengineered
plants, an updated web page was developed which shows the list of biotechnology
consultations that CFSAN has completed.
List of Completed Consultations on Bioengineered Foods
CFSAN's Office of Food Additive Safety has conducted outreach activities
to interested stakeholders. This includes individuals and groups from foreign
governments, industry groups, consumer interest groups, and academics. In
the period from January-May, 2004, CFSAN has held 13 meetings in order to
explain to the public FDA‘s policy for evaluation bioengineered crop plants.
- Food biotechnology Subcommittee Meeting: On September 24, 2003, CFSAN held the second meeting of the Food Biotechnology
Subcommittee of the Food Advisory Committee, in Washington, D.C. The subcommittee was asked to consider the use of molecular
biology data and information for assessing the safety of new bioengineered
foods and to provide suggestions to FDA on any new developments that might
enhance the agency's evaluation of these foods. The subcommittee generally
supported the new Codex guidelines and FDA's current approach that emphasizes
ensuring that new substances in food are safe for consumers.
- Guidance: Developed
guidance concerning the potential presence of unintended varieties of bioengineered
plant foods that may be present in the food supply, but that have themselves
not completed all regulatory steps for marketing:
Internal Processes
- CFSAN Adverse Events reporting System (CAERS): CFSAN now tracks, evaluates, and monitors all adverse events
and consumer complaints received about CFSAN regulated food and cosmetic
products.
- New
Data Sources: Identified three data sources to obtain exposure and other
missing data on dietary supplements and other CFSAN-regulated products. The
data sources are: (1) the Slone Survey, (2) the Behavioral Risk Factors
Surveillance Study (BRFSS) and (3) the American Association of Poison
Control Centers (AAPCC). Data collection from each source is underway.
- LACF Electronic Registration: In September 2004, pursuant to the Governmentwide Paperwork
Reduction Act (GPEA), CFSAN implemented "eLACF" (electronic submission
capability for Low Acid Canned Foods). "eLACF" gives all low-acid and
acidified food processing establishments the option to register their firm
with FDA electronically under the new FDA Unified Registration and Listing
System (FURLS).
- CFSAN Risk Management Framework: On February 5, 2004, completed CFSAN's Risk Management Framework. Risk management
is the process of weighing policy alternatives and implementing appropriate
control options, including regulatory measures. CFSAN's risk management
framework builds upon this foundation and includes how projects are identified,
prioritized, completed and procedures for monitoring and re-evaluating
outcomes of the decisions. The seven components of the CFSAN Risk Management
Framework are trigger/inputs, prioritization, process, decision, implementation,
outcome, and monitor/evaluate/modify. Training on implementation of the
Risk Management Framework for CFSAN management and staff is underway.
Focused, Economic-based Regulations
- Report to Congress – Standards of Identity for Yogurt: House Report 108-193 of the Agriculture Rural Development,
Food and Drug Administration and Related Agencies 2004 Appropriations Language
directs FDA to brief the Appropriation Committee on the comments received
and on Agency plans to issue a proposed rule on the standards of identity
for yogurt. CFSAN prepared a response to Congress
and it is currently under review by the Administration.
- Report to Congress – Unfiltered Milk and Milk Protein Concentrate: House
Report 108-193 and Senate Report 108-107 of the Agriculture Rural Development,
Food and Drug Administration and Related Agencies 2004 Appropriations Language
directs FDA to provide regular reports on the status of its review of any
petitions to use ultrafiltered milk, casein or MPC in dairy products that
have standards of identity. CFSAN prepared a response to Congress and
it is currently under review by the Administration.
EEO/Diversity Initiatives
- EEO/Diversity
Accomplishments: In FY 2004, CFSAN's Staff College met with members
of each Special Emphasis Group in CFSAN and with Center managers to determine
training, education, and development needs. Our Staff College also explored
other opportunities for raising cultural awareness, appreciating diversity
and fostering an inclusive environment in CFSAN. Listed below are activities
and events representing a continuation of the Center's past practices to
support EEO and Diversity.
- CFSAN's
EEO office was consolidated and moved to a central location under FDA's new
Office Shared Services.
- CFSAN held six workshops on conflict prevention and resolution for center managers.
- Several Special Emphasis Groups in
the Center put on cultural awareness programs for Black History Month,
Asian-American Pacific Islanders Month, and Hispanic Heritage Month.
- CFSAN
participated in the Emerging Leaders Program (ELP) - This is a DHHS recruitment
program for students who have post-baccalaureate degrees in the sciences.
Participants receive extensive training the first year, and are given specific
experience within a Center/office the second year. Staff College works with
Personnel and the program offices in CFSAN to represent the Center at a yearly
career fair, identify potentially suitable matches, and facilitate entry
on duty for participants coming to CFSAN.
CFSAN also participates annually in the Minorities
in Science and Technology (MIST) Career Fair. Our Staff College partnered
with BIG (Blacks in Government), Black Male Focus Group and the Hispanic
Initiative to achieve diverse Center representation.
Management Initiatives
- FY
2004 Budget Review: The budget review for FY 2004 is complete and all priority
funding needs have been identified.
- Decommissioning of FB-8: Continued the decommissioning effort of the Federal Building 8 facility,
all of the radioactivity surveys were completed in October 2004 and a final
report to the Nuclear Regulatory Commission (NRC) will be sent by December
of 2004. The majority of the chemical decommissioning is complete. The
last part, mercury removal from laboratory drain lines, is subject to the
GSA construction schedule.
- University Station: Construction
of the new CFSAN adjunct building: "University Station" is complete. CFSAN's
Office of Food Additive Safety formally located on Vermont Avenue in Washington, D.C. and CFSAN's Office
of Cosmetics and Color, formally located in Chantilly, Virginia will begin their move to University Station in November
2004.
- Realign
OMS: CFSAN's Office of Management Systems (OMS) has been
realigned to reflect the establishment of FDA's Office of Shared Services. OMS
now focuses on delivering efficient and effective services that do not
duplicate those offered at the Agency level but rather complement shared
services and fully meet the CFSAN user community's needs.
- Shared
Services Implementation: With
the advent of the Agency's Office of Shared Services, CFSAN has established,
within the OMS, a Shared Services Liaison Group. This group is responsible
for establishing service level agreements and monitoring services provided
by the Agency's Office of Shared Services to ensure that the CFSAN user
community is receiving the necessary administrative and information technology
services critical to the Center's mission. This liaison group has become
the "consumer advocate" for the CFSAN user community.
- Laboratory
Security: CFSAN has implemented programs and policies to ensure
full security over select agents used within CFSAN. All applicable laboratories
are electronically secured with limited access and the research facility
at Muirkirk road is a fully secure location.
- The President's Management Agenda: CFSAN personnel focused on 2 major initiatives in FY
2004 as part of the President's Management Agenda. The first is a-76 Implementation. The
Center completed all the work necessary to support the Agency's issuance
of a Performance Work Schedule (PWS) for clerical and administrative support
services. The 2nd initiative is the Unified
Financial Management System (UFMS). The Center is playing an active role
in implementation of this system.
- NTEU: This year has
been a productive one for the Labor-Management relationship within CFSAN. The
Cooperation Council has met on a regular schedule and successfully handled
such matters as Quality Step Increases (QSIs), Student loan repayments,
joint management - labor training and fuller implementation of the bargaining
unit contract. NTEU has worked in full partnership with CFSAN management
to ensure a smooth move of the Office of Food Additive Safety and the Office
of Cosmetics and Colors into the new University Station building. NTEU
has assisted in office assignments, furniture selections and move planning
for University Station.
- Leadership Legacy: On September
27, 2004, the report of the CFSAN Leadership Legacy
Steering Committee was completed. The Leadership Legacy Initiative
was established to address the significant and unprecedented number of
leaders and managers at all levels of the center who are eligible to
retire in the next five to ten years. The steering committee charge
prepared a report that takes a careful look at the subject of leadership
development in CFSAN, and outlines a series of recommendations and action
items that CFSAN will use as a template and framework for further developing
the Leadership Legacy Program at CFSAN.
2004 Program Priorities Part V:
"Carryovers into FY 2005"
- Issue a final rule for the establishment and maintenance
of records to identify immediate previous source and immediate subsequent
recipient of foods.
- Complete an evaluation of program performance
through the sixth year for Seafood HACCP with an emphasis on identifying factors that
may be inhibiting improvements in compliance rates, in order to assess
whether the program is accomplishing its objectives and to identify where
and how the program needs to be re-directed.
- Publish final Vibrio parahaemolyticus risk
assessment.
- Issue draft guidance for fresh cut produce.
- Prepare final report on modernizing the food Good
Manufacturing Practices (CGMPs).
- Publish proposal to permit "in-lid" labeling for
the Safe Handling Statement on shell eggs.
- Increase the number of state and local Retail
Food Regulatory Programs that are utilizing risk-base inspection programs
by 50%, as measured by the Voluntary National Retail Food Regulatory
Program Standards.
- Issue a proposal
to revise the bottled water quality standards for arsenic.
- Publish a Advance Notice of Proposed Rulemaking to solicit public
comment on how to give more prominence to calories on the food label,
i.e., increasing the font size for calories, including a percent Daily
Value (%DV) column for total calories, and eliminating the listing for
calories from fat.
- Publish a Advance Notice of Proposed Rulemaking to solicit comment
on (1) whether to require food packages that can reasonably be consumed
at one eating occasion to declare the whole package as a single serving,
(2) which, if any, Reference Amounts Customarily Consumed (RACCS) of
food categories need to be updated, and (3) whether to provide for comparative
claims for smaller portions of identical foods.
- Publish a final rule for dietary supplement Good
Manufacturing Practices (CGMPs).
- Publish a draft Compliance Policy Guide (CPG)
for chloropropanols.
- Alpha Hydroxy Acids (AHA): Publish final guidance
on labeling of AHA containing products.
- Publish proposed rule on general principles for
standards of identity in collaboration with USDA.
CFSAN 2004 Program Priorities