 |

Brain Cancer Study Explores Multi-Targeted Therapies
Targeted drugs such as imatinib (Gleevec) and erlotinib (Tarceva) have been tested against brain cancer, but few patients have benefited. A new study offers a possible explanation for the disappointing results and suggests that using the drugs in combination may be a more effective strategy against the deadly disease.
 |
 |
FDA News |
 |
|
FDA approves Evista for breast cancer chemoprevention. See story. |
|
The researchers found that brain cancer cells may simultaneously activate a number of proteins on the cell surface called receptor tyrosine kinases, or RTKs. These proteins relay growth-promoting signals into cells, sustaining their survival. RTKs have become popular drug targets because they are frequently overactive or mutated.
Read more 



Dr. Martin Abeloff
|
In Memory of Dr. Martin Abeloff
On Friday, September 14, 2007, the cancer community lost one of its truly outstanding leaders. Dr. Martin Abeloff, who directed the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University for the last 15 years, died after a year-long battle with leukemia.
He was among the finest clinician/researchers I have ever met. His legacy will be marked by his personal success as a leader in translational research, particularly with regard to the adjuvant treatment of breast cancer. Dr. Abeloff also was a proponent of cancer prevention and control research, establishing a formal program at Kimmel for research in early disease biomarkers and disease surveillance.
Read more 
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Brain Cancer Study Explores Multi-Targeted Therapies
Targeted drugs such as imatinib (Gleevec) and erlotinib (Tarceva) have been tested against brain cancer, but few patients have benefited. A new study offers a possible explanation for the disappointing results and suggests that using the drugs in combination may be a more effective strategy against the deadly disease.
The researchers found that brain cancer cells may simultaneously activate a number of proteins on the cell surface called receptor tyrosine kinases, or RTKs. These proteins relay growth-promoting signals into cells, sustaining their survival. RTKs have become popular drug targets because they are frequently overactive or mutated.
By simultaneously turning on a number of RTKs, cancer cells may reduce their dependence on any one, and thereby improve their chances of survival, the researchers reported online in Science on September 13. They first observed the phenomenon in cells from patients with glioblastoma and then in other major cancers.
"We have found that a number of RTKs are simultaneously activated in virtually all the cancer cells we've examined," says Dr. Ronald DePinho of the Dana-Farber Cancer Institute, who led the study. "When one is blocked another can step in and sustain the survival signal."
What may matter most in treating the disease is to reduce the overall level of these abnormal signals in cells, he adds.
The findings support the growing view that some RTKs are more or less interchangeable, and they may help explain the feeble clinical responses when RTK inhibitors are used against solid tumors, the researchers say. Even when the drugs elicit an initial response, most cancers eventually progress and patients need additional therapies.
Glioblastoma is usually fatal within a year of diagnosis. More than 100 clinical trials over the last decade have not improved survival, with brain cancer, with the notable exception of temozolomide (Temodar) for a subset of glioblastoma patients. The lack of progress stems from not knowing which genes are involved in the disease.
Dr. DePinho and his colleague Dr. Jayne Stommel were searching for RTKs that drive glioblastoma when they uncovered the multiple coactivated RTKs. Some of the kinases, such as EGFR and Met, were known to play a role in the disease, but others were not.
Researchers may need to look more globally at RTKs that work in conjunction with known factors such as EGFR, says Dr. John Laterra, a brain cancer researcher at Johns Hopkins Medical School.
This study is timely because many scientists are looking at the networks of RTKs and the pathways they control," Dr. Laterra continues, "and they are finding evidence of close crosstalk and interplay among the pathways."
In the study, three or more targeted drugs were typically needed to control abnormal cell growth. The researchers used an experimental Met inhibitor in combination with imatinib and erlotinib to block the flow of growth signals into cells and cause them to die. They confirmed the results using RNA interference to inhibit the RTKs.
Like many in the field, the researchers envision an individualized approach to cancer therapy. Patients would have their tumors profiled to see which RTKs are active, and a regimen would be designed based on the results.
A number of RTK inhibitors have been approved for cancer and others are in development. But the first Met inhibitors, for instance, are still in early-stage clinical testing. The costs and toxicities associated with using multiple targeted drugs would need to be addressed before clinical trials could be launched.
Nonetheless, if the basic hypothesis is confirmed by other studies, researchers will have to rethink how trials for glioblastoma and other solid tumors are designed, says Dr. Antonio Omuro of the Hôpital Pitié-Salpêtrière in Paris, who coauthored a recent commentary on targeted therapy for brain cancer.
"We will need to reinforce the policy of molecularly profiling every single patient enrolled in clinical trials," he says. "We have not always done this, but everyone knows that it is essential to move the field forward."
—Edward R. Winstead
|
 |
 |

In Memory of Dr. Martin Abeloff

Dr. Martin Abeloff
|
On Friday, September 14, 2007, the cancer community lost one of its truly outstanding leaders. Dr. Martin Abeloff, who directed the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University for the last 15 years, died after a year-long battle with leukemia.
He was among the finest clinician/researchers I have ever met. His legacy will be marked by his personal success as a leader in translational research, particularly with regard to the adjuvant treatment of breast cancer. Dr. Abeloff also was a proponent of cancer prevention and control research, establishing a formal program at Kimmel for research in early disease biomarkers and disease surveillance.
The tremendous achievements of the researchers, clinicians, and other staff at Hopkins over the past decade and a half are a tribute to Dr. Abeloff's tremendous abilities as a champion of cancer research and all it can accomplish. It was those strong beliefs that allowed Dr. Abeloff and his colleagues to attract the support needed to build a world-class research and treatment facility that encourages and fosters collaboration and excellence.
Despite his many responsibilities, Dr. Abeloff generously volunteered his considerable expertise, particularly at NCI, where he served for more than 10 years on various advisory boards and panels, including as chair of the Board of Scientific Counselors and as a member of the Advisory Committee to the Director.
In 1994, Dr. Abeloff gave the keynote address at the American College of Surgeon's "Commission on Cancer" meeting, outlining his thoughts on professionalism and cancer care. "Values essential to medical professionalism are honesty and integrity," he said. "The professional physician has an attitude of humility as well as accountability to patients, colleagues, and society."
Rarely do individuals ascend to the lofty standards they discuss in forums such as this. But that could never be said of Dr. Abeloff. His honesty and integrity were unparalleled. Former NIH director and current president of the Memorial Sloan-Kettering Cancer Center Dr. Harold Varmus described him well, calling Dr. Abeloff a man who "knew how to criticize without insult and praise without flattery."
Dr. Abeloff never forgot that patients are people, and that his hospital's responsibility was to treat the whole patient. From that belief arose the Art of Healing program at Kimmel, through which patients get to enjoy original works of art and musical performances, providing moments of serenity during a time often overflowing with anguish.
In a Washington Post article on Dr. Abeloff's death, a former patient recounted how 17 years ago, Dr. Abeloff helped to allay her fears about her pending treatment. He also gave her a small piece of torn paper with his phone number on it - he didn't have a business card - telling her to call him any time. Humble and accountable, indeed.
The remarkable outpouring of kind words and accolades from colleagues, former patients, and friends in the wake of his untimely passing is no surprise. He was a man who touched countless lives.
I'd like to offer the heartfelt condolences - and the eternal gratitude - of the entire NCI to Dr. Abeloff's family. He was a rare person, one who continually gave of himself, who believed in the constant pursuit of knowledge and excellence, and whose impact will continue to be felt for decades to come. As a very close friend and professional colleague, I will dearly miss him.
Dr. John E. Niederhuber
Director, National Cancer Institute
|
 |
 |
 |
 |
Presidential Proclamation for Prostate Cancer Awareness in September |
 |
|
Gene Profiling Identifies High-Risk Multiple Myeloma Patients
Researchers have identified a small subset of genes whose activity could predict high-risk cases of multiple myeloma and potentially guide therapy decisions in the future. They presented their data recently at the American Association for Cancer Research's second International Conference on Molecular Diagnostics in Cancer Therapeutic Development. NCI provided partial funding for the study.
The investigators, from the Myeloma Institute for Research and Therapy at the University of Arkansas for Medical Sciences, followed 532 multiple myeloma patients for 7 years after a blood stem cell transplant to create a genetic profile to chart the severity of the disease. The team determined that the activity of as few as 17 genes could mean the difference between high or low risk for a poor prognosis. About 30 percent of the genes that predict high risk are found on chromosome 1.
In addition, around 13 percent of all the patients they studied exhibited a genetic pattern that fit into the high-risk category, a frequency that rose to 76 percent among relapsed patients. An increase in the gene-expression risk score among relapsed patients provides evidence that there are likely to be small subsets of high-risk cells even in patients with low-risk disease, and that current therapeutics are suboptimal in that they kill off the low-risk cells, leaving behind cells that exhibit a high-risk genetic profile. "Gene expression profiles have now provided us with signposts that help us risk-stratify patients and tailor therapies accordingly," said lead researcher Dr. John D. Shaughnessy, Jr.
Bortezomib Multiple Myeloma Trial Halted after Analysis Shows Significant Survival Improvements
A phase III trial testing bortezomib (Velcade) plus the combination of two chemotherapy drugs for the first-line treatment of patients with newly diagnosed multiple myeloma has been stopped early based on recommendations of the trial's independent data monitoring committee (IDMC) so patients in the control arm can be switched over to the bortezomib treatment arm.
Millenium Pharmaceuticals, which manufactures bortezomib, announced last week that the IDMC halted the 682-patient trial, dubbed VISTA, after a planned interim analysis showed that the combination of bortezomib with melphalan and prednisone demonstrated a highly statistically significant improvement in overall survival and progression-free survival, as well as time-to-disease progression and complete remission rate compared with melphalan and prednisone alone.
"These results position [bortezomib]-based therapy as a new standard of care for newly diagnosed multiple myeloma patients," Dr. Paul Richardson, clinical director of the Jerome Lipper Multiple Myeloma Center at the Dana-Farber Cancer Institute and one of the trial's lead investigators, said in a statement.
During a conference call with investors and media, Millenium representatives said data from the analysis will be presented in December at the American Society of Hematology annual meeting. Dr. Nancy Simonian, Millenium chief medical officer, did say, however, that the results were consistent with those from a similar phase II trial presented in June at the International Myeloma Workshop in Greece, which showed a 43-percent complete response rate and a 3-year survival rate of 85 percent.
New FOBT Promising for Detection of Colon Cancer
In a large prospective study performed by investigators from three Northern California Kaiser Permanente medical centers, a type of fecal occult blood test (FOBT) called a fecal immunochemical test (FIT) showed high sensitivity and specificity for detecting left-sided colorectal cancer. The results were published in the September 25 Journal of the National Cancer Institute.
The investigators enrolled 7,394 eligible Kaiser Foundation Health Plan members aged 50 or older without a personal or family history of colon cancer into the study. All participants collected stool samples for use on three test cards, and the samples on each test card were used for three tests: a sensitive unrehydrated guaiac test (GT, a more sensitive version of the current standard FOBT), the FIT, and a combination of those two tests. Out of all participants, 5,841 prepared cards correctly and had at least 1 usable test result. Of these, 11 percent had at least 1 positive result.
Investigators recommended that all participants testing positive on the FIT or combination tests undergo colonoscopy, and all participants testing negative undergo sigmoidoscopy. Because most participants tested negative and underwent sigmoidoscopy, which can only visualize the left colon, only detection of left-sided colorectal cancer could be compared between tests. All participants were followed for 2 years, until diagnosis of a colorectal neoplasm, or until death, whichever came first.
The FIT had an 81.8 percent sensitivity for detecting colorectal carcinoma, and a 29.5 percent sensitivity for detecting advanced colorectal adenomas (noncancerous tumors). Specificity was 96.9 percent for carcinomas and 97.3 percent for adenomas, which increased to 98.1 percent and 98.4 percent in the combination test.
Although the investigators did not directly compare the FIT test with the current standard FOBT test, other recent comparisons have shown its superiority, they explain. "The FIT has high sensitivity and specificity for detecting left-sided colorectal cancer," they conclude, "and it may be a useful replacement for the [current standard test]."
|
 |
 |

FDA Approves Raloxifene for Breast Cancer Prevention
There are now two drugs approved for the prevention of breast cancer, after the U.S. Food and Drug Administration (FDA) last week approved raloxifene (Evista) to reduce the risk of invasive breast cancer in postmenopausal women at high risk of the disease. The new approval also covers raloxifene to reduce the risk of invasive breast cancer in postmenopausal women with osteoporosis.
Leading prevention researchers called the approval an important moment for women at high risk of breast cancer.
"I am excited that postmenopausal women at increased risk for breast cancer now have a choice of drugs to reduce their risk," says Dr. Leslie Ford, associate director for clinical research in NCI's Division of Cancer Prevention. "Women and their doctors are already familiar with raloxifene and should be comfortable using it for both breast cancer and osteoporosis prevention."
The agency's action follows recommendations in August by the FDA's Oncology Drugs Advisory Committee to approve the drug for both indications.
In a statement, Dr. Steven Galson, director of the FDA's Center for Drug Evaluation and Research, advised women at high risk of breast cancer to discuss raloxifene's risks and benefits with their physicians to determine "whether the drug is right for them."
Positive results from four different clinical trials formed the basis for the agency's approval of raloxifene, which was initially approved to prevent and treat osteoporosis in postmenopausal women.
Included among those was a pivotal, NCI-supported trial known as STAR, which demonstrated that raloxifene was as effective as tamoxifen - the first drug approved for breast cancer chemoprevention - at reducing breast cancer risk in postmenopausal women. In the trial, raloxifene was generally less toxic than tamoxifen, but it was not as effective at reducing the risk of a noninvasive condition that can be a precursor to cancer, known as ductal carcinoma in situ, or DCIS.
In the STAR trial, both raloxifene and tamoxifen reduced the risk of invasive breast cancer by approximately 50 percent. Women on raloxifene had a reduced risk of blood clots, endometrial cancer, and cataracts compared with women on tamoxifen, although not all were statistically significant reductions.
The three other clinical trial results the FDA took into consideration were the CORE, MORE, and RUTH trials. Although breast cancer risk reduction was not a primary endpoint for any of the trials, all three did demonstrate a significant risk reduction in women given raloxifene. |
 |
 |
A Conversation with Dr. M. Scott Lucia
 In 2003, finasteride became the first drug to prevent prostate cancer in a large, prospective study, the Prostate Cancer Prevention Trial (PCPT). Finasteride reduced the overall occurrence of prostate cancer by 25 percent, but concerns were raised because men taking the drug had a slightly higher number of high-grade prostate cancers, which tend to be aggressive. Two studies in the September 19 Journal of the National Cancer Institute provide reassurance that the drug was probably not causing the high-grade cancers. Rather, finasteride may have simply made it easier to detect high-grade cancers. Dr. M. Scott Lucia of the University of Colorado Health Sciences Center, who coauthored the PCPT results and led one of the new studies, discusses the findings.
In 2003, finasteride became the first drug to prevent prostate cancer in a large, prospective study, the Prostate Cancer Prevention Trial (PCPT). Finasteride reduced the overall occurrence of prostate cancer by 25 percent, but concerns were raised because men taking the drug had a slightly higher number of high-grade prostate cancers, which tend to be aggressive. Two studies in the September 19 Journal of the National Cancer Institute provide reassurance that the drug was probably not causing the high-grade cancers. Rather, finasteride may have simply made it easier to detect high-grade cancers. Dr. M. Scott Lucia of the University of Colorado Health Sciences Center, who coauthored the PCPT results and led one of the new studies, discusses the findings.
What did you learn from your study?
The data we collected best support the hypothesis that the use of finasteride enhances the detection of high-grade prostate cancers at biopsy. Finasteride decreased overall prostate volume by 27 percent in men diagnosed with high-grade cancer. We compared the results of prostatectomies and biopsies for 489 men. Of the men who had high-grade tumors at prostatectomy, biopsy correctly identified the cancer as high-grade 70 percent of the time in the finasteride group but only 50 percent of the time in the placebo group. This effect was likely a result of the decrease in prostate volume combined with a selective inhibition of low-grade cancer. Although we cannot rule out the possibility that finasteride might influence the growth of some high-grade prostate cancers, the results suggest that the impact of finasteride on high-grade cancer is less than originally thought, or perhaps even that it has no effect.
Have the new findings changed your view of the PCPT results?
One has to interpret the results of the original study with the knowledge that there is a bias in detecting high-grade cancers. We now know that finasteride interferes with two important measures that we use to detect prostate cancer - the prostate-specific antigen test and digital rectal exam - by making these tests more sensitive for cancer. This bias makes it very difficult to interpret the results. But finasteride is clearly a better drug than it initially appeared to be. We were also reassured to find that the high-grade cancers in the finasteride group appeared to be less extensive on biopsy than in the placebo group.
Are the results good news for men?
Yes. The PCPT results were important because we showed for the first time that you can prevent prostate cancer by taking an agent, in this case finasteride. Up until this point the concept of prostate cancer chemoprevention had been largely theoretical, but we have shown this can happen. The new results bring more good news because the risks of taking finasteride, which were a deal-breaker for many men back in 2003, are probably not as great as originally thought, though the precise risks are not known. Finally, many men take finasteride for benign prostatic hyperplasia (enlargement of the prostate), and the results are reassuring that the drug is safe for use in that setting. This may be the most important piece of good news for men.
|
 |
 |

Targeting Blood Cancers at the Source
Recent identification of cells with stem cell-like properties in solid tumors including breast, brain, lung, and prostate cancer has generated considerable excitement in the cancer research community. Scientists now believe that these cells - which can both self renew to maintain the population of stem cells and produce more differentiated progenitor cells which make up the bulk of a tumor - may play a role in the formation, recurrence, and metastasis of many solid cancer types and present a target that must be eradicated to potentially produce a cure.
This research in solid tumors is only now beginning to accelerate, but investigators studying cancers of the blood have known about the existence of cancer stem cells in these malignancies for over a decade, and tactics for destroying stem cells in hematologic cancers have begun to make their way into early human trials.
Cancer stem cells are thought to be at the root of therapeutic resistance for many hematologic cancers. For example, chronic myelogenous leukemia (CML) responds to treatment with the targeted drug imatinib, but patients inevitably relapse when they stop taking the drug, and many patients' cancers progress even during treatment.
Researchers speculate that resistance of CML stem cells to imatinib is due to their quiescence, a state of relative dormancy where the cells do not divide, protecting them from both traditional chemotherapy, which targets rapidly dividing cells, and therapies such as imatinib that target cell-signaling pathways.
"Slow cycling [of cancer stem cells] is probably one of the biggest challenges that we face in terms of targeting for therapy," says Dr. John Dick, director of the Program in Stem Cell Biology at the University of Toronto, whose laboratory first identified cancer stem cells in acute myeloid leukemia in 1994.
One approach to addressing this challenge has been to "wake up" the cancer stem cells - to force them to divide and enter the cell cycle. This idea is being tested by the United Kingdom's National Cancer Research Network, whose GIMI trial is based on the observation that granulocyte-colony stimulating factor applied intermittently to leukemia stem cells in vitro could stimulate them to proliferate, thus increasing their sensitivity to imatinib.
This approach is also being tested at Johns Hopkins University. "We're trying to bring the immature stem cells to maturity, to induce them to differentiate into mature cells," explains Dr. Carol Ann Huff, assistant professor of oncology at Johns Hopkins, whose group is working with granulocyte-macrophage colony stimulating factor in conjunction with cell cycle inhibitors in both myelodysplasia and leukemia.
But Dr. Huff's clinical trials group is also looking at another tactic - taking advantage of the differences between cancer stem cells and "normal" cancer cells, "to actually eradicate the quiescent cancer cells" rather than bring them into the cell cycle, she explains.
"One of the things we've learned in leukemia and multiple myeloma is that agents that are active against the differentiated cells have little effect on leukemia or myeloma stem cells and vice versa," she says. "We believe that's part of the reason why we haven't been able to eradicate the stem cells with the current drugs that we have." Dr. Huff and her colleagues are now testing combination therapies with drugs known to reduce the bulk of circulating tumor cells - such as imatinib in CML or cyclophosphamide in multiple myeloma - and drugs with particular affinity for cancer stem cells, such as rituximab in multiple myeloma, which targets a protein expressed on the surface of the stem cells but not the bulk of the cancer cells.
Additional research into the unique molecular properties of cancer stem cells is needed, explains Dr. Dick, to advance such targeted treatment regimens. "We need to understand their properties," he says. "We need to understand what the mechanisms are that make them resistant to therapy, and...we need to understand the self-renewal mechanisms in these cells, because in the end that's the one property I think everyone would accept that a cancer stem cell has - the ability to renew itself in perpetuity. That's what makes the cancer stem cell so potent and really so different from the other cells in the tumor."
—Sharon Reynolds |
 |
 |

New Chemo Agents Travel Winding Road
At a time when there is no shortage of studies that identify potential molecular targets for new cancer therapies and many pharmaceutical and biotechnology companies are banking on targeted cancer agents to ensure future success, cytotoxic chemotherapy still represents the foundation of treatment for the vast majority of cancers.
In fact, a number of pharmaceutical companies are busily developing new chemotherapeutic agents.
Among those under development, a class of drugs known as epothilones has generated intense interest because of promising results from preclinical studies and early-phase clinical trials, and because they work similarly to a class of highly effective chemotherapy drugs, the taxanes, but don't require premedication with steroids to fend off allergic reactions. In June, the U.S. Food and Drug Administration (FDA) granted priority review to Bristol Myers Squibb's application to market ixabepilone, the epothilone furthest along in clinical testing, for the treatment of metastatic breast cancer. Priority review means FDA intends to complete the review within 6 months.
 |
 |
Watch Your E-Mail Box! |
 |
|
Keep an eye out for an e-mail later this week asking you to complete an online survey about the NCI Cancer Bulletin.
By completing this short questionnaire, you'll help us to better meet the needs of our readers. Your feedback is vital in shaping future issues of the Bulletin.
All survey responses are confidential and respondents can choose to answer or skip any questions in the survey. For more information, please contact Nina Goodman at goodmann@mail.nih.gov or 301-435-7789. |
|
It's unclear at this point, though, what FDA approval of ixabepilone would mean for the epothilones' long-term prospects. Treatment options for some of the cancers in which epothilones are being studied have expanded dramatically. And, as a handful of researchers have argued, in the absence of tests that indicate which patients are the best candidates for which treatments, it may prove difficult for any new chemotherapeutic agent that doesn't demonstrate clear superiority to other available agents to establish a foothold in the clinic.
In August, results of five phase II clinical trials testing ixabepilone were published in the Journal of Clinical Oncology (JCO) - four trials in metastatic breast cancer and one in advanced non-small-cell lung cancer (NSCLC). In all five trials, the drug showed comparable efficacy to what has historically been seen with the standard-of-care drugs, including taxanes such as paclitaxel and docetaxel, that are the cornerstones of treatment for metastatic breast cancer.
And, as important, it had what's considered an "acceptable" toxicity profile - one that produced toxicities that, although not negligible, were manageable and didn't prohibit the drug's use in most patients for long enough to provide a clinical benefit.
"The evidence to date on ixabepilone [to treat metastatic breast cancer] suggests that it is extremely effective either as a first-line therapy or after patients have undergone multiple treatments, especially with an anthracycline or after taxanes," explains Dr. Sandra Swain, medical director of the Washington Cancer Institute in Washington, DC, who has led several phase II trials involving ixabepilone.
Ixabepilone also produced favorable results in one of two ongoing phase III breast cancer trials in which it is being investigated, comparing it plus capecitabine to capecitabine alone in patients with metastatic breast cancer who have progressed after standard treatments. Presented at the American Society of Clinical Oncology annual meeting in June, the results of the trial were not staggering - a 1.5 month improvement in progression-free survival and a 2.5-fold increase in overall response rate - but the improvements compared favorably enough with the current standard to secure an FDA priority review.
Although they are not generally considered "targeted" drugs, most chemotherapeutic agents do have a target: rapidly dividing cells. In the epothilones' case, just like the taxanes and the vinca alkaloids, another class of chemotherapy agents, they target a component of cells' cytoskeleton known as microtubules, which are critical to cell division. All three types of agents work by stabilizing microtubules; this disrupts the process of cell division (and thus, further proliferation) and eventually leads to cancer cell death.
"We know the tubulins have been a good target," says Dr. Edith Perez, director of the Breast Cancer Program at the Mayo Clinic Jacksonville and investigator on one of the JCO-published ixabepilone trials.
But the epothilones, of which there are four being tested in phase II or III trials (see table), "appear to bind in a different location compared with other tubulin-targeting agents," she adds. That may explain why these drugs have shown activity in patients who have developed resistance to other tubulin-targeting agents like
the taxanes.
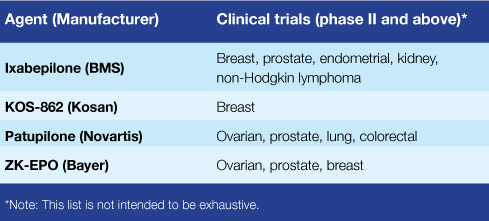
That was the case in four of the five trials published last month in JCO (the fifth used ixabepilone as a first-line treatment). In the advanced NSCLC trial, ixabepilone demonstrated results comparable to those seen with three other drugs already FDA-approved for patients whose tumors are no longer responding to the first-line treatment.
And therein lies part of the difficulty for any new chemotherapeutic agent, notes Dr. Alan Sandler, medical director of Thoracic Oncology at Vanderbilt University and co-investigator on the trial.
"It's not like the old days where you didn't have anything for these patients," he says. "We now have three agents for second line, two chemotherapeutic and one targeted agent."
In the case of ixabepilone for the treatment of NSCLC, he says, it's still unclear where it can or will go from here. For example, there is potential in the second-line arena because
two of the approved second-line agents, pemetrexed and docetaxel, are now being investigated as first-line treatments. If they succeed, that could free up space in the second-line arsenal for an agent like ixabepilone that has already shown some activity in that setting.
If approved by the FDA, Dr. Perez says, it appears as if ixabepilone at least would present new options. In the case of the phase II trial in which she was involved, for example, there is no approved therapy for the type of patients it enrolled, all of whom were resistant to anthracyclines, taxanes, and capecitabine.
"These patients are now treated with unapproved drugs," she notes, "so it was nice to demonstrate that ixabepilone has clear activity, thus addressing an unmet patient need."
Information from NCI’s PDQ Cancer Clinical Trials Registry about epothilone clinical trials can be found at: Ixabepilone clinical trials, Patupilone clinical trials, and ZK-EPO clinical trials.
—Carmen Phillips
|
 |
 |

Pomegranate Juice for PSA-Only Prostate Cancer Recurrence
Name of the Trial
Phase III Randomized Study of Pomegranate Juice in Patients with Rising Prostate-Specific Antigen Levels after Surgery or Radiotherapy for Localized Prostate Cancer (UCLA-0507059-01). See the protocol summary at http://cancer.gov/clinicaltrials/UCLA-0507059-01.
 Principal Investigators
Principal Investigators
Drs. Allan Pantuck and Arie Belldegrun, Jonsson Comprehensive Cancer Center at UCLA
Why This Trial Is Important
Surgery and radiotherapy are common treatments for localized prostate cancer (cancer confined to the prostate gland). Following such treatments, doctors may monitor the blood level of a protein called prostate-specific antigen (PSA). An increase in PSA level may be an early indicator that prostate cancer has returned. Research has shown that the length of time it takes for a rising PSA level to double (called PSA doubling time) can be useful in predicting the risk of prostate cancer progression and death.
Currently, there is no standard treatment for men who have recurrent prostate cancer detected by an increase in PSA level only. Consequently, doctors want to develop treatments that can slow down or reverse increases in PSA without causing serious side effects.
In this trial, researchers are exploring the potential of pomegranate juice to slow or reverse increasing PSA levels in men who have undergone treatment for localized prostate cancer. Pomegranate juice is rich in phytochemicals, substances that have been shown in laboratory studies to inhibit cancer growth and spread. Men in this trial will be randomly assigned to drink a special preparation of pomegranate juice or a placebo drink daily for up to 1 year.
"In a phase II trial we conducted, daily consumption of pomegranate juice resulted in a significant lengthening of PSA doubling time and disease stabilization," said Dr. Pantuck. "We hope to verify those results in a phase III, double-blinded, placebo-controlled study."
Who Can Join This Trial
Researchers seek to enroll 250 patients aged 18 and over with prostate cancer that have completed prior surgery, cryotherapy, or radiotherapy but have rising PSA levels. See the list of eligibility criteria at http://cancer.gov/clinicaltrials/UCLA-0507059-01.
Study Site and Contact Information
Study sites in the U.S. are recruiting patients for this trial. See the list of study contacts at http://cancer.gov/clinicaltrials/UCLA-0507059-01 or call 1-800-4-CANCER (1-800-422-6237) for more information.
An archive of "Featured Clinical Trial" columns is available at http://cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |
Following are newly released NCI research funding opportunities:
The Cooperative Human Tissue Network
Announcement Number: RFA-CA-08-503
Letter of Intent Receipt Date: Sept. 30, 2007.
Application Receipt Date: Oct. 30, 2007.
This is a renewal of RFA-CA-01-009 and will use the U01 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3772. Inquiries: Dr. Yaffa Rubinstein - runinsty@mail.nih.gov
Multidisciplinary Fellowships in Cancer Nanotechnology Research
Announcement Number: RFA-CA-08-003
Application Receipt Date: Dec. 20, 2007.
This is a renewal of RFA-CA-05-025 and will use the F32 and F33 award mechanisms. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3771. Inquiries: Dr. Jerry S. H. Lee - leejerry@mail.nih.gov; Dr. Piotr Grodzinski - grodzinp@mail.nih.gov
Research on Malignancies in the Context of HIV/AIDS
Announcement Number: PA-07-454 and PA-07-455
Application Receipt Dates: Jan. 7, May 7, and Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7 and May 7, 2010.
This is a renewal of PA-07-173 and will use the R01 and R21 award mechanisms. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3770. Inquiries: Dr. Geraldina Dominguez - domingug@mail.nih.gov
|
 |
 |

If Memory Serves...
In 1937, little was known about the origins of cancer. Some doubted that the cause of the disease would ever be understood and discouraged investment in this research. But scientists already understood that environmental exposures could cause tumors and that there may be a genetic component to certain types
of cancer. (Read more)
For more information about the birth of NCI, go to http://www.cancer.gov/
aboutnci/ncia.
2007 Pioneer and New Innovator Awards Announced
On September 19, NIH Director Dr. Elias Zerhouni announced the recipients of the 2007 NIH Director's Pioneer Awards and the New Innovators Awards. This is the first group of New Innovator Awards and the fourth group of Pioneer Awards. Both programs are part of an NIH Roadmap for Medical Research initiative that tests new approaches to supporting research.
Pioneer Awards support scientists at any career stage, while New Innovator Awards are for new investigators who have not received an NIH regular research (R01) or similar grant. The 12 new Pioneer Award recipients will each receive $2.5 million in direct costs over 5 years. The 29 New Innovator Award recipients will each receive $1.5 million in direct costs over the same period.
More information about these awards can be found at http://nihroadmap.nih.gov/.
Four New Members Appointed to DCLG
NCI Director Dr. John Niederhuber announced his intention to appoint four new members to the Director's Consumer Liaison Group (DCLG):
Marie Dahlstrom, of Portland, OR, represents De La Mano Frente Al Cancer: Latino Cancer Coalition. She is also a member of the Komen Breast Foundation National Hispanic/Latina Advisory Council and conducts research in cancer prevention, access to care, and domestic violence in the Latino community.
Everett Dodson, of Silver Spring, MD, represents Prostate NET. He is also a clinical research associate at the Howard University Cancer Center, where he serves as the director of prostate cancer screening.
Joyce Wilcox Graff, of Brookline, MA, is the Executive Director of the VHL Family Alliance, a nonprofit support organization that serves more than 15,000 patients and their family members dealing with von Hippel-Lindau (VHL) disease.
Arlene Wahwasuck, of Horton, KS, represents the Four Tribes Women's Wellness Coalition. She is a nurse and retired U.S. Public Health Service officer.
Teleconference Playback Available
Toll-free playback of the most recent "Understanding NCI" teleconference from the Office of Liaison Activities is available until October 12 at 1-866-443-8027. The call featured a report from NCI Director Dr. John Niederhuber with information about NCI's budget and latest programs. DCLG Chair Doug Ulman addressed the role advocates play at NCI through the DCLG.
The next teleconference will take place on October 31 from 1:00-2:00 p.m., ET. It will feature a discussion of the President's Cancer Panel report on cancer risk reduction. The call can be accessed toll free at 1-800-857-6584; the pass code is PCP. More information about the teleconference series is available at http://ola.cancer.gov/activities/teleconferences.
NCI Releases New Tool for Finding Cancer-Related Proteins
NCI recently released the Computational Proteomics Analysis System (CPAS), a new software tool for the cancer Biomedical Informatics Grid that enables cancer proteomics researchers to store, analyze, and share clinical proteomics data, thus driving the discovery of cancer-related proteins for developing new diagnostic tools and therapies.
LabKey Software, in collaboration with the Fred Hutchinson Cancer Research Center, developed CPAS with funding from the NCI Clinical Proteomic Technologies Initiative for Cancer, which was launched in 2006 to help build the foundation of analytical systems, technologies, data, reagents, standards, and infrastructure to advance the understanding of protein biology in cancer and accelerate discovery research and clinical applications.
Researchers can obtain the CPAS software at https://cabig.nci.nih.gov/tools.
|
|
 |
 |

The Cancer Institute of New Jersey
Interim Director: Dr. Joseph R. Bertino • 195 Little Albany Street, New Brunswick, NJ 08903 • Phone: 732-235-2465 • Web site: http://www.cinj.org/
Background
 The Cancer Institute of New Jersey (CINJ) was established in 1991 as a Center of Excellence of the University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) with the mission to create a cancer center of extraordinary quality for the citizens of New Jersey. Since that time, CINJ has experienced remarkable growth to its current 225,000-square-foot facility, housing state-of-the-art cancer research laboratories, a clinical facility that will host more than 80,000 outpatient visits in the coming year, and specialized areas dedicated to clinical, population, and bioinformatics cancer research. CINJ became an NCI-designated Clinical Cancer Center in 1997, followed by the NCI Comprehensive Cancer Center designation 5 years later, establishing CINJ as the only such facility in the state of New Jersey. The Cancer Institute of New Jersey (CINJ) was established in 1991 as a Center of Excellence of the University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) with the mission to create a cancer center of extraordinary quality for the citizens of New Jersey. Since that time, CINJ has experienced remarkable growth to its current 225,000-square-foot facility, housing state-of-the-art cancer research laboratories, a clinical facility that will host more than 80,000 outpatient visits in the coming year, and specialized areas dedicated to clinical, population, and bioinformatics cancer research. CINJ became an NCI-designated Clinical Cancer Center in 1997, followed by the NCI Comprehensive Cancer Center designation 5 years later, establishing CINJ as the only such facility in the state of New Jersey.
Research Activities
CINJ's researchers have made major scientific contributions toward the national goal of reducing the incidence of cancer and improving the outcomes for cancer patients. CINJ members, including scientists from UMDNJ-RWJMS and Rutgers University, work side by side with clinicians to ensure the rapid translation of laboratory results into new cancer therapies and prevention techniques. New therapies to kill cancer cells are being translated into novel clinical trials through research programs in breast and prostate cancers, malignant melanoma, and hematologic malignancies, and are being tested in a phase I clinical trials group. Collaborative research programs in cancer pharmacology, prevention and chemoprevention, and epidemiology have identified new therapies, markers of cancer risk, and preventive approaches, such as the utility of green tea in cancer prevention. CINJ is also developing expertise in cancer bioinformatics to facilitate the discovery of the next generation of diagnostics and therapeutics.
Patient Care Specialties
CINJ provides state-of-the-art clinical cancer care through disease-specific teams that offer sophisticated techniques in diagnosis, surgical options, chemotherapy, hormonal therapy, biological therapy, and radiation therapy. Special clinical expertise in areas such as urologic, gynecologic, and pediatric oncology, high-risk breast cancer, and cancer in young adults complements the institute's strengths in treatment of all major adult malignancies. And through a network of hospitals across New Jersey, CINJ ensures that all patients have access to the most advanced cancer treatments available.
Other Notable Programs
CINJ has an outstanding phase I clinical trials program offering more than 130 active clinical trials that use therapies developed at CINJ and through strong collaborative ties with the pharmaceutical and biotechnology industries. The institute has developed a Web site, New Jersey Cancer Trial Connect (www.njctc.org), which is available in English and Spanish, to match patients with cancer clinical research studies conducted in New Jersey. Since its inception, NJCTC has had more than 6 million page views.
|
 |
|