|
CDER 2007 Update
Pediatric Drug Development
President Bush signed into law the FDA Amendments Act of 2007 in September. This law reauthorizes and amends the Best Pharmaceuticals for Children Act of 2002 (BPCA) and the Pediatric Research Equity Act of 2003 (PREA). Both laws encourage more research into developing treatments for children. Some highlighted changes to BPCA and PREA are:
- Authorization to establish an internal review committee. The Pediatric Review Committee will review requests for waivers and deferrals, review pediatric assessments and pediatric plans prior to approval and pediatric written requests prior to issuance.
- The clinical, clinical pharmacology and statistical reviews are to be made public for applications submitted in response to both PREA and BPCA.
- Adverse event reporting now affects both PREA and BPCA. The review of reports has been modified to occur one year after labeling is approved.
Pediatric Research Equity Act. In 2007, CDER granted 86 waivers and 32 deferrals. There were 32 applications with PREA requirements fulfilled. As of June 2007, there have been nine PREA-related labels identified and posted. Since FDAAA, there were seven pediatric assessments approved, six deferrals and 23 waivers granted.
Pediatric Review Committee. In 2007, there were 23 CDER products reviewed by the committee.
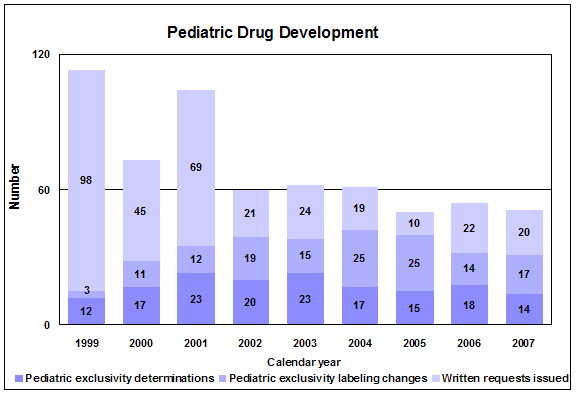
Pediatric exclusivity. We issued 20 on-patent written requests and one off-patent. During 2007, there were 14 exclusivity determinations, 13 of which were granted exclusivity and 17 labels with new pediatric information approved.
Improved safety, dosing information. The failure to produce drugs in dosage forms that can be taken by young children—such as liquids or chewable tablets—can deny children access to important medications. As a result of pediatric testing under BPCA, we now have 15 drugs with new pediatric formulations and seven drugs with recipes in their labels to provide directions for the pharmacist to compound an age-appropriate formulation.
Public disclosure. We have posted 83 summaries of the medical and clinical pharmacology reviews responsive to BPCA 2002. These summaries are located at http://www.fda.gov/cder/pediatric/Summaryreview.htm.
Adverse events reporting. Seventy-seven drugs have been presented to the Pediatric Advisory Committee. The law mandates review of all adverse event reports for a one-year period after pediatric exclusivity is granted.
2007 Pediatric exclusivity statistics
- 20 written requests issued
- 17 pediatric exclusivity labeling changes granted
- 14 exclusivity determinations made
Click image for larger view. Accessible text.
Maternal Health Team
The Maternal Health Team mission is to increase knowledge about the safe and effective use of medicines during pregnancy and breast-feeding. We encourage research in this area and provide scientific guidance to industry and FDA reviewers. When more information about medicine use during pregnancy and breastfeeding becomes available, we work with FDA reviewers and drug manufacturers to update medicine labels.
Women who are pregnant or breast-feeding often need to use prescription and/or over-the-counter medicines. During pregnancy, treating a disease or condition with a medicine may be safer for the woman and her baby than not treating the condition. It is important that health-care providers and pregnant women have the information they need to make the best medicine choices. Breastfeeding offers many health benefits to mother and baby, and these benefits should be considered along with the possible risks of infant exposure to medicine through breast milk.
In 1997, FDA started reviewing the pregnancy and breast-feeding sections of prescription medicine labels and the regulations that describe how the label is written and the information it must include. Based on feedback from government agencies, medical experts, clinicians and the public, FDA developed a new format for pregnancy and breast-feeding sections of medicine labels that will be presented to the public for comment as a proposed rule in the Federal Register.
Scientific guidance
- Determining the appropriate dose of a drug for pregnant women. In 2004, we published draft guidance for industry that provides a basic framework for designing, conducting and analyzing pharmacokinetic and pharmacodynamic studies in pregnant women.
- Evaluating study results on approved drugs when used during pregnancy. In 2005, we issued our final guidance for FDA reviewers about evaluating the effects of medicines on the growing fetus.
- Lactation studies in women. In 2005, we published draft guidance for industry describing a basic framework for designing, conducting and analyzing clinical lactation studies. We reviewed public comments received in response to this publication and held an Advisory Committee Meeting on November 29, 2007 to obtain expert advice.
- Pregnancy exposure registries. In 2002, we published a final guidance for industry that provides advice on how to establish a registry that prospectively monitors outcomes of pregnancies in women exposed to a specific drug. The Maternal Health Team is updating this guidance to clarify recommendations on reporting of major and minor congenital malformations, nonteratogenic endpoints, patient recruitment procedures and control group considerations.
Next Page
 Back
to Top
Back
to Top  CDER 2007 Update Table of Contents CDER 2007 Update Table of Contents
 PDF requires the free
Adobe Acrobat Reader PDF requires the free
Adobe Acrobat Reader
Date created: July 31, 2008 |