|
CDER 2007 Update
Drug Review
New Drug and Biologic Review
Drug review definitions
- Review and approval times. Review time is time spent examining the application. Approval time represents review time plus industry’s response time to our requests for additional information.
- Priority reviews. These products represent significant improvements compared with marketed products. We have a goal of reviewing 90 percent of these applications within six months.
- Standard reviews. These products have therapeutic qualities similar to those of already marketed products. We have a goal of reviewing 90 percent of these applications within 10 months.
- Actions and filings. An application is filed when we determine it is complete and accept it for review. We make a filing decision within 60 days of receiving an application. Approval is one of the actions that we can take once an application is filed. Another action is seeking more information from the sponsor. There is no direct connection between applications filed in one year and actions in the same year.
- Orphan drugs. We administer a program that provides incentives to develop drugs for use in patient populations of 200,000 or fewer. Sponsors of orphan drugs receive the following inducements: seven-year marketing exclusivity, tax credit for the product-associated clinical research, research design assistance from FDA and grants of up to $200,000 a year.
- Accelerated approval. This program makes products for serious or life-threatening diseases available earlier in the development process by relying on an effect on a surrogate end point to predict clinical benefit. An effect of the drug on a surrogate end point can be observed significantly sooner than can a long-term clinical benefit. Sponsors must perform additional studies to demonstrate long-term clinical benefit.
- Fast-track development. This program facilitates the development and expedites our review of new medicines that demonstrate the potential to address unmet medical needs for serious or life-threatening conditions. Fast track emphasizes our close, early communication with sponsors.
- Median times. Our charts show review and approval times as medians. The value for the median time is the number that falls in the middle of the group after the approval times are ranked in order. It provides a truer picture of our performance than average time, which can be unduly influenced by a few very long times. Our guide to understanding median approval time statistics is available at http://www.fda.gov/cder/present/MedianAPtime/index.htm.
- Tentative approval. This program is issued to the drug company when the application is approvable prior to the expiration of any patents or exclusivities accorded to the reference listed drug product. A tentative approval does not allow the applicant to market the product and postpones the final approval until all patent or exclusivity issues have expired.
- New Molecular Entities (NMEs) contain an active substance that has never before been approved for marketing in any form in the United States. Because of high interest in truly new medicines, we report approvals of NMEs and new biologic license applications (BLAs). The charts for all new drug applications (NDAs) and all BLAs include NMEs and new BLAs.
New drug applications
NDAs are the formal submissions of data that sponsors send us when they are seeking approval to market a new drug in the United States. Some NDAs are for NMEs; however, NDAs can also be for an active substance previously sold in a different form.
Biologic license applications
BLAs are the formal submissions of data that sponsors send us when they are seeking approval to market a biologic in the United States. A new BLA is an application for a biologic that has never been approved for marketing in the United States.
New drug and biologic review statistics
Beginning with 2004, our charts incorporate data on the review of therapeutic biologics transferred to us in late 2003. These include:
- Monoclonal antibodies
- Cytokines
- Growth factors
- Enzymes
- Other therapeutic immunotherapies
Additional review statistics are available at http://www.fda.gov/cder/rdmt/default.htm.
Approval totals in 2007:
- 78 drugs and biologics
- 18 truly new medicines
- 16 drug NMEs
- 2 new biologic NMEs
- 13 tentative NDA approvals
- 10 priority PEPFAR new combinations
- 3 standard tentative approvals
- 8 total orphan condition approvals
- 7 new drugs or biologics
- 4 priority reviews of new drugs (including 3 NMEs)
- 1 priority review of a new biologic
- 3 standard reviews of new drugs
- 4 new or expanded uses for orphan conditions
- 2 priority reviews for drugs
- 2 standard reviews for drugs
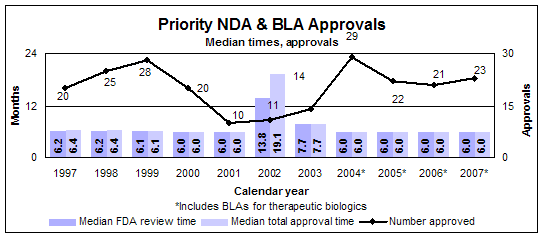
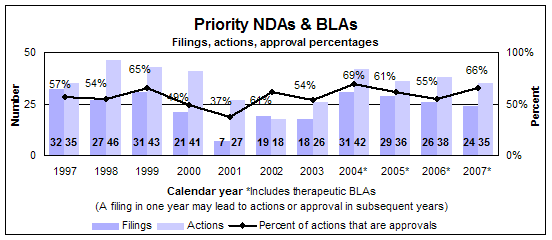
Priority new drugs and biologics
- 23 approvals
- Median review time: 6.0 months
- Median approval time: 6.0 months
- 24 filings
- 35 actions
Click image for larger view. Accessible text.
Click image for larger view. Accessible text.
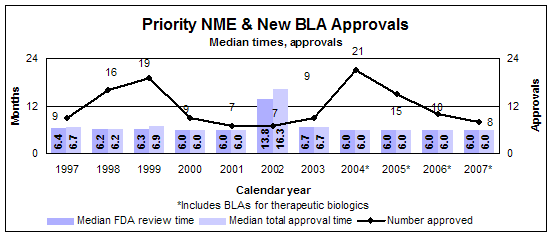
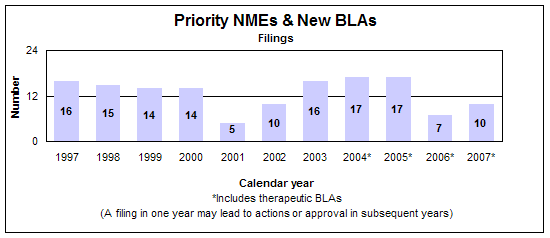
Priority new molecular entities and new biologics
- 8 approvals
- Median review time: 6.0 months
- Median approval time: 6.0 months
- 10 filings
Click image for larger view. Accessible text.
Click image for larger view. Accessible text.
Standard drugs and biologics
- 55 approvals (all NDAs)
- Median review time: 10.2 months
- Median approval time: 10.4 months
- 86 filings
- 125 actions
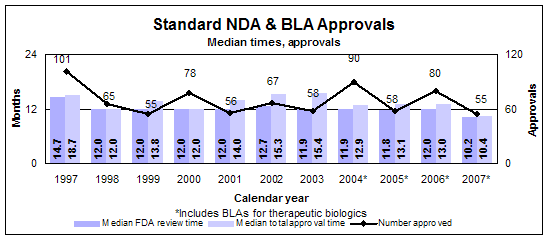
Click image for larger view. Accessible text.
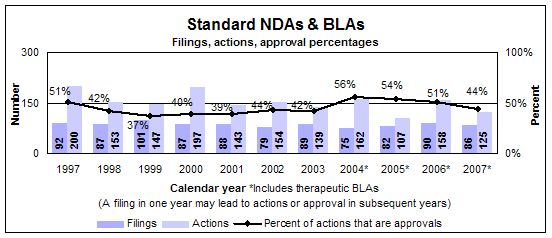
Click image for larger view. Accessible text.
Standard new molecular entities and new biologics
- 10 approvals (all NMEs)
- Median review time: 12.9 months
- Median approval time: 12.9 months
- 26 filings

Click image for larger view. Accessible text.
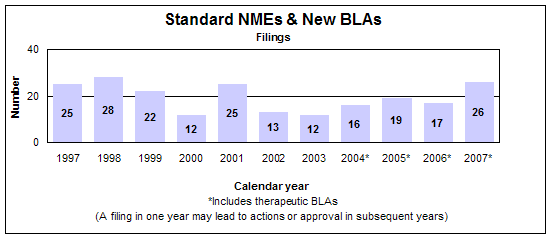
Click image for larger view. Accessible text.
New or Expanded Use Review
Applications for a new or expanded use, often representing important new treatment options, are formally called efficacy supplements to the original new drug application. We have a goal of reviewing standard supplements in 10 months and priority supplements in six months.
Approval totals
- 127 reviews of drugs and biologics
- 119 reviews of drugs
- 8 reviews of biologics
Priority new or expanded uses (efficacy supplements)
- 36 approvals
- 33 reviews of drugs
- 3 reviews of biologics
- Median review time: 7.6 months
- Median approval time: 6.0 months
- 42 actions

Click image for larger view. Accessible text.
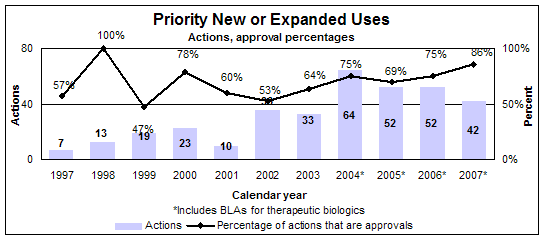
Click image for larger view. Accessible text.
Standard new or expanded uses (efficacy supplements)
- 91 approvals
- 86 reviews of drugs
- 5 reviews of biologics
- Median review time: 10.0 months
- Median approval time: 11.8 months
- 109 actions
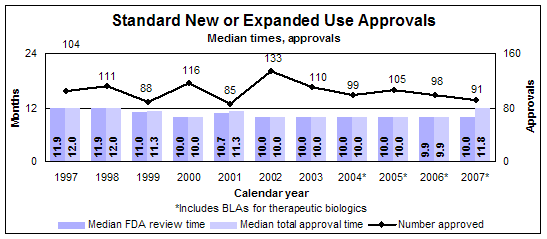
Click image for larger view. Accessible text.
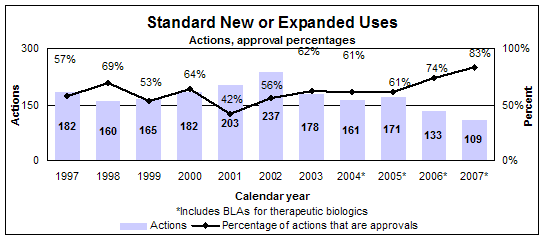
Click image for larger view. Accessible text.
Next Page
 Back
to Top
Back
to Top  CDER 2007 Update Table of Contents CDER 2007 Update Table of Contents
 PDF requires the free
Adobe Acrobat Reader PDF requires the free
Adobe Acrobat Reader
Date created: July 31, 2008 |