|
Chapter 13A
Staphylococcal Enterotoxins:
![]()
Among the metabolites produced by Staphylococcus aureus, and other staphylo-coccal spp., enterotoxins (5,16,27) present the greatest foodborne risk to the health of consumers. Staphylococcal enterotoxins are basic proteins produced by certain Staphylococcus strains in a variety of environments, including food substrates. These structurally-related, toxicologically similar proteins are produced primarily by Staphylococcus aureus, although S. intermedius and S. hyicus also have been shown to be enterotoxigenic (1). Normally considered a veterinary pathogen (36,40), S. intermedius was isolated from butter blend and margarine in a food poisoning outbreak (15,29). A coagulase negative S. epidermidis was reported to have caused least one outbreak (17). These incidents support testing staphylococci other than S. aureus for enterotoxigenicity, if they are present in large numbers in a food suspected of causing a food poisoning outbreak.
When large numbers of enterotoxigenic staphylococci grow in foods, they may elaborate enough toxin to cause food poisoning after the foods are ingested. The most common symptoms of staphylococcal food poisoning, which usually begin 2-6 h after contaminated food is consumed, are nausea, vomiting, acute prostration, and abdominal cramps. To date, 8 enterotoxins (types A, B, C1, C2, C3, D, E, and H) have been identified as distinct serological entities. Current methods to detect enterotoxins use specific polyclonal or monoclonal antibodies (33,42,43).
The threshold amount of enterotoxin for causing illness in humans is not known. However, information from food poisoning outbreaks (16,25) and human challenge studies (24) indicates that individuals experiencing illness probably consumed at least 100 ng of enterotoxin A, the serotype most frequently involved in foodborne staphylococcal illness (20). The microslide gel double diffusion technique requires at least 30-60 ng of enterotoxin per gram of food. Chromatographic purification and concentration are used to achieve this toxin concentration so that the serological assay can be performed (4).
The microslide method is approved by AOAC International (4) and is the current standard for evaluating new methods. Other methods used for food extracts should be at least as sensitive as the microslide method, which requires concentrating extracts from 100 g food in as much as 600 ml to about 0.2 ml. Less sensitive methods are inadequate.
Techniques such as radioimmunoassay (RIA), agglutination, and enzyme-linked immunosorbent assay (ELISA), require less concentration of the food extracts ; thus, they save time and are more sensitive. Latex agglutination (16) appears promising as a serological tool for identifying staphylococcal enterotoxins. Several ELISA methods (26,28,30,32,37,38,39) have been proposed for the identification of enterotoxins in foods, but, except for a polyvalent ELISA (7,9), their specificity has not been studied extensively. Among ELISA methods, the "double antibody sandwich" ELISA is the method of choice, because reagents are commercially available in polyvalent and monovalent formats for both toxin screening and serotype specific identification(22). An automated enzyme-linked fluorescent immunoassay (ELFA) has been developed and is commercially available. This method has undergone specificity and sensitivity evaluations and has proven to be an effective serological system for the identification of staphylococcal enterotoxin in a wide variety of foods (14). Other methods, which have been used in the identification of the staphylococcal enterotoxins and may have application in foods, are the T-cell proliferation assay (35), and polyacrylamide gel electrophoresis (PAGE) combined with Western blotting (2).
Examining staphylococci isolated from foods for enterotoxin production helps establish potential sources of enterotoxin in foods. Of the methods developed for laboratory testing of enterotoxin production, the semisolid agar procedure (19) is approved by AOAC International. It is simple to perform and requires minimal, routine laboratory equipment. Another simple approach is the use of pH 5.5 brain heart infusion (BHI) broth (14). The major problem with identifying enterotoxins in foods is that minute concentrations are sufficient to cause food poisoning. Pasteurization and thermal processing may render most toxin serologically unreactive. Consequently, false negatives may result, if detection methods lack sufficient sensitivity to detect active toxin (6).
This chapter presents a technique for the routine culturing of suspect staphylococci, procedures for the extraction of enterotoxin from foods and selected serological methods (Microslide gel double diffusion precipitation test, two manual ELISAs [TecraTM, TransiaTM], an automated qualitative "enzyme-linked fluorescent immunoassay" [ELFATM, VidasTM], and sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]-immunoblotting) for the identification of staphylococcal enterotoxin from isolates and from foods.
Recommended for routine analysis of foods for staphylococcal enterotoxin is the use, initially, of two different polyvalent ELISA kits. If results from different polyvalent ELISA methods yield conflicting results, retest using another method (e.g., another polyvalent ELISA method or the SDS-polyacrylamide gel electrophoresis-immunoblot assay for S. aureus enterotoxin A). Methods were developed to restore serological activity to heat-altered toxin in extracts of heat-processed foods (3,10,11,12,18,41,44). However, current toxin detection assays (described above) are sensitive enough to detect unaltered toxin that may persist after heat without such treatment (2).
| These procedures are to be performed with extreme caution. Staphylococcal enterotoxins are highly toxic and procedures that may create aerosols should be performed in appropriate containment facilities, such as a biosafety hood. |
Chromatographic Separation of Toxin from Foods for Micro-Slide Double Diffusion
Note: Chloroform is hazardous. Wear gloves, avoid contact with skin, and perform extraction in a chemical fume hood.
Note: This procedure and other procedures that may generate aerosols of pathogenic microorganisms should be performed in an approved biohazard hood. |
Grind 100 g food in Waring blender at high speed for 3 min with 500 ml 0.2 M NaCl. Use Omnimixer for smaller quantities. Adjust pH to 7.5 with 1 N NaOH or HCl if food is highly buffered, and 0.1 N NaOH or HCl if food is weakly buffered (e.g., custards). Let slurry stand for 10 to 15 min, recheck pH, and readjust if necessary.
Transfer slurry to two 285 ml stainless steel centrifuge bottles. Centrifuge at 16,300 x g for 20 min at 5°C. Lower speeds with longer centrifuge time can be used, but clearing of some foods is not as effective. Separation of fatty materials is ineffective unless food is centrifuged at refrigeration temperature. Decant supernatant fluid into 800 ml beaker through cheesecloth or other suitable filtering material placed in a funnel. Re-extract residue with 125 ml of 0.2 M NaCl by blending for 3 min. Adjust pH to 7.5 if necessary. Centrifuge at 27,300 x g for 20 min at 5°C. Filter supernatant through cheesecloth, and pool filtrate with original extract.
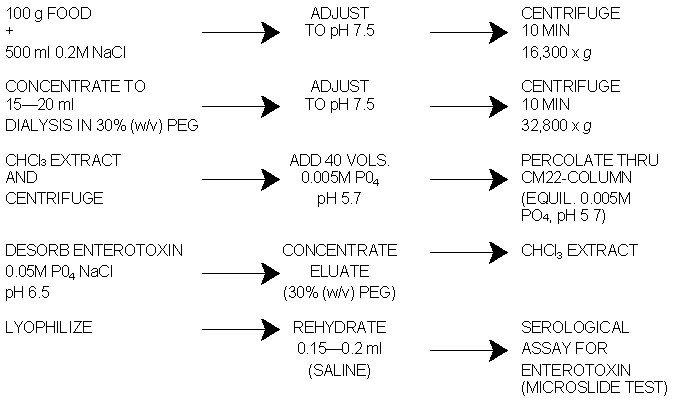
Fig. 1 Schematic diagram for the extraction and serological assay of enterotoxin in food.
Place pooled extracts in dialysis sac. Immerse sac in 30% (w/v) PEG at 5°C until volume is reduced to 15-20 ml or less (usually overnight). Remove sac from PEG and wash outside thoroughly with cold tap water to remove any PEG adhering to sac. Soak in distilled water for 1-2 min and in 0.2 M NaCl for a few min. Pour contents into small beaker.
Rinse inside of sac with 2-3 ml 0.2 M NaCl by running fingers up and down outside of sac to remove material adhering to sides of tubing. Repeat rinsing until rinse is clear. Keep volume as small as possible.
Adjust pH of extract to 7.5. Centrifuge at 32,800 x g for lO min. Decant supernatant fluid into graduated cylinder to measure volume. Add extract with < to = volume of CHCl3 to separatory funnel. Shake vigorously 10 times through 90 degree arc. Centrifuge CHCl3 extract mixture at 16,300 x g for 10 min at 5°C. Return fluid layers to separatory funnel. Draw off CHCl3 layer from bottom of separatory funnel, and discard. Measure volume of water layer and dilute with 40 volumes of 0.005 M sodium phosphate buffer, pH 5.7. Adjust pH to 5.7 with 0.005 M H3PO4 or 0.005 M Na2HPO4. Place diluted solution in 2 liter separatory funnel.
Place stopper (attached to bottom of separatory funnel) loosely into top with liquid from separatory funnel. Tighten stopper in top of tube and open stopcock of separatory funnel. Let fluid percolate through CMC column at 5°C at 1-2 ml/min by adjusting flow rate with stopcock at bottom of column so that percolation can be completed overnight. If all liquid has not passed through column overnight, stop flow when liquid level reaches glass wool layer. If all liquid has passed through overnight, rehydrate column with 25 ml distilled water.
After percolation is complete, wash CMC column with 100 ml 0.005 M sodium phosphate buffer (1-2 ml/min); stop flow when liquid level reaches glass wool layer. Discard wash. Elute enterotoxin from CMC column with 200 ml 0.05 M sodium phosphate buffer, pH 6.5 (0.05 M phosphate-0.05 M NaCl buffer, pH 6.5), at flow rate of 1-2 ml/min at room temperature. Force last of liquid from CMC by applying air pressure to top of chromatographic tube.
Place eluate in dialysis sac. Place sac in 30% (w/v) PEG at 5°C and concentrate almost to dryness. Remove sac from PEG and wash. Soak sac in 0.2 M phosphate buffer, pH 7.4. Remove concentrated material from sac by rinsing 5 times with 2-3 ml 0.01 M sodium phosphate buffer, pH 7.4-7.5. Extract concentrated solution with CHCl3. Repeat CHCl3 extractions until precipitate is so lacy that it falls apart in CHCl3 layer in cheesecloth.
Place extract in short dialysis sac (about 15 cm). Place sac in 30% (w/v) PEG, and let it remain until all liquid is removed from inside sac (usually overnight). Remove sac from PEG and wash outside with tap water. Place sac in distilled water for 1-2 min. Remove contents by rinsing inside of sac with 1 ml portions of distilled water. Keep volume below 5 ml. Place rinsings in test tube (18 x 100 mm) or other suitable container and freeze-dry. Dissolve freeze-dried test sample in as small an amount of saline as possible (0.1-0.15 ml). Check for enterotoxins by microslide method.

To examine foods, use procedures described for detecting coagulase- positive staphylococci (see Chapter 12). Test isolates for enterotoxigenicity as described in E, below. To examine food in a suspected staphylococcal food poisoning outbreak, however, the following method is recommended:
NOTE: To determine presence of enterotoxin producers in food, add enough 0.2 M NaCl to slurry (1:5 dilution) to obtain 1:6 dilution, e.g., add additional 100 ml of 0.2 M NaCl to 1:5 dilution of slurry containing food and 400 ml of 0.2 M NaCl.
Turbidity of suspension should be equivalent to No. 1 on McFarland nephelometer scale (approx. 3.00 x 108 organisms/ml). Using sterile 1.0 ml pipet, spread 4 drops of aqueous culture suspension over entire surface of BHI agar plate with sterile spreader and incubate at 35°C. Good surface growth is obtained after 48 h incubation, when pH of culture should have risen to 8.0 or higher. Transfer contents of petri dish to 50 ml centrifuge tube with wooden applicator stick or equivalent. Remove agar and organisms by high speed centrifugation (10 min at 32,800 x g). Examine supernatant for presence of enterotoxin by filling depots in slide gel diffusion assembly (see E, below).

Figure 3. Arrangement of antiserum (antisera) and homologous reference enterotoxins for assay of test preparation(s) for presence of 2 serologically distinct enterotoxins (simultaneously (bivalent detections system) or for assay of dilutions of a test preparation system (monovalent detection system).
Adjust dilutions of reagents to give distinct but faint lines of precipitation for maximum sensitivity (see C-9, above). Prepare control slide with only reference toxin and antitoxin. Fill wells to convexity with reagents, using Pasteur pipet (prepared by drawing out glass tubing of about 7 mm outside diameter) or disposable 30 or 40 1 pipet. Remove bubbles from all wells by probing with fine glass rod. Make rods by pulling glass tubing very fine, as for capillary pipets; break into 2-1/2 inch lengths and melt ends in flame. It is best to fill wells and remove bubbles against a dark background. Insert rods into all wells to remove trapped air bubbles that may not be visible. Before examination , keep slides in covered petri dishes containing moist sponge strips at room temperature for 48-72 h or at 37°C for 24 h

Fig. 4. Microslide gel diffusion test as bivalent detection system. Antisera to staphylococcal enterotoxins A and B are in well 1; known reference enterotoxins A and B are in wells 3 and 5, respectively, to produce reference lines of A and B; test preparations are in wells 2 and 4. Interpret 4 reactions as follows: (1) No line development between test preparations and antisera--absence of enterotoxins A and B; (2) coalescence of preparation line from well 4 with enterotoxin A reference line (intersection of test preparation line with enterotoxin B reference line)-- absence of enterotoxins A and B in well 2, presence of enterotoxin A and absence of enterotoxin B in well 4; (3) presence of enterotoxin A and absence of enterotoxin B in both test preparations; and (4) absence of enterotoxins A and B in test preparation in well 2, presence of enterotoxins A and B in well 4.
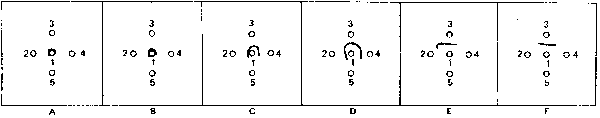
Fig. 5. Effect of amount of staphylococcal enterotoxin in test preparation on development of reference line of precipitation. Diagram A demonstrates inhibition (suppression) of reference line when 10 and 4 µg enterotoxin/ml, respectively, are used. Diagrams B-E show precipitate patterns when successively less enterotoxin (test preparation) is used. Diagram F shows typical formation of reference line of precipitation observed in slide test control system.
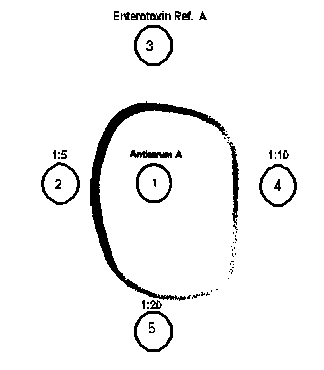
Fig. 6. Microslide gel double diffusion tests as monovalent detection system in which varying dilutions of test preparation are assayed for the presence of staphylococcal enterotoxin.
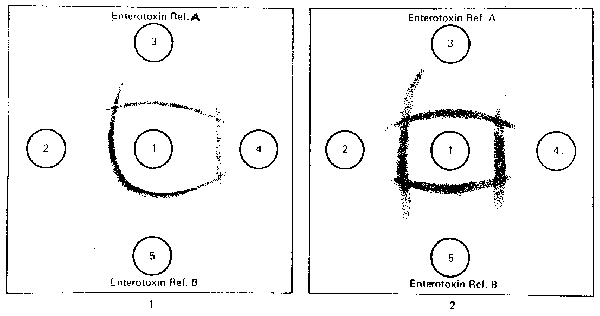
Fig. 7. S. aureus enterotoxin: Precipitate patterns in microslide gel diffusion test demonstrate nonspecific (atypical) lines of precipitation caused by other antigens reacting with antienterotoxin antibodies. In pattern 1, test preparation in well 4 produces atypical reaction indicated by nonspecific line of precipitation (lines of nonidentity with enterotoxin references A and B) which intersects with enterotoxin reference lines. In pattern 2, both test preparations (wells 2 and 4) are negative for enterotoxins A and B but, produce nonspecific lines of precipitation, which intersect enterotoxin A and B reference lines of precipitation.
When examining processed foods with obvious can defects which might result in the growth of organisms that produce peroxidase, test the extract for peroxidase production and inactivate as described above before testing for staphylococcal enterotoxin.
Note: Raw food (e.g., vegetables), see, General Precautions, above. Follow directions under 5. Other Foods, below
Visual ELISA: Polyvalent (Types A-E) Screening for Determining
Enterotoxigenicity and Identifying Staphylococcal Enterotoxins in Foods
This visual immunoassay provides a rapid (4 h), sensitive (1.0 ng or greater per ml or g), specific screening test for the simultaneous identification of staphylococcal enterotoxins A-E. However, this kit cannot be used to distinguish among specific toxin serotypes. The ELISA is performed in a "sandwich" configuration. The kit is commercially available as TECRATM (TECRA Diagnostics, 28 Barcoo St., NSW, P.0. Box 20, Roseville, 2069, Australia) and is distributed by International Bioproducts Inc., 14796 N.E. 95th St., Redmond, WA 98052. This method has been adopted "First Action" by AOAC International (13).
Materials supplied in kit:
Materials/equipment supplied by user:
Materials supplied in kit:
Reagents supplied by user:
Secure desired number of anti-SET antibody-coated Removawells in holder provided. Allow 1 well for each food sample, 1 well for negative control, and 1 well for positive control. Additional wells are required if optional positive (food) and negative controls are prepared. Fill each well with wash solution and let stand 10 min at room temperature (20-25°C). Empty wells by quickly inverting holder; remove residual liquid by firmly striking holder face-down on paper towel several times.
Transfer 200 µl aliquots of controls and samples (food extracts or culture fluids) into individual wells; record position of each sample on sample record sheet (original provided in kit). Gently tap holder containing test wells to ensure homogeneous distribution and contact of test material with walls of wells. Agitation of wells on microtiter plate shaker for 30 s is optional. To prevent evaporation, cover wells with plastic film or plate sealers (Dynex Technologies, Inc., 14340 Sullyfield Circle, Chantilly, VA 20151-1683) and incubate 2 h at 35-37°C. Wash well liberally with wash solution from squeeze bottles as follows: Press Removawells firmly into holder. Quickly invert holder, emptying contents into trough containing 2% (v/v) sodium hypochlorite. Remove residual liquid by firmly striking holder face-down on paper towel several times. Completely fill each well with wash solution. Repeat liberal washing 2-3 more times. Finally, empty wells.
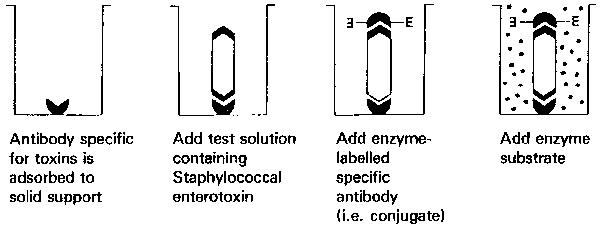
Figure 8. Typical double antibody "sandwich" ELISA scheme.
Add 200 µl reconstituted enzyme conjugate into each well. Cover tray and incubate 1 h at room temperature (20-25°C). Empty wells and wash them thoroughly 5 times, as above. Empty wells and remove residual liquid as described above.
Add 200 µl reconstituted substrate to each well. Leave at room temperature (20-25°C) for at least 30 min until positive control reaches absorbance greater than 1.0 or color darker than panel No. 4 on Color Comparator. Color development tends to concentrate around edge of wells. For accurate results, tap sides of plate gently to mix contents before reading. Add 20 µl of stop solution to each well. Tap sides of plate gently to mix contents. Assay is now complete. Determine results visually or with microtiter tray reader.
Sample is considered positive when the following criteria are met:
(1) negative control is within negative range on Color Comparator, and
(2) sample has green (or blue) color greater than negative range on Color Comparator.
Sample is considered negative for enterotoxin when the following criteria are met:
(1) negative control is within negative range on Color Comparator, and
(2) sample is colorless or has color within negative range on Color Comparator.
Sample is considered positive if absorbance is > 0.200.
Sample is considered negative if absorbance is <= 0.200.
Generally, culture fluids that contain toxin have absorbance readings significantly greater than 0.200. Some strains of S. aureus produce intrinsic peroxidase, which can be inactivated with sodium azide.
If confirmation of serotype by the AOAC method is necessary, use CHCl3 to extract the food extract as previously described, and proceed with remaining steps in procedure. Analyze chromatographed eluate with microslide gel double diffusion test. A faster and more sensitive option would be confirmation by SDS-PAGE-Western blotting described in this Chapter.
This kit can be purchased from biomerieux Vitek, Inc.,545 Anglum Dr., Hazelwood, Missouri 63042-2395.
Materials and reagents supplied in kit:
The SET Reagent Strip (refer to the table below) is a polypropylene strip of 10 wells covered with a foil seal and paper label. The first well of the strip is for the sample. The last well of the strip, an optically clear cuvette, is for the fluorometric determination. The eight wells in the center of the strip contain the various reagents for the assay. (See description of reagent strip below).
| DESCRIPTION OF THE STAPH ENTEROTOXIN REAGENT STRIP | |
| Wells | Reagents |
|---|---|
| 1 | Sample Well: 0.5 ml of food extract is placed into the well |
| 2 | Pre-Wash Solution (0.4 ml): TBS - Tween with 0.1% (w/v) sodium azide |
| 3-4-5-7-8-9 | Wash Solution (0.6 ml): TBS - Tween with 0.1% (w/v) sodium azide |
| 6 | Conjugate (0.4 ml): alkaline phosphatase labeled polyclonal antibodies with 0.1% (w/v) sodium azide |
| 10 | Cuvette with substrate (0.3 ml): 4-methyl-umbelliferyl phosphate with 0.1% (w/v) sodium azide |
| The name of the test, the lot number, and the expiration date of the kit are included on a bar code which is printed on the SET Reagent Strip. The test identification, lot number and calibration parameters are both clearly indicated in the kit's specification sheet and printed with a bar code. | |
The interior of the SET SPR is coated at the time of manufacture with anti-enterotoxin antibodies.
Purified staphylococcal enterotoxin B (5 ng/ml) with 0.1% (w/v) sodium azide and protein stabilizers.
CAUTION: HANDLE WITH CARE!
Purified staphylococcal enterotoxin B (5 ng/ml) with 0.1% (w/v) sodium azide and protein stabilizers. Control range indicated on the vial label. CAUTION: HANDLE WITH CARE!
TRIS buffered saline (TBS) - Tween with 0.1% (w/v) sodium azide.
2.5 mol/1 TRIS - 1% (w/v) Tween with 1% (w/v) sodium azide.
MATERIALS REQUIRED BY USER BUT NOT PROVIDED IN KIT:
Staphylococcal enterotoxins A, B, C1, C2, C3, D, E are detected by the VIDAS SET Assay at the sensitivity of at least 1 ng/ml.
In addition to the food extraction procedures described here, a greater variety of food extraction procedures are described by the kit manufacturer. Prepare food extracts immediately before testing.
Dispense 500 µl of control reagent provided in the kit. Run positive control whenever assay is performed to indicate that all reagents are functional and that the assay has been conducted correctly.
Use negative control solution provided in kit. No dilution of negative control solution is necessary. Add 500 µl of negative control reagent to test strip.
Add aliquot of positive control provided in kit to known enterotoxin-negative food product to serve as positive food control. Extract and assay sample under same conditions as suspect sample.
Use same type of food as suspect food, but which is known to be toxin-free. Prepare negative food control in exactly the same manner as suspect food. This control will ensure that washing of wells was adequate and that no food components will interfere with test results. Extract and assay sample under same conditions as suspect sample.
Important: A standard must be run in duplicate for every lot of kits. The result is stored in the computer and automatically used for assay analysis. A standard may be run with each SET work list, or a stored standard result (stored in the computer) may be used. See the VIDAS Operator's Manual for complete instructions.
A positive and negative control are provided to validate kit performance.
Test the positive and negative controls with each new lot or shipment to ensure that assay performance has remained unimpaired throughout shipping and storage. Test the controls as specified by your laboratory's regulatory guidelines. Controls are provided in ready-to-use form and must be thoroughly mixed and pipetted directly into the sample well of a reagent strip.
The expected positive control value will be: included in the range indicated on the vial label. If the results from testing the controls do not fall within this range, do not report sample results. NOTE: if the standard is out of range, the test value can be recalculated with another standard. See the VIDAS Operator's Manual for complete information.
Two instrument readings for fluorescence in the Reagent Strips's optical cuvette are taken for each specimen tested. The first reading is a background reading of the cuvette and substrate before the SPR is introduced into the substrate. The second reading is taken after the substrate has been exposed to the enzyme conjugate remaining on the interior of the SPR. The background reading is subtracted from the final reading to give a Relative Fluorescence Value (RFV) for the test result. A test value is generated for each sample by forming a ratio from the RFV of the sample to that of a standard. Test values from test samples and control samples are compared to a set of thresholds stored in the computer. The table below shows the thresholds and the interpreted results.
| Test Value Threshold | Interpretation |
| < 0.13 | Negative |
| >0.13 | Positive |
A report is printed that records the type of test performed, the sample identification, the date and time, the lot number and expiration date of the reagent kit being used and each sample's RFV, test value and interpreted result.
Results with test values less than the low threshold indicate sample without detectable enterotoxin. Samples with test values greater than (or equal to) the high threshold are reported as positive.
Invalid results are reported when the background reading is above a pre-determined cut-off (indicating low-level substrate contamination). In this case, repeat the assay with the original sample.
An invalid result is also seen if there is no standard available for the lot number of the sample test strip. In this case, run a standard in duplicate in SET strips with the same lot number as the invalid sample test. The sample test result can then be recalculated using the new stored standard. See the VIDAS Operator's Manual for complete information.
If confirmation of serotype by the AOAC method is necessary, use CHCl3 to extract the food extract as previously described, and proceed with remaining steps in procedure. Analyze chromatographed eluate with microslide gel double diffusion test. A more sensitive and faster option is the SDS-PAGE-Western blotting method described in this Chapter.
This kit is produced by Transia-Diffchamb S.A. Lyon, France and is distributed by Idetek, Inc., Sunnyvale, CA. in the U.S.A.
Materials and reagents supplied in kit:
CAUTION: use gloves to handle.
Equipment
- Chlorine bleach or 1N soda solution.
For preparation of 1 liter, add 30.28 g TRIS hydroxymethy - aminomethane to approx. 800 ml. of distilled H20.Adjust to pH 8.0 and adjust volume to 1 liter.
Dissolve 30 g of polyethylene glycol (PEG) in 100 ml of distilled water or use dry flakes of PEG
Bleach: dilute 50 ml of concentrated bleach in 950 ml of water.
NaOH 1N: dissolve 40 g in 1 liter of distilled water.
In addition to the food extraction procedures described here, a greater variety of food extraction procedures are presented in manufacturer's directions.
NOTE: Prepare food extracts immediately before testing.
Positive control solution is prepared by adding 10 µl positive control to 500 µl of wash buffer in a polypropylene tube. Run positive control whenever assay is run to verify that all reagents are functional and that assay has been conducted correctly. Discard unused diluted toxin control into sodium hypochlorite solution.
Use negative control solution provided in kit. No dilution of negative control solution is necessary. Use 500 µl of all controls.
Add aliquot of positive control provided in kit to known enterotoxin-negative food product to serve as positive food control. Extract and assay sample under same conditions as suspect sample.
Use same type of food as suspect food, but which is known to be toxin-free. Prepare negative food control in exactly the same manner as suspect food. This control will ensure that washing of wells was adequate and that no food components will interfere with test results. Extract and assay sample under same conditions as suspect sample.
Recommendation for Use
Immunoenzymatic test
See immunoenzymatic test flow chart (Figure 9)
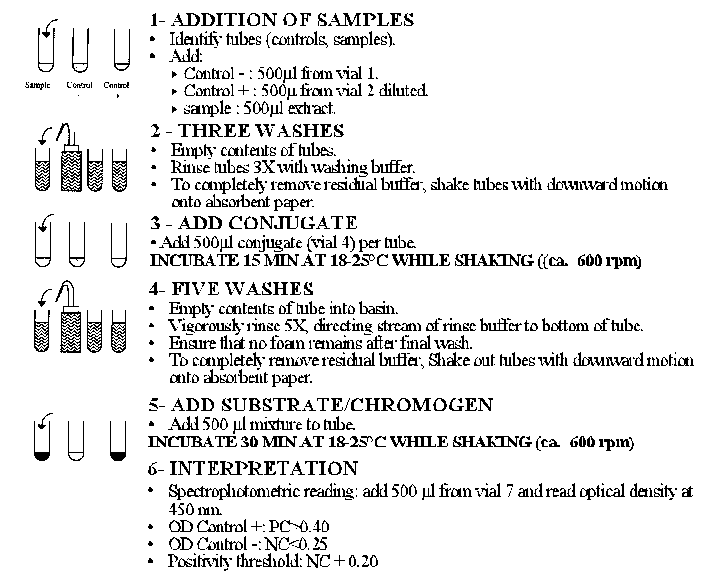
Figure 9. Scheme for Immunoenzymatic test.
Allow:
1 tube for the negative control (Vial 1),
1 tube for the positive control (Vial 2),
1 tube per sample.
NOTE: Increasing the first incubation period from 15 to 60 min improves the detection of enterotoxins.
NB: Separate addition of the substrate and the chromogen can be done: distribute successively 250 µl of substrate (Vial 5) and then 250 µl of chromogen (Vial 6).
The optical density of the positive control (PC) has to be higher than or equal to 0.40. The optical density of the negative control (NC) has to be lower than or equal to 0.25. The test can be validated if the optical densities of the controls meet the requirements defined above. If not, start the test again.
Spectrophotometric reading
Read the optical densities at ![]() = 450 nm
against an air blank. If you
do not have a spectrophotometer for tubes, read the optical densities
after having transferred the contents of the tubes into identified
microcuvettes with 1 cm of optical pathway.
= 450 nm
against an air blank. If you
do not have a spectrophotometer for tubes, read the optical densities
after having transferred the contents of the tubes into identified
microcuvettes with 1 cm of optical pathway.
Microtiter plate reading
At FDA, a microtiter plate reader set at a wavelength of 450 nm is used to determine the optical density of each sample. This is accomplished by removing 200 µl of the test sample after addition of the stop solution and adding this volume (200 µl) to plain (unsensitized) flat bottom polystyrene microwells (Removawell, Dynatech) designed to fit a Removawell strip Holder (Dynatech Laboratories, Inc.). Place holder containing the sample extracts in microtiter plate reader and determine their absorbances. Record the results from the microtiter plate reader printout on the worksheet.
NOTE: Some food samples containing peroxidase, protein A or endogenous substances may interfere with this method. False positives are rare and inconsistent.
If confirmation of serotype by the AOAC method is necessary, use CHCl3 to extract as previously described, and proceed with remaining steps in procedures described in BAM. Analyze chromatographed eluate with microslide gel double diffusion test. A faster, more sensitive option would be to confirm with the SDS-PAGE-Western blotting method described in this chapter.
1. Adesiyun, A.A., S.R. Tatini, and D.G. Hoover. 1984. Production of enterotoxin(s) by Staphylococcus hyicus. Vet. Microbiol. 9:487-495.
2. Anderson, J.E., R.R. Beelman and S. Doores. 1996. Persistence of serological and biological activities of staphylococcal enterotoxin A in canned mushrooms. J. Fd. Prot. 59:1292-1299.
3. Anderson, J.E.1996. Survival of the serological and biological activities of staphylococcal enterotoxin A in canned mushrooms. UMI Dissertation Services, Ann Arbor, Michigan.
4. Association of Official Analytical Chemists. 1990. Official Methods of Analysis, 15th ed. AOAC, Gaithersburg, MD.
5. Baird-Parker, A.C. 1990. The staphylococci: An Introduction. J. Appl. bacterial Symp. Suppl. 15-85.
6. Bennett, R.W., and M.R. Berry, Jr. 1987. Serological reactivity and in vivo toxicity of Staphylococcus aureus enterotoxins A and D in selected canned foods. J. Food Sci. 52:416-418.
7. Bennett, R.W., and V. Atrache. 1989. Applicability of visual immunoassay for simultaneous indication of staphylococcal enterotoxin serotype presence in foods. ASM Abstracts, p. 28.
8. Bennett, R.W., D.L. Archer, and G. Lancette. 1988. Modified procedure to eliminate elution of food proteins under seroassay for staphylococcal enterotoxins. J. Food Safety 9:135-143.
9. Bennett, R.W., M. Ash, and V. Atrache. 1989. Visual screening with enzyme immunoassay for staphylococcal enterotoxins in foods: an interlaboratory study. AOAC Abstracts, p. 72.
10. Bennett, R.W. 1992. The biomolecular temperament of staphylococcal enterotoxins in thermally processed foods. J. Assoc. Off. Anal. Chem. 75:6-12.
11. Bennett, R.W., K. Catherwood, L.J. Luckey and N. Abhayaratna. 1993. Behavior and serological identification of staphylococcal enterotoxin in thermally processed mushrooms. In: S. Chang, J.A. Buswell and S. Chiu (eds.). Mushroom Biology and Mushroom Products. Chapter 21 (p. 193-207). The Chinese University Press, Hong Kong.
12. Bennett, R.W. 1994. Urea renaturation and identification of staphylococcal enterotoxin. In: R.C. Spencer, E.P. Wright and S.W.B. Newsom (eds.) RAMI-93. Rapid Methods and Automation in Microbiology and Immunology. Intercept Limited, Andover, Hampshire, England.
13. Bennett, R.W. and F. McClure. 1994. Visual screening with immunoassay for staphylococcal enterotoxins in foods: Collaborative study. JAOAC International. 77:357-364.
14. Bennett, R.W. and R.N. Matthews. 1995. Evaluation of polyvalent ELISA's for the identification of staphylococcal enterotoxin in foods. AOAC International Abstracts 1995:17-B-016.
15. Bennett, R.W. 1996. Atypical toxigenic Staphylococcus and Non-Staphylococcus aureus species on the Horizon? An Update. J. Food Protection. 59:1123-1126.
16. Bergdoll, M.S. 1990. Staphylococcal food poisoning. In Foodborne Diseases. D.O. Cliver (Ed.) Academic Press, Inc. San Diego, CA. p. 86-106.
17. Breckinridge, J.C., and M.S. Bergdoll. 1971. Outbreak of foodborne gastroenteritis due to a coagulase negative enterotoxin producing staphylococcus. N. Engl. J. Med. 248:541-543.
18. Brunner, K.G. and A.C.L. Wong. 1992. Staphylococcus aureus growth and enterotoxin production in mushrooms. J. Food Sci. 57:700-7033.
19. Casman, E.P., and R.W. Bennett. 1963. Culture medium for the production of staphylococcal enterotoxin A. J. Bacteriol. 86:18-23.
20. Casman, E.P., R.W. Bennett, A.E. Dorsey, and J.A. Issa. 1967. Identification of a fourth staphylococcal enterotoxin--enterotoxin D. J. Bacteriol. 94:1875-1882.
21. Casman, E.P., R.W. Bennett, A.E. Dorsey, and J.E. Stone. 1969. The microslide gel double diffusion test for the detection and assay of staphylococcal enterotoxins. Health Lab. Sci. 6:185-198.
22. Chen Su, Yi and A.C.L. Wong. 1997. Current perspectives on detection of staphylococcal enterotoxins. J. Fd. Prot. 60:195-202.
23. Crowle, A.J. 1958. A simplified micro double-diffusion agar precipitin technique. J. Lab. Clin. Med. 52:784-787.
24. Dangerfield, H.G. 1973. Effects of enterotoxins after ingestion by humans. Presented at the 73rd Annual Meeting of the American Society for Microbiology. May 6-11, Miami Beach, FL.
25. Evenson, M.L., M.W. Hinds, R.S. Berstein, and M.S. Bergdoll. 1988. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak in staphylococcal food poisoning involving chocolate milk. Int. J. Food Microbiol. 7:311-316.
26. Freed, R.C., M.L. Evenson, R.F. Reiser, and M.S. Bergdoll. 1982. Enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. Appl. Environ. Microbiol. 44:1349-1355.
27. Genigeorgis, C.A. 1989. Present state of knowledge on staphylococcal intoxication. Int. J. Food Microbiol. 9:327-360.
28. Kauffman, P.E. 1980. Enzyme immunoassay for staphylococcal enterotoxin A. J. Assoc. Off. Anal. Chem. 63:1138-1143.
29. Khambaty, F.M., R.W. Bennett, and D.B. Shah. 1994. Application of pulsed field gel electrophoresis to the epidemiological characterization of Staphylococcus intermedius implicated in a food-related outbreak. Epidemiol. Infect. 113:75-81.
30. Kuo, J.K.S., and G.J. Silverman. 1980. Application of enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. J. Food Prot. 43:404-407.
31. McFarland, J. 1907. The nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. 49:1176-1178.
32. Notermans, S., H.L. Verjans, J. Bol, and M. Van Schothorst. 1978. Enzyme-linked immunosorbent assay (ELISA) for determination of Staphylococcus aureus enterotoxin type B. Health Lab. Sci. 15:28-31.
33. Notermans, S., R. Boot, and S.R. Tatini. 1987. Selection of monoclonal antibodies for detection of staphylococcal enterotoxin in heat processed foods. Int. J. Food Microbiol. 5:49-55.
34. Oda, T. 1978. Application of SP-Sephadex chromatography to the purification of staphylococcal enterotoxins A, B, C2. Jpn. J. Bacteriol. 33:743-752.
35. Rasooly, L., R. Noel, D.B. Shah and A. Rasooly. 1997. In vitro assay of Staphylococcus aureus enterotoxin activity in food. Appl. Environ. Microbiol. 63:2361-2365.
36. Raus, J., and D.N. Love. 1983. Characterization of coagulase-positive Staphylococcus intermedius and Staphylococcus aureus isolated from veterinary clinical specimens. J. Clin. Microbiol. 18:789-792.
37. Saunders, G.C., and M.L. Bartlett. 1977. Double-antibody solid-phase enzyme immunoassay for the detection of staphylococcal enterotoxin A. Appl. Environ. Microbiol. 34:518-522.
38. Simon, E., and G. Terplan. 1977. Nachweis von staphylokokken enterotoxin B Mittles ELISA-test. Zentralbl. Veterinaemed. Reihe B. 24:842-844.
39. Stiffler-Rosenberg, G., and H. Fey. 1978. Simple assay for staphylococcal enterotoxins A, B, and C: modification of enzyme-linked immunosorbent assay. J. Clin. Microbiol. 8:473-479.
40. Talan, D.A., D. Staatz, E.J. Goldstein, K. Singer, and G.D. Overturf. 1989. Staphylococcus intermedius in canine gingiva and canine-inflicted human wound infections: laboratory characterization of a newly recognized zoonotic pathogen. J. Clin. Microbiol. 27:78-81.
41. Tatini, S.R. 1976. Thermal stability of enterotoxins in food. J. Milk Food Technol. 39:432-438.
42. Thompson, N.E., M.S. Bergdoll, R.F. Meyer, R.W. Bennett, L. Miller, and J.D. MacMillian. 1985. Monoclonal antibodies to the enterotoxins and to the toxic shock syndrome toxin produced by Staphylococcus aureus, pp. 23-59. In: Monoclonal Antibodies, Vol. II, A.J.L. Macario and E.C. Macario (eds). Academic Press, Orlando, FL.
43. Thompson, N.E., M. Razdan, G. Kunstmann, J.M. Aschenbach, M.L. Evenson, and M.S. Bergdoll. 1986. Detection of staphylococcal enterotoxins by enzyme-linked immunosorbent assay and radioimmunoassays: comparison of monoclonal and polyclonal antibody systems. Appl. Environ. Microbiol. 51:885-890.
44. Van der Zee, H. and K.B. Nagel. 1993. Detection of staphylococcal enterotoxin with Vidas automated immunoanalyzer and conventional assays. In 7th International Congress on Rapid Methods and Automation in Microbiology and Immunology. RAMI-93. Conference Abstracts 1993. PI127, p38.
Hypertext Source: Bacteriological Analytical Manual, 8th Edition, Revision A, 1998. Chapter 13A.
*Author:
Reginald W. Bennett
Hypertext updated by kwg/cjm 2001-OCT-24