|
January 2001
![]()
Although serological confirmation is not necessary for regulatory identification of Listeria monocytogenes, it is useful for determining the prevalence of specific serotypes in epidemiological studies and for environmental recontamination tracking. Early attempts to confirm L. monocytogenes serologically were generally not successful because of cross-reactions with other organisms (1). Serology, therefore, should always follow cultural and biochemical identification (see Chapter 10). Serological assays of Listeria, reviewed by Gray and Killinger (4), include agglutination, precipitation, and complement fixation assays as well as automated fluorescent antibody techniques, using flow cytometry (3) and rapid method ELISA kits, which are based on monoclonal and polyclonal antibodies (5). However, these serological systems were developed to detect all Listeria spp. They have been used successfully to screen foods for the genus (see Chapter 10), but they do not differentiate L. monocytogenes from other Listeria species.
This chapter presents procedures for the serological identification of L. monocytogenes by flagellar and somatic antigenic profiles (2), using agglutination as the serological tool.
The uniform approach to serotyping is first to determine the flagellar (H) serotype, and then to determine the somatic (O) serotype and/or subserovar, depending on the refinement of the antisera. Specific somatic types are associated with specific flagellar serogroups. The relationship of somatic and flagellar antigenic factors for L. monocytogenes is shown in Table 1.
| Table 1. Diagnostic scheme showing relationship of somatic (O) and flagellar (H) antigenic factors of L. monocytogenes | |
|---|---|
| H factors | O factors |
| A | 1a (1/2a) 3a [1a(1); 1a(1,2);3a (4)](b) |
| C | 1b (1/2b)a;3b [4a(7,9); 4b(5,6); 4b(6); 4d(8)](b) |
| D | 2 (1/2c)(a)
3C |
| a Seeliger and Donker-Voet designations. | |
| b Brackets indicate that antisera to somatic antigens are available. | |
| Antisera to H antigens are also available. | |
Scheme for routine serodiagnosis of L. monocytogenes, based on H and O antigenic factors.
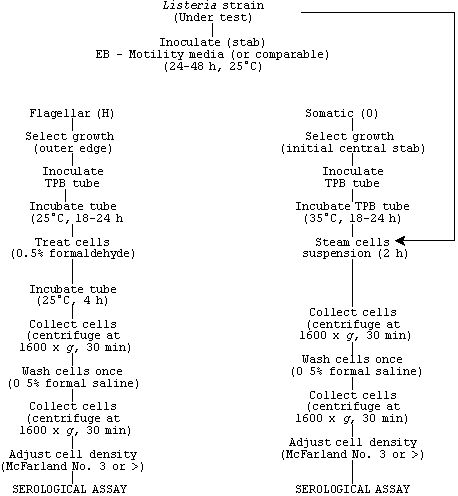
2. Routine serological typing for flagellar antigens
Remove growth from agar slant with straight inoculating needle and stab tube of EM motility agar if both flagellar and somatic antigens are required for serodiagnosis. See scheme for growth and treatment events. Incubate inoculated motility agar at 25°C for 24-48 h. Pick colonies from outer edge of motile growth on EB motility agar (Fig. 1) and inoculate tube of TPB or similar agar. Incubate inoculated TPB for 18-24 h at 25°C.
Fig. 1. Typical umbrella-like growth of L. monocytogenes on motility agar.

Add formaldehyde solution (37%) for final volume of 0.5% to broth culture (0.04 ml of formaldehyde to 8.0 ml of broth culture). Allow formaldehyde-treated broth to incubate 4 h at 25°C or treat as live culture. Collect cells by centrifugation (1600 x g, 30 min); then resuspend cells in 0.5% formal saline to turbidity equal to McFarland No. 3 standard. The broth cell suspension works equally as well as the washed standardized cells for the agglutination test.
In 6 x 50 mm tubes, mix cells (antigen) to be tested with equal volumes (100 l) of predetermined dilutions of H antisera with serological factors, A, C, and D. To prepare negative control, place 100 l of test cells in equal volume of saline. Incubate tubes containing bacterial cells and antisera as well as negative control in water bath preset at 48°C.
Observe tubes for agglutination after 1 h incubation. If agglutination occurs, a sediment will form and the supernatant will be as clear as the negative control (or clearer). Agitate tubes slightly (execute gently with finger) to resuspend sediment. Typical tube agglutination reaction is shown in Fig. 2.
Fig. 2. Comparative tube agglutination test showing positive agglutination reaction (tube on left with granular appearance) and typical negative reaction (tube on right with smooth, homogenous appearance in the serodiagnosis of L. monocytogenes.

2. Routine serological typing for somatic antigens
Remove growth from agar slant or comparable media with inoculating needle and inoculate tube(s) of TPB if flagellar serodiagnosis is not being done. If both flagellar and somatic antigen profiles are being determined, see scheme for routine serodiagnosis of L. monocytogenes based on H and O factors. Collect cells (antigen) by centrifugation (1600 x g, 30 min). Wash cells once in TPB for slide test or once with 0.5% formal saline for tube agglutination testing.
For slide testing, resuspend cells in minimal amount of 0.5% formal saline to prepare heavy cell suspension (cell turbidity equal to or greater than that of McFarland No. 3). Place 25 µl of factor serum on slide with equal volume of cells (antigen). To prepare negative control, mix 25 µl of cell suspension with 25 µl of saline. Mix antiserum and cells together while rocking slide back and forth.
To observe agglutination, hold slide against black background near a desk lamp. Typical positive (agglutination) and negative (smooth) reactions are shown in Fig. 3. If reaction is not smooth or fails to give good observable agglutination, test cells by tube agglutination, which is considerably more sensitive. The tube test for routine serotyping of O antigens is the same as that used for testing H antigen factors. For somatic serotyping, incubate tubes for 2 h in 48°C water bath and refrigerate overnight, or incubate overnight in 48°C water bath.
Fig. 3. Comparative slide agglutination test showing typical positive agglutination reaction (left section of slide) and negative serological reaction (right section of slide) in the serological typing of L. monocytogenes.

References
1. Bennett, R.W. 1986. Detection and quantitation of Gram-positive nonsporeforming pathogens and their toxins. In: 1985 IFT Basic Symposium Series, Microorganisms and Their Toxin - Developing Methodology. N.J. Stern and M.D. Pierson (eds). Marcel Dekker, New York.
2. Bennett, R.W. 1988. Production of flagellar (H) and somatic (O) subfactor antibodies to Listeria monocytogenes. IFT Abstracts, 1988:176.
3. Donnelly, C.W., and G. Baigent. 1985. Use of flow cytometry for the selective identification of Listeria monocytogenes. ASM Abstracts, p. 254.
4. Gray, M.L., and H.H. Killinger. 1966. Listeria monocytogenes and Listeria infections. Bacteriol. Rev. 30:309-382.
5. Mattingly, J.A., B.T. Butman, M.C. Plank, and R.J. Durham. 1988. A rapid monoclonal antibody-based ELISA for the detection of Listeria in food products. J. Assoc. Off. Anal. Chem. 71:679-681.
Hypertext Source: Bacteriological Analytical Manual, 8th Edition, Revision A, 1998. Chapter 11.