|
CDER 2007 Update
Drug Promotion Review
Information about a drug available to physicians and consumers is critically important for its safe use. We ensure that drug advertisements and other promotional materials are truthful and balanced. We operate a comprehensive program of education, voluntary compliance, surveillance and enforcement for drug advertising and promotion.
Surveillance of drug promotion activities
Drug advertising and promotion must be truthful, fairly balanced and not misleading. When we find advertising and promotion that is not, we issue two types of letters to ensure compliance with our regulations. These are advisory comments when a company requests review of draft promotional materials and regulatory letters resulting from our surveillance.
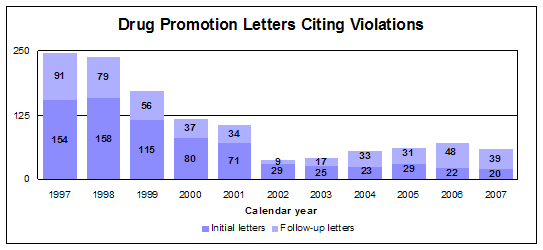
Regulatory letters citing violations
We issued 20 regulatory action letters to companies for prescription drug promotions determined to be false, misleading, lacking in fair balance of risks and benefits or that promoted unapproved uses. These were either untitled letters for general violations or warning letters for more serious or repeat violations. Examples of promotional violations include promotion at a Pharmacy & Therapeutics Committee, Internet sites, e-mails, plus traditional materials such as journal advertisements, sales brochures and TV ads.
Launch campaign advisory letters
When requested, we review advertisements and other promotional materials before drug companies launch marketing campaigns that introduce new drugs or new indications or dosages for approved drugs. In 2007, we issued 129 advisory letters to companies regarding their promotional materials for launch campaigns.
Other advisory letters
We issued 391other advisory letters to the industry regarding proposed promotional pieces, both professional and consumer directed. We also issued 135 other types of correspondence to the pharmaceutical industry, such as letters of inquiry, closure letters or acknowledgement letters.
Drug promotion letters in 2007
In 2007, we issued 714 letters concerning drug promotion. These were:
- 59 letters citing violations
- 655 other drug promotion letters
- 129 launch campaigns
- 526 others
Click image for larger view. Accessible text.

Click image for larger view. Accessible text.
Direct-to-consumer promotion
We are conducting research to help develop policy for our oversight of direct-to-consumer advertising. Data from our studies as well as those conducted by consumer groups and other entities shows that direct-to-consumer ads may encourage some patients to seek care for under treated conditions. This often results in patients obtaining treatment for serious medical conditions of which they were unaware. However, physicians and consumers have expressed concerns that direct-to-consumer ads may not always provide a balanced view of the benefits and risks of a product.
FDA Amendments Act of 2007
On September 27, 2007 the FDA Amendments Act of 2007 became law. The act modifies the Food, Drug and Cosmetic Act to specifically address direct-to-consumer prescription drug advertising for the first time. It contains provisions that will change existing requirements for direct-to-consumer prescription drug advertisements and the authority FDA has to oversee this area.
For example, the act gives FDA new authority to require submission of direct-to-consumer television drug advertisements for review before the ads are aired publicly. It also gives FDA authority to seek civil monetary penalties against companies who run false or misleading direct-to-consumer prescription drug advertisements. The act further requires that the statement of risks in broadcast direct-to-consumer advertisements for prescription drugs be presented in a clear, conspicuous and neutral manner, and that print direct-to-consumer drug advertisements include information on how to report adverse drug events to FDA. CDER is working actively to meet its mandate under the various direct-to-consumer drug advertising provisions of the law in the time frames expected.
This act also reauthorized the Prescription Drug User Fee Act (PDUFA) for five more years, through fiscal year 2012. It created a new voluntary user fee program for review of direct-to-consumer prescription drug television advertisements. Under this new program, the pharmaceutical industry would have paid user fees when they voluntarily submitted draft prescription drug television advertisements to CDER or CBER for advisory review. These fees would have enhanced CDER and CBER resources for certain prescription drug promotional review activities. However, the Consolidated Appropriations Act of 2007 failed to provide FDA with the authority to collect user fees for this new program, and as a result it was not able to commence.
Ongoing Direct-to-consumer promotion research
We continue work on three studies to help find the best way or ways to present information in the “brief summary,” the page of risk information in a print ad:
- Purpose. The first study will concern the purpose of the brief summary—how do people use it and what topics do they find most useful. This study is underway and we expect to have data collected for this study by September 2008.
- Content. The second study will address content issues in the brief summary, including the amount of common side effect information and the inclusion of numerical context.
- Format. The third study will examine format issues, such as graphics, layout and font. We expect to have data collected for these two studies by the fall 2008.
We have designed research to investigate the role of distraction in broadcast television ads. CDER will explore the collective role of the audio, textual and visual portions of the ad in the understanding of the risk and benefit information in ads. This study was published in the Federal Register for comment in August 2007. After extensive revision and external peer review, the second comment period is expected to commence by summer 2008.
New Direct-to-consumer research
We are developing a new study that will investigate the communication of effectiveness information on the main advertising page of print advertisements. By varying the presentation of this information, we will determine which manipulations bring consumers closer or farther away from an independent physician assessment of the effectiveness of the drug product in actual practice. This multi-phase study is in early development.
Direct-to-consumer promotion letters

Click image for larger view. Accessible text.
We issued guidance on direct-to-consumer broadcast advertisements in 1997. Since then, the number of letters addressing direct-to-consumer promotion, and the percentage of the total letters addressing promotion, have been:
- 2007: 188 (26%)
- 2006: 150 (20%)
- 2005: 203 (30%)
- 2004: 217 (27%)
- 2003: 254 (34%)
- 2002: 188 (27%)
- 2001: 190 (22%)
- 2000: 215 (24%)
- 1999: 247 (19%)
- 1998: 282 (44%)
- 1997: 240 (31%)
Next Page
 Back
to Top
Back
to Top  CDER 2007 Update Table of Contents CDER 2007 Update Table of Contents
 PDF requires the free
Adobe Acrobat Reader PDF requires the free
Adobe Acrobat Reader
Date created: July 31, 2008 |