|
How can you be sure your shampoo that claims to have all natural ingredients does not also contain some synthetic chemicals? Or that your hand lotion actually does contain the vitamin hit claims? The logical response should be, "Read the ingredient label on the back of the product." Logical, if you happen to be a chemist or a cosmetic scientist. Perplexing, if you are the average cosmetic consumer.
A quick glance at the back of the cosmetic label is all it takes to see that the ingredients are written in the language of chemistry. (See Chemical Translations.) Unless you know that one of the shampoo ingredients--methyl paraben--is a synthetic preservative derived from a petroleum base, or that tocopherol is vitamin E, you may never be able to check the claims against the contents.
John Bailey, Ph.D., director of the Food and Drug Administration's Office of Cosmetics and Colors, understands such consumer dilemmas. He and the scientists on his staff admit that most of us don't recognize the names of the ingredients listed. But there's no way to change that and still accurately identify the ingredients.
Chemical names are the only way ingredients can be listed because that's what they are. Most are cosmetic formulations, but in some products, such as an underarm deodorant that also claims to stop perspiration, the first chemical listed may be a drug ingredient and FDA would classify the product as a drug as well as a cosmetic.
Many ingredients are marketed with trade names, but these often provide little clue to the identity and intended use of the material. Trade names in the ingredient list could be confusing to consumers purchasing a cosmetic because they would have no way to compare similar ingredients in similar products. Also, some trade names include mixtures of raw materials--for example, an ingredient could be combined with a preservative.
Despite the highly technical language of the ingredient list, Bailey says it's entirely possible for consumers to get valuable information about a product by checking the label-front and back. To decode the cosmetic label, here's what you need to know.
Don't be fooled by claims made for certain cosmetic ingredients. Their presence in the products could be pure puffery because the law does not require cosmetic manufacturers to substantiate performance claims.
"Image is what the cosmetic industry sells through its products," Bailey says, "and it's up to the consumer to believe it or not." (See "Cosmetic Ingredients: Understanding the Puffery" in the May 1992 FDA Consumer.)
FDA considers the labeling of vitamins in cosmetics a separate issue, however, and does not recognize health claims for them in cosmetics. A product that features a vitamin--for example, vitamin E--must list it by its chemical name TOCOPHEROL on the ingredient list. Listing it as a vitamin in the ingredient statement would give the misleading impression that vitamin E in the product offers a nutrient or health benefit. (Vitamin E is usually added as an antioxidant to prevent chemical deterioration of the product.)
Consumers can get important health and value information by checking the ingredient list. For example, if you need fragrance-free hair spray because you have a sensitivity, a product containing a fragrance--even one that just masks the chemical odors of the raw materials--could be a waste of money if you can't use it.
Ingredient statements on cosmetics were first required in 1973 under the Fair Packaging and Labeling Act, enforced by FDA. Before then, consumers could only guess what was in a cosmetic product or if the product contained what it claimed. That requirement is especially valuable today with the industry competition for new ingredients.
The law allows a manufacturer to ask FDA to grant "trade secret" status for a particular ingredient. FDA grants this status under vary limited circumstances and after careful review of the manufacturer's data. The manufacturer must prove that the ingredient imparts some unique property to a product and that the ingredient is not well-known in the industry. If trade secret status is granted, the ingredient does not have to be listed on the label, but the list must end with the phrase "and other ingredients."
Consumers can also check value by comparing ingredient lists of similar products. Ingredients are listed in descending order, starting with the greatest amount in the product. A lotion with a featured ingredient close to the beginning of the list, for example, would have more of that ingredient than any other ingredient. A featured ingredient listed close to the end suggests that not much of that ingredient is present.
Anyone curious about an ingredient in a cosmetic can find answers in the International Cosmetic Ingredient Dictionary, published by the Cosmetic, Toiletries, and Fragrance Association. The dictionary provides a complete list of the most widely known cosmetic ingredients and their definitions and trade names. The dictionary, and all other compendia FDA recognizes to name ingredients, are available for reference at many public libraries, or at the Office of the Federal Register, 1100 L St., N.W., Washington, DC 20408.
Cosmetic ingredient declaration regulations apply only to retail products intended for home use. Products used exclusively by beauticians in beauty salons or cosmetic studios, and cosmetic samples such as those distributed free at hotels are not subject to the ingredient labeling rules. They must, however, state the name and address of the manufacturer, packer or distributor, and give an accurate statement of quantity and all necessary warning statements, as do all other cosmetics that weigh over one-fourth ounce or one-eighth fluid ounce.
Cosmetics making therapeutic claims that they may affect the structure or function of the body are regulated as drugs and cosmetics and must meet the labeling requirements for both. One way you can tell if you're dealing with such a product is if the first entry in the ingredient list says "Active Ingredient." (The active ingredient is the chemical that makes the product effective, and it must be safe for its intended use.) However, active ingredients are not legally required to be identified by this term. The law does require the active ingredient(s) to be listed first, followed by a list of all inactive cosmetic ingredients.
Examples of products that are both cosmetics and drugs are shampoos that treat dandruff, fluoride toothpastes to prevent dental decay, and sunscreens and sunblocking cosmetics, including foundations that contain sunscreens. (See "Dodging the Rays" in the July-August 1993 FDA Consumer.)
A product with a drug and cosmetic classification must be scientifically proven safe and effective for its therapeutic claims before it is marketed. If the product is not, FDA considers it to be a misbranded drug and can take regulatory action.
Under FDA's good manufacturing practice guidelines, even cosmetic products that are not regulated as drugs should be thoroughly tested for safety and subject to quality control during manufacture. But the law does not require the agency to review these tests before the cosmetics are marketed. Nevertheless, FDA does require safety warnings when problems become apparent.
Misuse of some cosmetic products can cause problems that range in severity from a mild rash to skin burns, or from burning eyes to blindness.
Look for warnings about the consequences of misuse required on products that could be hazardous, in addition to the detailed directions for use that appear on almost all cosmetics.
For example, products containing halocarbon or hydrocarbon propellants, such as aerosol hairsprays or deodorants, must bear the exact wording:
"Warning--Use only as directed. Intentional misuse by deliberately concentrating and inhaling the contents can be harmful or fatal."
All cosmetics in self-pressurized containers, such as shaving creams, must have specifically worded warnings against spraying near the eyes, puncturing, incinerating, storing, and intentionally misusing.
"Keep out of the reach of children" is also required for all products in pressurized containers. In the case of products intended for use by children, such as foaming soap, the phrase "except under adult supervision" may be added.
The following products require explicit warnings, though not with specific wording:
Manufacturers often use warning statements on labels when there is even a small chance of a problem. Baby products often contain such warnings. Baby powder. for example, if used carelessly and accidentally inhaled by the baby in large amounts, can block the infant's bronchial and lung passages and cause suffocation. (For more about cosmetic safety, see "Cosmetic Safety: More Complex Than at First Blush" in the November 1991 FDA Consumer.)
Cosmetic labels are more than product advertising. They connect cosmetic science with consumer protection by providing a means for consumers to know what's in a product and how to safely use it. A wise consumer will take the time to read the label to get the best value and results without incurring any of the possible harmful effects.
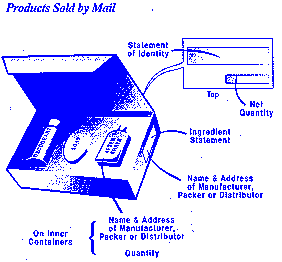
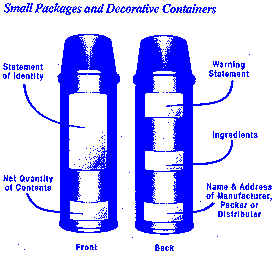
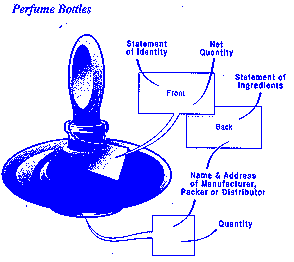
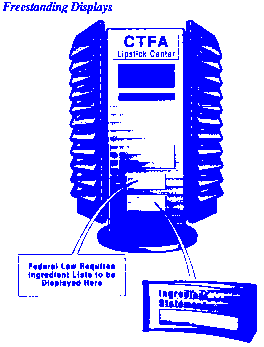
The placement of required information on the cosmetic label may vary with the packaging and container.
Written by Judith E. Faulke
FDA CONSUMER, May 1994