|
July 19, 2005
One of the benefits of performing a quantitative product pathway risk assessment is that the model can be used to estimate the likely impact of intervention strategies on the predicted number of illnesses. The impact of different harvesting methods, season (i.e., water and air temperatures), time until refrigeration, and length of storage before consumption were included in the baseline model. By changing one or more of the input parameters and measuring the resulting change in the model outputs, the likely impact of new or different processing procedures or regulatory actions can be evaluated. These changes to the baseline model are commonly referred to as conducting "what-if" scenarios.
The what-if scenarios evaluated include the following:
Strategies to reduce levels of V. parahaemolyticus in oysters after harvest include those associated with post-harvest treatments including immediate refrigeration, freezing, mild heating, and ultra high pressure. These procedures have varying degrees of effectiveness in reducing levels of V. parahaemolyticus in oysters. Potential mitigation strategies are summarized in Table VI-1 and described in greater detail below.
| Mitigation | Description | Log10 Reductiona |
|---|---|---|
| Irradiation | Exposure of oysters to up to 3 kGy Cobalt-60 gamma radiation | 6 |
| Ultra high pressure | Treatment of oysters with high pressure such as 345 MPa for 30 seconds | 6 |
| Hot water/cold shock | Oysters are heated (hot water pasteurization) to 50°C and held for 10 minutes followed by cold shock | 5 |
| Mild heat | Oysters are heated to 50°C and held for 5 minutes | >4.5 |
| Freezing | Rapid freezing and frozen storage (35 days at -20°C) | 2 |
| Immediate refrigeration | Placing oysters under refrigeration immediately after removal from the water at harvest | <1 |
| Relaying | Transfer of oysters to "clean" growing areas for various lengths of time | <1 |
| Depuration | Transfer of oysters (various lengths of time) to tanks containing seawater treated with UV light to inactivate bacteria. | 0 to 2 |
a These log reductions are based on studies described in this chapter and are specific to Vibrio parahaemolyticus but may not necessarily apply to 03:K6. Individual processors would need to conduct validation studies for their particular processing to measure log reduction under those specific conditions.
Irradiation. Gamma irradiation was investigated as an alternative post harvest treatment (PHT) for raw shell stock oysters (Andrews et al., 2002). Live oysters, with naturally incurred and artificially inoculated Vibrios, were exposed to 0-3 kGy dose Cobalt-60 gamma radiation. Vibrio parahaemolyticus TX03:K6 required 1.0 kGy to reduce the level of the microorganism in oysters to non detectable levels (a 6-log10 reduction). Vibrio vulnificus required 0.75 kGy to achieve a similar reduction. Sensory quality was maintained with irradiation exposure up to 1.5 kGy. Higher exposure levels affected the mortality of the oyster.
Hydrostatic Pressure. Inactivation of pathogenic microorganisms by high hydrostatic pressure was first demonstrated by Hite (1899). High hydrostatic pressure has been shown to be lethal to V. parahaemolyticus when suspended in various liquid media (Styles et al., 1991; Berlin et al., 1999). Styles et al. (1991) reported D-values of 5.1 min and 4.0 min for V. parahaemolyticus cells treated with 170 MPa at 23 °C (73.4 °F) in PBS and clam juice, respectively. Berlin et al. (1999) treated various pathogenic Vibrio species (approximately 107 cfu/g) including V. parahaemolyticus with 200 to 300 MPa at 25 °C (77 °F) in artificial seawater and reported that all strains tested were below detectable levels after 15 minutes at 250 MPa and 5 minutes at 300 MPa. A similar response was observed with oyster homogenates. Viable but non-culturable (VBNC) V. parahaemolyticus cells appeared to be more resistant than culturable V. parahaemolyticus but these differences were not statistically significant. At least a 5 to 6-log10 decrease in the level of V. parahaemolyticus in oysters was observed by Calik et al. (2002) depending on the time and pressure applied to oysters. After treatment for 30 seconds at 345 MPa, there was a 6-log10 reduction in the level of V. parahaemolyticus resulting in <10 CFU/ml. After 10 min at 240 MPa, the levels in the oysters ranged from <10 cfu/ml to ~30 cfu/ml (Calik et al., 2002). Vibrio parahaemolyticus strains vary in their resistance to high pressure; with serotype O3:K6 strains being more resistant than other pathogenic strains (Cook, 2003). For serotype O3:K6, the average reduction was approximately 6-log10 after 5 minutes at 250 MPa in PBS with a range of 5-log10 to >9.6-log10. For other (non-O3:K6) pathogenic strains, the average log10 reduction under the same conditions was ~12-log10 reduction with a range of 9.6-log10 to >15-log10.
Hot Water Pasteurization Followed by Cold Shock. The use of hot water pasteurization followed by cold shock has been reported to be effective in eliminating environmental strains of V. vulnificus and V. parahaemolyticus from naturally and artificially infected raw oysters (Andrews et al., 2000). More recently this hot water/cold shock process was performed on V. parahaemolyticus O3:K6 (Andrews et al., 2003). The investigators found that a 5-log10 reduction in the levels of environmental strains was achieved by heating oysters until an internal temperature of 50 °C had been reached and then holding them at that temperature for 10 minutes. The total process time, including the "come-up" time, was 18 minutes. The oysters had to be held at 50 °C for 12 minutes, which resulted in a total treatment time of 22 minutes, to achieve similar reductions with O3:K6 strains (Andrews et al., 2003).
Mild Heat Treatment. Cook and Ruple (1992) observed a 6-log10 reduction of V. vulnificus levels when shucked oysters were heated to an internal temperature of 50 °C (122 °F) for 5 minutes. Vibrio parahaemolyticus and V. vulnificus have been reported to have similar sensitivity to heat (Cook, 1999; Cook, 2002c). Other studies have shown that a 4.5 to 6-log10 (1,000,000-fold) reduction of V. parahaemolyticus densities could be expected by treating shucked oysters for 5 minutes at 50 °C (122° F) (Cook, 1999; Cook, 2002c). However, these studies observed that there is substantial variability in heat resistance among different strains. For example, when strains of serotype O3:K6 in phosphate buffered saline solution (PBS) were subjected to a mild heat treatment, there was a ~2-log10 reduction. However, when non O3:K6 pathogenic strains were treated similarly a much greater reduction (~6-log10) was observed (Cook, 2002c).
Freezing. A two-phase inactivation occurs when V. parahaemolyticus are frozen; the effect of an initial cold shock followed by further declines during frozen storage conditions (Johnson and Liston, 1973; Cook, 1999). Estimates of the effect of cold shock and frozen storage conditions were determined by performing a regression analysis on data reported by Johnson and Liston (1973). Based on such an analysis, freezing combined with frozen storage for 30 days at -30 °C (-22 °F) and -15 °C (5 °F) is projected to result in a 1.2 and 1.6-log10 reduction of V. parahaemolyticus numbers in oysters, respectively. A similar decline (2 to 3-log10) of V. parahaemolyticus (natural population and dosed with pathogenic O3:K6 serotype) was observed in oysters frozen 35 days at -20 °C (-4 °F) (Cook, 1999). In this study, oysters with high natural levels of TDH-negative V. parahaemolyticus were dosed with high levels of TDH+ V. parahaemolyticus (O3:K6) and then frozen. Based on these studies, freezing combined with frozen storage for 30 days would be expected to produce approximately a 2-log10 reduction of pathogenic V. parahaemolyticus. Both pathogenic strains (TDH-positive) and non-pathogenic (TDH-negative) V. parahaemolyticus respond similarly to freezing (Cook, 1999).
Immediate refrigeration. Gooch et al. (2002) found that the levels of V. parahaemolyticus in oysters increase with the length of time oysters are left unrefrigerated (26 °C) after harvest. That is, the levels can increase at least 50-fold in the warmer months when left at ambient temperatures for 10 h after harvest. Levels can in fact approach 105 to 107 viable cells (Cook and Ruple, 1989). However, since the levels of V. parahaemolyticus in freshly harvested oysters are generally low and growth does not occur at or below 10 °C, cooling oysters to that temperature soon after harvest will reduce any potential for bacterial growth. Furthermore, once the oysters are refrigerated, the levels decrease after prolonged refrigeration (six-fold after 14 days) (Gooch et al., 2002). A reduction in the extent of growth of up to 50-fold in V. parahaemolyticus densities could be achieved by immediate cooling depending on the initial V. parahaemolyticus levels, ambient air temperature and time-to-refrigeration (Cook and Ruple, 1989; Gooch et al., 2002). The extent of reduction of V. parahaemolyticus in oysters by immediate refrigeration is variable and approximately 1-log10 reduction. Immediate cooling would involve icing or otherwise refrigerating oyster shellstock immediately upon harvest.
Relaying. Relaying is the process by which shellfish are cleansed by transferring them to "clean" shellfish growing areas. It has been used most commonly with shellfish harvested from water having marginal bacteriological quality. There is little information available on this approach in relation to reducing the levels of V. parahaemolyticus. Relaying is not likely to have a significant impact since V. parahaemolyticus is ubiquitous in estuarine environments. Son and Fleet (1980) demonstrated a decrease from 18 V. parahaemolyticus/g to < 5 V. parahaemolyticus/g in relayed oysters after 6 days.
Depuration. In the United States, depuration is conducted exclusively with UV light disinfection (Richards, 1988). There is a broad spectrum of conditions under which shellfish are depurated. Optimal times, temperatures and salinities for effective depuration vary among shellfish species. Depuration has been generally reported to have no significant effect on decreasing the level of Vibrio spp. in naturally infected oysters or clams, and these microbes may even multiply in depurating shellfish, tank water, and plumbing systems (Eyles and Davey, 1984; Greenberg and Duboise, 1981). However, a 1-log10 reduction of V. parahaemolyticus was observed in the hardshell clam, Mercinaria mercinaria, after 72 h of depuration at room temperature (Greenberg and Duboise, 1981), and >2-log10 reduction at 15 °C (59 °F) (Greenberg et al., 1982). Son and Fleet (1980) observed a 5-log10 reduction in lab-infected oysters (from 9x107 to 8x102 within 72 h).
The impact of post-harvest mitigations that reduce levels of pathogenic V. parahaemolyticus in oysters was evaluated. The reduction levels, representing the range of potential mitigation controls, were as follows.
As shown in Figure VI-1, these mitigations would be implemented post-harvest and at different steps in the sequence of events occurring from harvest to retail. For example, immediate refrigeration would occur on the boat, immediately after harvest and freezing would occur prior to storage.
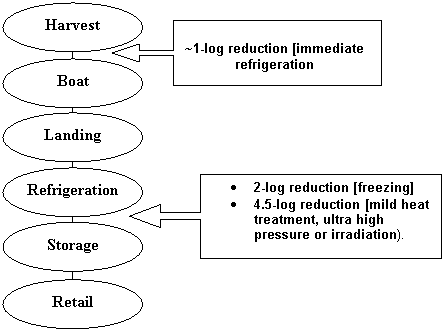
Figure VI-1. Schematic Representation from Harvest to Retail Showing Steps at which Evaluated Mitigations Occur
Immediate refrigeration was modeled by assuming that oysters would be cooled to no growth temperatures immediately following harvest. Assuming that this mitigation practice was followed without exception, post-harvest growth of V. parahaemolyticus in oysters would occur only during the period of cooldown required for the oyster meat to reach no growth temperatures. The time unrefrigerated was assumed to be zero and growth was considered to occur only during the cooldown period. The distribution of cooldown duration was assumed to be the same as that specified with respect to the baseline assessment.
The potential effects of mild heat treatment, irradiation, and/or high hydrostatic pressure and that of freezing were modeled by reducing the density predictions of the baseline model (i.e., no mitigation, at retail) downward by factors of 4.5-log10 and 2-log10, respectively. These effects correspond to dividing predicted total and pathogenic densities per gram by 31,623 and 100 for the 4.5-log10 and 2-log10 reductions, respectively. Implicitly, it was assumed that the effect of treatment on log10 V. parahaemolyticus densities is uniform with no induced change in the variance of log10 densities. If the variance of log10 densities actually increases after mitigation, even as the mean log10 density is decreased by the specified amount, then the potential degree of risk reduction is overstated.
The results of these "what-if" scenarios are summarized by harvest region in Table VI-2. See Appendix 6 for the results for each of the 24 region/season combinations. All three types of mitigation strategies were found to have a substantial effect on the probable number of illnesses likely to occur in comparison to the baseline (no mitigation). The scenarios indicate that implementing a mitigation that reduces V. parahaemolyticus levels in oysters after harvest by 4.5-log10 would be expected to reduce the number of predicted illnesses to less than one per year for all regions and that immediate refrigeration would be expected to reduce the number of predicted illnesses by about 90%.
| Region | Season | Predicted Mean Number of Illnesses per Annuma | |||
|---|---|---|---|---|---|
| Baseline | Immediate Refrigerationb | 2-log10 Reductionc | 4.5-log10 Reductiond | ||
| Gulf Coast (Louisiana | Spring | 505 | 54 | 5.2 | <1.0 |
| Summer | 1,406 | 139 | 15 | <1.0 | |
| Fall | 132 | 8.8 | 1.3 | <1.0 | |
| Winter | 6.7 | <1.0 | <1.0 | <1.0 | |
| Gulf Coast (Non-Louisiana) | Spring | 193 | 29 | 2.0 | <1.0 |
| Summer | 299 | 42 | 3.1 | <1.0 | |
| Fall | 51 | 7.7 | <1.0 | <1.0 | |
| Winter | 2.9 | <1.0 | <1.0 | <1.0 | |
| Mid-Atlantic | Spring | 4.4 | <1.0 | <1.0 | <1.0 |
| Summer | 6.9 | <1.0 | <1.0 | <1.0 | |
| Fall | 3.8 | <1.0 | <1.0 | <1.0 | |
| Winter | <1.0 | <1.0 | <1.0 | <1.0 | |
| Northeast Atlantic | Spring | 3.0 | <1.0 | <1.0 | <1.0 |
| Summer | 14 | 1.7 | <1.0 | <1.0 | |
| Fall | 1.7 | <1.0 | <1.0 | <1.0 | |
| Winter | <1.0 | <1.0 | <1.0 | <1.0 | |
| Pacific Northwest (Dredged) | Spring | <1.0 | <1.0 | <1.0 | <1.0 |
| Summer | 3.9 | <1.0 | <1.0 | <1.0 | |
| Fall | <1.0 | <1.0 | <1.0 | <1.0 | |
| Winter | <1.0 | <1.0 | <1.0 | <1.0 | |
| Pacific Northwest (Intertidal) | Spring | 18 | 10 | <1.0 | <1.0 |
| Summer | 173 | 96 | 2.1 | <1.0 | |
| Fall | 1.0 | <1.0 | <1.0 | <1.0 | |
| Winter | <1.0 | <1.0 | <1.0 | <1.0 | |
a Values rounded to significant digits. See Appendix 7 for actual values of numbers presented as <1.0.
b Represents conventional cooling immediately after harvest; the effectiveness of varies both regionally
and seasonally and is typically approximately 1-log reduction.
c Represents any process which reduces levels of Vibrio parahaemolyticus in oysters 2-log, e.g.,
freezing.
d Represents any process which reduces levels of Vibrio parahaemolyticus in oysters 4.5-log, e.g.,
mild heat treatment, irradiation, or ultra high hydrostatic pressure.
The uncertainty in the estimates is shown in Figure VI-2, using the Gulf Coast summer harvest as an example. Although the distribution of predicted illness is reduced substantially under these mitigations, the variance of the predicted number of illnesses (compared to the baseline) remains relatively unchanged. This is a consequence of the effect of specified model uncertainties, particularly with respect to the dose-response, growth rate and the percentage of total V. parahaemolyticus that are pathogenic.
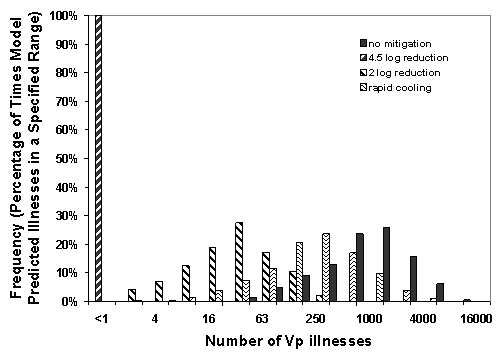
Figure VI-2. Effect of Potential Mitigations on the Distribution of Probable Number of Illnesses Associated with
Vibrio parahaemolyticus in Oysters Harvested from the Gulf Coast (Louisiana) in the Summer
The effects of the mitigations on the mean risk per serving are shown in Figures VI-3 through VI-8 for the six region harvest areas. With the exception of immediate refrigeration, the effect of the potential mitigations on the number of illnesses is similar for the six regions and four seasons. The effectiveness of immediate refrigeration for the Pacific Northwest (Intertidal) is predicted to be much less than that in the Pacific Northwest (Dredge) and the other harvest regions. This is a consequence of the intertidal harvesting method as oysters are exposed to ambient air temperatures (e.g. on mud flats) for various time periods unrefrigerated. The 4 to 8 hours when the intertidal oysters are exposed to ambient air are included in the 1 to 11 hours harvest duration modeling. This period on the tidal flat allows for additional V. parahaemolyticus growth that cannot be effectively inhibited by refrigeration during the period of intertidal exposure. Immediate refrigeration is effective in the Gulf Coast but the effectiveness of the immediate refrigeration mitigation was found to be seasonal in the Mid-Atlantic, Northeast Atlantic and Pacific Northwest regions but not in the Gulf Coast regions. This is an apparent consequence of considerably lower air temperatures (which may be at or below the growth temperature threshold for V. parahaemolyticus) during the winter season in those regions compared to the Gulf Coast regions.
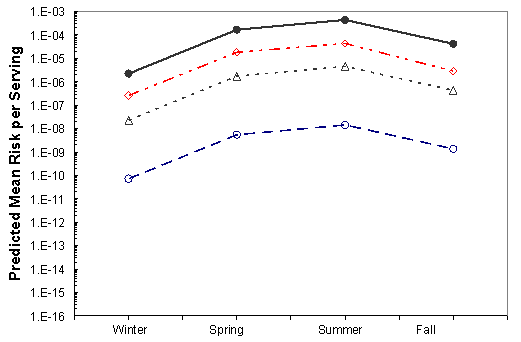
Figure VI-3. Effect of Potential Mitigations on Mean Risk of Vibrio parahaemolyticus Illnesses
per Serving Associated with the Gulf Coast (Louisiana) Harvest
[No mitigation (•); immediate refrigeration upon harvest (◊); treatment resulting in a 2-log10
reduction (Δ); treatment resulting in a 4.5-log10 reduction (Ο).]
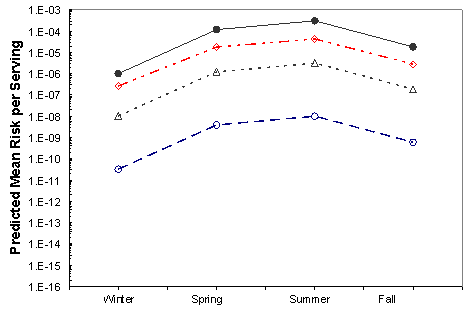
Figure VI-4. Effect of Potential Mitigations on Mean Risk of Vibrio parahaemolyticus
Illnesses per Serving Associated with the Gulf Coast (Non-Louisiana) Harvest
[No mitigation (•); immediate refrigeration upon harvest (◊); treatment resulting in a 2-log10
reduction (Δ); treatment resulting in a 4.5-log10 reduction (Ο).]
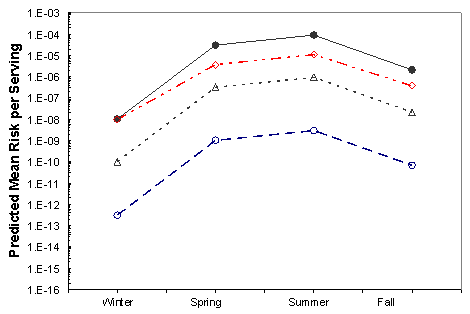
Figure VI-5. Effect of Potential Mitigations on Mean Risk of Vibrio parahaemolyticus Illnesses per
Serving Associated with the Mid-Atlantic Harvest
[No mitigation (•); immediate refrigeration upon harvest (◊); treatment resulting in a 2-log10
reduction (Δ); treatment resulting in a 4.5-log10 reduction (Ο).]
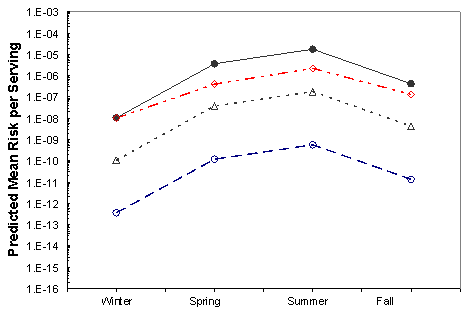
Figure VI-6. Effect of Potential Mitigations on Mean Risk of Vibrio parahaemolyticus Illnesses per
Serving Associated with the Northeast Atlantic Harvest
[No mitigation (•); immediate refrigeration upon harvest (◊); treatment resulting in 2-log10
reduction (Δ); treatment resulting in a 4.5-log10 reduction (Ο).]
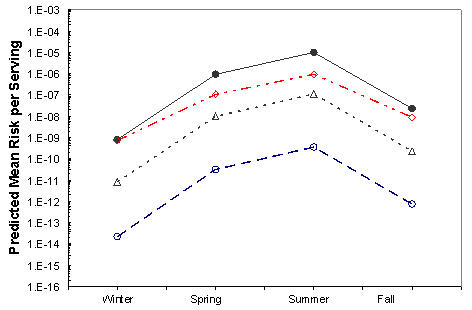
Figure VI-7. Effect of Potential Mitigations on Mean Risk of Vibrio parahaemolyticus Illnesses per
Serving Associated with the Pacific Northwest (Dredged) Harvest
[No mitigation (•); immediate refrigeration upon harvest (◊); treatment resulting in 2-log10
reduction (Δ); treatment resulting in a 4.5-log10 reduction (Ο).]
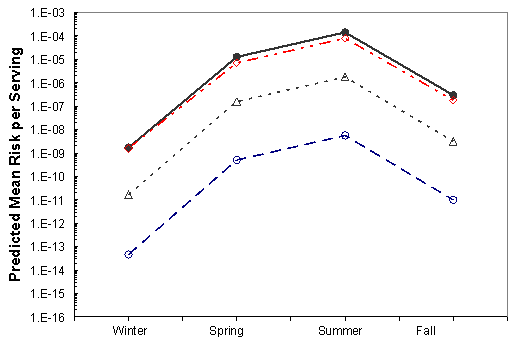
Figure VI-8. Effect of Potential Mitigations on Mean Risk of Vibrio parahaemolyticus Illnesses per
Serving Associated with the Pacific Northwest (Intertidal) Harvest
[No mitigation (•); immediate refrigeration upon harvest (◊); treatment resulting in 2-log10
reduction (Δ); treatment resulting in a 4.5-log10 reduction (Ο).]
The effect of reducing the time that oysters are unrefrigerated was further investigated by comparing the impact on predicted illness for different times from harvest to when oysters are refrigerated. The predicted effect of "rapid" cooling (e.g., using ice or an ice slurry) was also compared to "conventional" cooling (e.g., immediate refrigeration after harvest). For conventional cooling, it is estimated to take up to 10 hours for oysters to cool to a temperature at which V. parahaemolyticus will no longer grow (Cook, 2002b). For rapid cooling, there is a much shorter time for oysters to reach a no-growth temperature for V. parahaemolyticus; it is about 1 hour (Schwarz, 2003b).
For the rapid cooling scenario, a one hour cooldown time to no-growth temperature was assumed after oysters
are placed on ice or ice slurry. This estimate was based on studies by the Seafood Safety Laboratory,
Texas A & M University at Galveston (Schwarz, 2003a). The average growth rate occurring during the one
hour cooldown period was assumed to be equal to half the growth rate corresponding to the (variable) air
temperature at the time of harvest. With the one hour cooldown time the mean times to "no-growth" temperature
were approximately 2.0, 2.9, 3.7, and 4.3 hours over the set of 4 simulations.
For the conventional cooling scenario the same 1 to 4 hour range of maximum time unrefrigerated was combined
with the assumed range of 1 to 10 hours to reach no-growth temperatures. This range was based on preliminary
experiments (De Paola, 1999) and later confirmed by Cook (2002b) and Schwarz (2003b) for oysters in conventional
(air-circulated) coolers. The amount of growth occurring during the cooldown period corresponded to that
associated with the baseline model. Thus, for this scenario, the mean times to reach no-growth temperature
were 5.5, 6.4, 7.2, and 7.8 hours over the set of 4 simulations corresponding to maximum times until first
refrigeration of 1, 2, 3, and 4 hours, respectively.
Model simulations were run assuming maximum times of 1, 2, 3, and 4 hours for the time between harvest and first refrigeration. Specifically, the baseline distribution of duration of time from initial harvest until the initiation of oyster cooling was truncated at selected maximum times of 1, 2, 3, and 4 hours. All other variables (e.g., air and water temperatures) and uncertainties (e.g., dose-response) were taken to correspond to that specified in the baseline assessment.
For illustration, the results for the Gulf Coast (Louisiana and non-Louisiana) summer harvest are shown in Figure VI-9. As shown in the figure, the predicted reduction in V. parahaemolyticus illness from summer harvest of Gulf Coast oysters ranges from 46% to 97%, depending upon the specifics of the scenario. The results for all 24 region/season combinations are provided in Appendix 10.
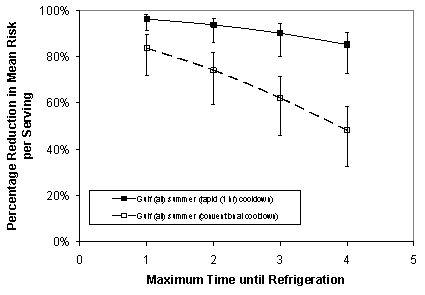
Figure VI-9. Predicted Effectiveness of Rapid versus Conventional Cooling on Vibrio parahaemolyticus
Risk for Gulf Coast Summer Harvest
[The scenario represents a simultaneous consideration of both the Gulf Coast (Louisiana) and Gulf Coast
(non-Louisiana) regions in the summer.]
The impact of overnight submersion of oysters after intertidal harvesting on the predicted risk of illness was evaluated. The baseline model predicts the levels of Vibrio parahaemolyticus in intertidally-harvested oysters, i.e., oysters are placed into baskets and removed after the tide rises, a typical practice in the Pacific Northwest. Studies of intertidally harvested oysters have shown that V. parahaemolyticus levels increase 4 to 8 -fold in oysters during intertidal exposure (Nordstrom et al., 2004; Herwig et al., 2001). However, Nordstrom et al. (2004) also demonstrated that after overnight submersion for a single tidal cycle, V. parahaemolyticus levels were reduced to levels similar to those measured prior to the intertidal exposure.
The baseline risk assessment model estimates that in the summer the risk of illness increases from 1.1 x 10-5 for dredged to 1.5 x 10-4 for intertidal harvesting because of intertidal exposure and heating. Delaying harvest overnight until near the end of the next tidal cycle just before oysters are re-exposed again to ambient air reduces the risk to a level predicted for oysters harvested by dredge (1.0 x 10-5) (see Appendix 10). The calculation for the percent reduction in risk obtained if the oysters are submerged overnight is based on the assumption that if V. parahaemolyticus levels after overnight submersion are similar to those in dredged oysters, then the risk decreases to that of dredged oysters. Results revealed that a 90% reduction in risk of illness could be obtained if intertidally harvested oysters were left submerged in the water overnight (Table VI-3). Further research is needed to determine whether this reduction could actually be achieved when oysters are stacked in baskets or by other means such as relaying or depuration.
| Type of Harvest | Season | Reduction in Illness (%) |
|---|---|---|
| Overnight Submersion of Intertidal Harvesta | Winter | 51.5 |
| Spring | 93.3 | |
| Summer | 93.0 | |
| Fall | 93.2 |
aThis assumes levels of V. parahaemolyticus in oysters after submersion overnight are similar to dredged.
The level of total V. parahaemolyticus in oysters is useful as a convenient surrogate indicator of the risk of illness due to the level of pathogenic V. parahaemolyticus in oysters. The FDA guidance for V. parahaemolyticus in seafood recommends that levels not exceed 10,000 viable cells per gram (ISSC/FDA, 1997). The 1999 V. parahaemolyticus Interim Control Plan (ICP) for molluscan shellfish adopted by the ISSC in 1999 and revised in 2001 included a microbiological criterion that if >10,000 cells/g are found in oysters, the area would need to be resampled for the presence of TDH+ strains. If any pathogenic (TDH+) V. parahaemolyticus were found in oysters, the harvest waters would be closed. In the 2001, revised plan, the number of total V. parahaemolyticus/g for resampling harvest waters was changed from 10,000 to 5,000.
The risk assessment cannot completely evaluate the effectiveness of such control plans because, as the model is constructed, there is no mechanism included to account for the possibility of persistence of either pathogenic or total V. parahaemolyticus in specific oyster harvesting areas and not others within the same region/season. The structure of the risk assessment does, however, allow consideration of the hypothetical impact on the incidence of disease if it were possible to exclude oysters from the raw market (or subjected to preventive controls) which have greater than any particular level of total V. parahaemolyticus at the time of harvest or at retail. The percentage of oyster harvest exceeding selected criteria levels for total V. parahaemolyticus can also be determined, giving an indication of the percentage of oysters that would no longer be available for raw consumption or for which preventative measures would need to be implemented to reduce V. parahaemolyticus growth under the assumption that the control plan could be implemented with 100% efficiency. For illustration, the results for the Gulf Coast (Louisiana) summer harvest are shown and the results for other region-season combinations can be found in Appendix 10.
Changes in the risk after removing varying percentages of the harvest greater than selected criteria levels were also determined in the simulations. Removal was simulated as occurring when a given criteria level was exceeded and the harvester/processor was compliant to that level. Varying levels of compliance (100%, 90%, 70%, 50%) were considered. For each criteria level and compliance probability, the proportion of harvest lost to the raw consumption market was estimated as the fraction of 10,000 simulated exposures for which initial V. parahaemolyticus levels exceeded the criteria level and the harvester/processor was compliant. The impact of deviation from compliance with these guidance levels was also evaluated, using the Gulf Coast region (Louisiana)/ Summer harvest as an example. As might be anticipated, the effectiveness of the guidance level to reduce illnesses is dependant on to the level of compliance (see Appendix 10).
At-Harvest Scenario. The at-harvest scenario included selected levels of 10 up to 100,000 total V. parahaemolyticus/g in order to estimate the relationship between illnesses potentially averted and harvest that would have to be diverted from the "raw market" (or subjected to preventive controls). The effect of uncertainties on this analysis was evaluated by considering the results of each uncertainty realization (sample) separately and then computing both a central estimate of probable effectiveness and a 90% uncertainty interval.
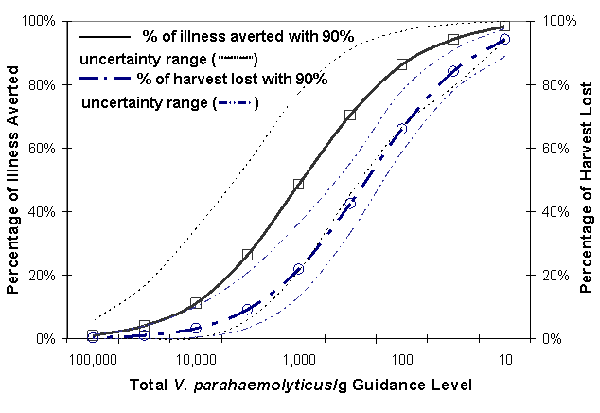
Figure VI-10. Predicted Effect of Control of Total Vibrio parahaemolyticus per Gram Oysters at
Time of Harvest for the Gulf Coast (Louisiana) Summer Harvest
[The term in Figure VI-10 "harvest lost" refers to the portion of the harvest that would have to be diverted
from the "raw market."]
Based on the means of the uncertainty distributions, the simulation results suggest that if all shellstock could be evaluated for total V. parahaemolyticus at time of harvest, excluding all oysters that had levels of 10,000 viable cells per g or more would reduce illness by 16% and 3% of the harvest would have to be diverted from the "raw market" or subjected to preventive controls. A 5,000 V. parahaemolyticus per g standard at time of harvest could (potentially) eliminate 28% of the illnesses associated with the consumption of oysters from this region/season with 6% of the harvest having to be diverted from the "raw market" or subjected to preventive controls. The relatively low (potential) reduction of illness is attributable to the large proportion of the harvest that would remain with a lower level of V. parahaemolyticus that would still grow to more significant levels after harvest. In comparison, the simulation results suggest that in the absence of subsequent post-harvest mitigations, "at-harvest" guidance levels of 5-log10 (105 or 100,000), 3-log10 (1,000 or 103) and 2-log10 (100 or 102) total V. parahaemolyticus per g could (potentially) reduce the illness rate by 1.6%, 68% and 98% with corresponding impact of 0.25%, 21% and 66% of the harvest, respectively. There is, however, uncertainty associated with these predictions as indicated by the uncertainty bounds shown in Figure VI-10. It is important to note that these estimates are based on the consideration of the baseline model only and do not take into account any other potential mitigations such as those evaluated earlier in this chapter.
At-Retail Scenario. The hypothetical impact on the incidence of disease if it were possible to exclude oysters (from the raw market) which have greater than any particular level of total V. parahaemolyticus at retail was also evaluated for different guidance levels following the same method described above for at-harvest control. The results are shown in Figure VI-11 for the Gulf Coast (Louisiana) summer harvest, with selected levels of 10 to 100,000 total V. parahaemolyticus/g included in order to estimate the relationship between illnesses potentially averted and harvest that would have to be diverted from the "raw market."
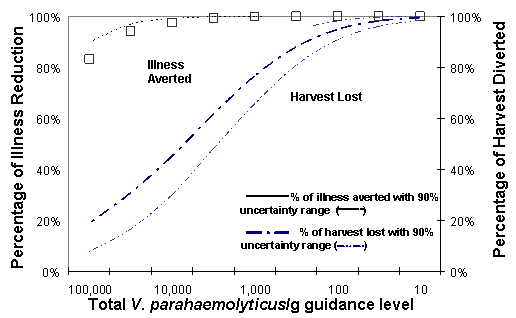
Figure VI-11. Predicted Effect of Control of Total Vibrio parahaemolyticus per Gram Oysters at
Retail for the Gulf Coast (Louisiana) Summer Harvest
[The term in Figure VI-10 "harvest lost" refers to the portion of the harvest that would have to be
diverted from the "raw market."]
The effect of uncertainties on this analysis was evaluated as for the at-harvest control scenario. The simulation results suggest that at the same control levels, many more illnesses would be potentially eliminated, but with a much higher loss in harvest diverted from the raw market. For example, excluding all oysters that had levels of 10,000 viable cells per g at retail would reduce illness by 99% and 43% of the harvest would have to be diverted from the "raw market", compared to 11% and 3%, respectively, for at-harvest control levels of 10,000 V. parahaemolyticus per gram. A 5,000 V. parahaemolyticus per g standard at retail could (potentially) eliminate almost 100% of the illnesses associated with the consumption of oysters from this region/season with 70% of the harvest having to be diverted from the "raw market." The greater effectiveness of guidance level applied at retail than at harvest with respect to illness aversion is because the former is applied after the effects of temperature abuse during harvesting operation.