|
July 19, 2005
The Hazard Characterization component of a risk assessment describes the adverse effects on the host of a particular substance, organism, or other hazard. In the current risk assessment, a quantitative evaluation was conducted of the dose-response relationship between the levels of V. parahaemolyticus ingested and the frequency and severity of illness. The dose-response relationship for V. parahaemolyticus was derived using human clinical feeding trial studies and epidemiological surveillance data. The probability of illnesses (gastroenteritis and septicemia) and the incidence of severe disease (septicemia) were evaluated.
Dose-response relationships are influenced by three factors: the pathogen (e.g., virulence characteristics), the environment (e.g., the food matrix), and the host (e.g., susceptibility and immune status). These factors are described below.
Currently, the only trait that has definitively been demonstrated to reliably distinguish pathogenic from non-pathogenic V. parahaemolyticus is the production of TDH. The tdh gene was first cloned from a Kanagawa-positive strain by Kaper et al. (1984). The so-called, Kanagawa Phenomenon (KP) is the exhibition of β-hemolysis induced by this haemolysin on a special blood agar (Wagatsuma) medium. This phenotype is strongly associated with clinical strains (Miyamoto et al., 1969). Pathogenic strains possess a tdh gene and produce TDH, whereas non-pathogenic strains lack the gene and the trait. For the purpose of this risk assessment, pathogenic V. parahaemolyticus are defined as those strains that produce TDH.
Food matrix factors such as fat levels, acidity, salt content, and other characteristics can have a significant impact on the ability of a pathogen to cause disease (Foegeding, 1997). For example, gastrin, the most potent stimulant of gastric acid secretion, is released after eating a protein-rich meal, such as oysters (West, 1985). Because most enteric pathogens, including V. parahaemolyticus, are sensitive to acids, the increased production of gastric acid actually provides a protection against infection. On the other hand, consumption of highly buffered foods (such as cooked rice) or antacids may decrease the number of microorganisms needed to cause illness because of their effects on gastric pH. For example, the ID50 (the dose at which 50% of infected subjects become ill) observed in feeding trials with V. cholerae O1 was substantially lower when the microorganism was ingested with antacid vs. no antacids (Levine et al., 1981).
Host factors such as the general health status, presence of underlying disease, nutritional status, or physical stress can play an important role in an individual's response to infections. The immune status, especially of those individuals who are immunocompromised due to disease or medical treatments can influence occurrence and/or severity of foodborne diseases. Intrinsic factors such as age, sex, and genetics further influence the immune system, and thus the susceptibility of an individual to disease. For illness associated with V. parahaemolyticus infection, the severity of the disease is strongly associated with the presence of underlying medical conditions. The impact of immune status on the initial colonization and infection of the gastrointestinal tract is less clear-cut.
Several human clinical feeding trials were conducted prior to 1974 using pathogenic V. parahaemolyticus. The available data from these studies are briefly summarized here. Information on non-O1 V. cholerae is also provided as this represents a possible surrogate microorganism with respect to future investigations.
Takikawa (1958) used a Kanagawa-positive strain in a human volunteer study and showed that V. parahaemolyticus caused diarrhea in 1 of 2 individuals fed a dose of approximately 106 cells. Diarrhea occurred in both individuals fed approximately 107 cells. The ingested doses were not directly determined, but were instead estimated assuming that V. parahaemolyticus cultures can reach maximum growth densities of approximately 1010 cells per milliliter. These data were selected for the dose-response model.
In a study by Aiso and Fujiwara (1963), three clinical isolates (2 Kanagawa-negative strains and 1 Kanagawa-positive strain) and one shell fish isolate (Kanagawa-negative strain) were tested. The cultures were suspended in salted milk and were fed just prior to eating a normal meal. Illness only occurred with the Kanagawa-positive strain fed at a dose of 109 organisms. Symptoms developed 5 to 11 hours after challenge. Typical symptoms included violent abdominal pain, diarrhea and vomiting in each of the 4 volunteers. The data for the Kanagawa-positive strain were selected for the dose-response model.
In a third study (Sanyal and Sen, 1974), three Kanagawa-negative strains isolated from cases of gastroenteritis were fed to groups of four volunteers each. No illness was observed in any of the volunteers at doses as high as 2 x 1010 cells. A Kanagawa-positive strain also isolated from a gastroenteritis case produced no symptoms at a low dose of 200 viable cells; however, abdominal discomfort was reported by 1 of 4 volunteers at a dose of 2 x 105 viable cells, and 2 of 4 volunteers experienced abdominal discomfort and diarrhea at 3 x 107 viable cells. All volunteers received antacid tablets prior to challenge with cultures suspended in gelatin. Only the data from the Kanagawa-positive strains were used in the dose-response model.
In another study, human exposure to 15 Kanagawa-negative strains isolated from fish produced no illnesses when doses as high as 109 viable cells were used (Sakazaki et al., 1968). It was not reported how many volunteers were challenged in this study. These data were not used in the dose-response model.
A personal communication from Kasai (1971) reports that it took 6 to 8 hours incubation for a V. parahaemolyticus Kanagawa-positive strain to cause disease whereas a Kanagawa-negative strain required approximately 18 hours to cause disease after challenge. The infecting dose was reported to be approximately 106 organisms. No information was provided in the communication about the dose level or number of volunteers in the study. These data were not used in the dose-response model.
Two human clinical feeding studies have been conducted with non-O1 Vibrio cholerae, a potential surrogate for Vibrio parahaemolyticus. In one study, healthy volunteers were fed 105 to 109 levels of non-O1 V. cholerae. One of the three strains caused no diarrhea in 2 volunteers fed 105 cells, 2 of 3 fed 106, 1 of 2 fed 107 and 3 of 3 fed 109. Two other strains produced no disease at doses as high as 109 cells (Morris et al., 1990). In a second study, Vibrio cholerae O139 Bengal fed to volunteers caused diarrhea in 2 of 4 fed 104 cells and in 7 of 9 fed 106 cells (Morris et al., 1995). The pathogenicity of this serotype more closely resembles Vibrio cholerae O1, and as such may be less useful as a potential surrogate.
Animal studies using V. parahaemolyticus or a surrogate microorganism are potentially useful as a basis for extrapolating dose-response estimates for humans. Animal studies can also be useful for assessing the virulence potential of different strains and serotypes, susceptibility of sensitive subpopulations (i.e., immunocompromised), and the role of specific virulence determinants. Several V. parahaemolyticus animal studies have shown the virulence potential of TDH-negative strains. However, it remains to be determined whether the virulence potential of these strains also applies to humans. The effect of food matrices and other environmental factors on virulence and the dose-response relationship can be evaluated more readily in animal studies than in human studies. Potentially relevant animal dose-response data and identified factors influencing the infectivity of V. parahaemolyticus in animal models are described in this section. Although potentially informative, animal data were not utilized in the dose-response model for this risk assessment because the measures of the severity of illness in relevant animal studies did not correspond with definitions of human illness on which reporting statistics are based and therefore provided little additional information with respect to quantitative risk prediction/characterization of human illness.
A limited number of animal studies have been conducted using V. parahaemolyticus. In one study, suckling rabbits infected orally with a Kanagawa-positive strain at doses of 109 to 1010 had positive blood cultures in 9 of 36 tested, positive spleen cultures in 11 of 21 tested and positive liver cultures in 14 of 21 tested (Calia and Johnson, 1975). Similar doses of a Kanagawa-negative crab isolate were negative for bacteremia, liver or spleen invasion in all 12 animals challenged (Calia and Johnson, 1975).
Hoashi et al. (1990) conducted 7 experiments in which mice were challenged intraperitoneally with 4 TDH+and 3 TDH- strains. In the combined results of all 7 experiments, no deaths were reported with a dose of 105 cells; 4% deaths with a dose of 106; 61% deaths with a dose of 107, and 90% deaths with a dose of 108 cells. Combined results of 2 experiments in which mice were challenged orally with TDH-positive strains resulted in 38% deaths with a dose of 107 cells, 57% deaths with a dose of 108 and 80% deaths with a dose of 109 cells (Hoashi et al., 1990). There were no significant differences in mortality between the TDH+ and TDH- strains at any of the doses.
In rabbit ileal loop model the effective dose required to produce ileal loop dilation in 50% of rabbits for three Kanagawa-positive strains ranged from 2.6 x 105 to 7.7 x 106 cells (Twedt et al., 1980). It was estimated that the initiation of positive loops occurred with doses from 102 to 105 cells (Twedt et al., 1980). Seven clinical isolates were tested belonging to four different serotypes that possess one or more virulence factors: TDH, TRH, and urease, in relation to the ability to cause diarrhea (Kothary et al., 2000). All strains were found to induce fluid accumulation in suckling mice and diarrhea in a ferret model after oral inoculation in a dose-dependent manner. The relationship between clinical and environmental origins of these strains was not evaluated.
Epidemiological investigations of V. parahaemolyticus provide directly relevant information on the dose-response in humans. These data may be somewhat limited if there is a lack of information for the ingested dose associated with reported cases of illness. However, even when epidemiological data is not informative as to dose-response, such data often provide valuable information on the likelihood of illness (gastroenteritis) progressing to more severe outcomes (i.e., septicemia, death) in susceptible versus otherwise healthy populations. Information on the annual incidence of illness from surveillance data and outbreak investigations is provided in "Chapter II. Hazard Identification."
CDC estimated the annual illness burden from pathogenic V. parahaemolyticus associated with the consumption of raw oysters as 2,790 cases of illness per year (Painter, 2003). For additional information, see Chapter II: Hazard Identification.
The selection of data for use in the Dose-Response model considered the availability of the data and limitations of data sources. Consideration was given to using the dose-response of an appropriate surrogate bacteria and/or host (i.e., animal model), which could provide a more suitable basis for risk prediction/characterization if uncertainties such as immune status and food matrix effects were substantially reduced. If a surrogate dose-response is to be more informative than the available feeding trials data, then better information is needed with respect to response rates associated with low dose exposure (including knowledge of relevant biomarkers) and the effect of the (oyster) food matrix on the dose-response relationship. However, the potential difference between a surrogate dose-response and that of V. parahaemolyticus adds an additional uncertainty with respect to risk prediction/characterization. For the purpose of this risk assessment, human clinical feeding studies with pathogenic V. parahaemolyticus were used. A summary of the selection criteria and evaluation of each identified human clinical feeding study is provided in Table III-1.
| Study | Selection Criteria | Used in Dose-Response Model? | ||
|---|---|---|---|---|
| Dosed with Vibrio parahaemolyticus | Pathogenic strains?a | Dose Level Reported? | ||
| Aiso and Fujiwara, 1963 | Yes | Yes | Yes | Yes |
| Takikawa, 1958 | Yes | Yes | Yes | Yes |
| Sanyal and Sen, 1974 | Yes | Yes | Yes | Yes |
| Sakazaki et al, 1968 | Yes | No | Yes | No |
| Kasai, 1971 | Yes | Yes | No | No |
| Morris et al., 1990 | No (V. cholerae) | Not applicable | Yes | No |
| Morris et al., 1995 | No (V. cholerae) | Not applicable | Yes | No |
a For the purpose of this risk assessment, pathogenic Vibrio parahaemolyticus strains are those characterized as Kanagawa Phenomenon-positive.
The limitations of the available human feeding trial and surrogate studies for use in dose-response modeling are summarized below. Some of the studies were performed using uncharacterized strains.
The human feeding studies were performed prior to 1974 and it is unlikely that any future human feeding studies with V. parahaemolyticus will be undertaken to resolve these issues due to an apparent cardiotoxicity of TDH in animal models (Honda et al., 1976a; Seyama et al., 1977).
The structure of the dose-response model is shown in Figure III-1. The V. parahaemolyticus dose-response model was developed by fitting a distribution to the selected human feeding trial data. The resulting estimate of the shape of the dose-response relationship was then modified by "anchoring" the mean risk predictions to be consistent with epidemiological surveillance data. The probability of cases of gastroenteritis progressing to septicemia was also calculated.
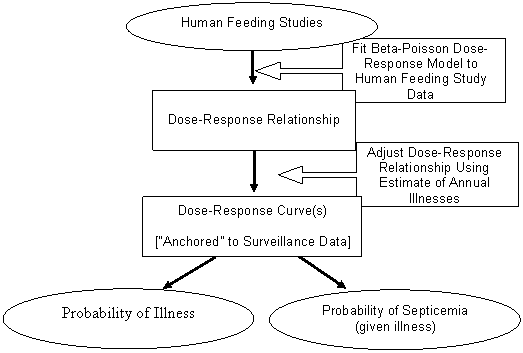
Figure III-1. Schematic Representation of the Development of the Vibrio parahaemolyticus Dose-Response Model
First, the available human feeding trial data for the incidence of gastrointestinal illness from the three selected studies [Takikawa (1958), Aiso and Fujiwara (1963), and Sanyal and Sen (1974)] were pooled. Collectively, a total of 20 healthy volunteers were administered pathogenic V. parahaemolyticus at doses ranging from 2.3 to 9-log10 cfu in a bicarbonate buffer. In these three studies, 9 of 20 subjects developed symptoms of gastroenteritis. No illnesses were reported for the lower doses of 2x102 and 2x105 cfu of V. parahaemolyticus. However, at higher doses (>1x106 V. parahaemolyticus organisms) between 50% and 100% of the human subjects became ill. A summary of the dose levels, number of subjects, and number that develop illness is provided in Table III-2.
| Dose (cfu) | Number of Subjects | Number of Illnesses | Rate of Observed Illness | Reference |
|---|---|---|---|---|
| 2 x 102 | 4 | 0 | 0 | Sanyal and Sen (1974) |
| 2 x 105 | 4 | 0 | 0 | Sanyal and Sen (1974) |
| 1 x 106 | 2 | 1 | 0.5 | Takikawa (1958) |
| 1 x 107 | 4 | 2 | 0.5 | Takikawa (1958) |
| 3 x 107 | 2 | 2 | 1.0 | Sanyal and Sen (1974) |
| 1 x 109 | 4 | 4 | 1.0 | Aiso and Fujiwara (1963) |
| Total Subjects = 20 | Total Illnesses = 9 | |||
Secondly, the dose-response models were selected. Dose-response models are used to define the shape of the dose-response curves, allowing the extrapolation from the observed data from the human feeding trials to other (lower) dose levels. Three dose-response models, Beta-Poisson, Gompertz, and Probit, were evaluated. These models exhibit different behaviors at low dose levels; that is they would predict different probability of illness for the same exposure levels. These models are parametric, meaning that they can be described by a mathematical (i.e., algebraic) equation. The mathematical equations for these three models are shown in Table III-3. Additional details about the model selection are provided in Appendix 4.
| Dose-Response Model | Equation a |
|---|---|
| Beta-Poisson | Pr(ill | d) = 1 - (1 + d/β)-α |
| Probit | Pr(ill | d) = Φ(α + β * log10(d)) |
| Gompertz | Pr(ill | d} = 1 - exp[-exp[α + β * log10(d)]] |
a For the Beta-Poisson, α and β are the shape (steepness) and location parameters, respectively. The approximation used for the Beta-Poisson dose-response function applies when α << β (and β>>1). For the Probit and Gompertz models, α and β are the location and shape (steepness) parameters, respectively. For all three models, d denotes the dose. For the Probit model Φ denotes the cumulative distribution function of a standard normal random variable.
Next, the dose response models were fit to the observed feeding trial data as shown in Figure III-2. The models were fit to the data by the maximum likelihood criteria; that is, the values chosen for the model equation parameters shown in Table III-3 were the values which maximized the likelihood of the model predicting data similar to the observed data. The adequacy of model fits to the data was evaluated using a likelihood ratio based goodness-of-fit measure. All of the models provided an adequate statistical fit to the data. For more information about estimated model parameters and the statistical evaluation of the model fits, see Appendix 4.
The Maximum Likelihood Estimate (MLE) is the most likely value of all possible outcomes (i.e., the best estimate of the probability of illness). The best estimates of the dose corresponding to a 50% probability of illness (i.e., the MLE of the ID50) were determined to be 2.8×106, 4.0×106, and 3.2×106 organisms/serving for the Beta-Poisson, Gompertz and Probit dose-response models, respectively. Although these estimates are not substantially different at the ID50, the differences are much more substantial at low dose levels as can be seen in Figure III-2. For example, the estimated risk of illness is approximately 5 cases per 10,000 servings for the Beta-Poisson model at a dose of 1,000 V. parahaemolyticus organisms/ serving. However, at the same dose, the estimated risk is approximately 10-fold higher based on the Gompertz and approximately 10-fold lower based on the Probit. The differences between these models are less substantial for high doses that exceed 100,000 organisms per serving.
An evaluation of the uncertainty distributions of the risk predications for the three dose-response models was conducted (Appendix 4). This comparison indicated that considering the residual predictions of uncertainty, the three models were comparable. Therefore, for simplicity, one model was chosen to use in the risk characterization. Of the three models evaluated, the Beta-Poisson model is the only one that meets the mechanistic criteria identified by FAO/WHO (2003). The criteria include consideration that there is no threshold level (i.e., a single cell can cause illness). The Beta-Poisson model was therefore considered the most appropriate model to use for this risk assessment.
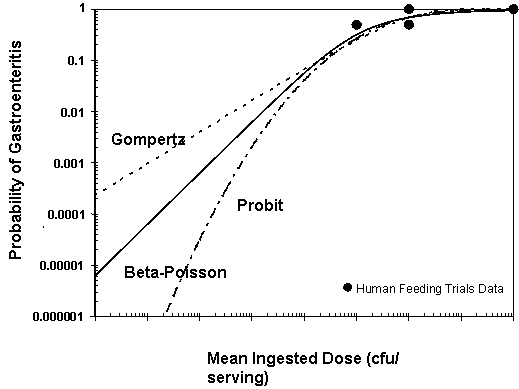
Figure III-2. Comparison of the Beta-Poisson, Gompertz, and Probit Dose-Response Models Fit to Data from Human
Feeding Studies
The V. parahaemolyticus human feeding trial data is the most complete data set available to describe the relationship between dose and the probability of illness. However, there are apparent biases in these data relative to what may be expected from exposure to V. parahaemolyticus by a diverse population consuming raw oysters. For example, the human feeding trials included concurrent antacid administration and no concurrent administration of oysters (food matrix) with the V. parahaemolyticus dose, which potentially changes the infective dose. Thus, the ID50 observed in feeding trials would be expected to be lower than that of the general population based on effect of the food matrix vs. buffer on the infective dose.
Figure III-2 shows the relationship between dose and the probability of illness. Using the Beta-Poisson curve and the predicted exposure levels (see Chapter IV Exposure Assessment), the model would predict too many illnesses in comparison to epidemiological data. For example, using the Gulf Coast summer harvest, the mean exposure to pathogenic V. parahaemolyticus from oysters is predicted to be 20,000 organisms per serving (~100 cells per gram) (see Chapter IV: Exposure Assessment). At this level of exposure, the risk of illness would be predicted to be substantially greater than 0.001 (i.e., >1 illness in 1,000 servings). Accounting for the number of servings per year, this rate of illness would be approximately equivalent to 4,000 illnesses/year associated with the Gulf Coast summer harvest. This predicted rate is too high, considering that CDC estimates there are only 2,790 cases/year (Painter, 2003) for the entire United States population.
Based on the above considerations, the dose-response model was adjusted or "anchored' to be consistent with both the CDC's estimate of the average annual number of cases occurring per year and the estimated number of servings consumed (Chapter IV: Exposure Assessment). This adjustment factor represents the effect of the apparent differences between the dose-response observed in human volunteers under controlled conditions versus that in the general population when exposure is associated with the oyster food matrix.
The shape of the dose-response curve (i..e., the slope or steepness) was assumed to be the same for both the controlled feeding trials and oyster-related exposure situations. However, the location of the curve was shifted, using the adjustment factor. For the Beta-Poisson model, the resulting expression used for risk prediction was taken to be:
Pr(ill | d) = 1 - (1 + (d/(γ*β)))-α
where γ is the dose-response adjustment factor.
The magnitude of the adjustment factor was estimated by iteratively running the risk characterization model and adjusting the location of the curve to be consistent with CDC's estimated average annual illness burden of approximately 2,800 cases (Painter, 2003). For the Beta-Poisson model, the resulting dose-response adjustment factor was estimated to be 27, which corresponds to a difference of 1.4-log10 between the ID50 under the controlled versus oyster-related exposure scenarios. The difference between the adjusted and unadjusted curves is shown in Figure III-3.
The solid line shown in Figure III-3 is the MLE of the Beta-Poisson model fit to the pooled human feeding studies data and the dashed line shows the shift adjustment (location) made so that the model predictions agree with the epidemiological surveillance data. From Figure III-3, it can be seen that the dose corresponding to a 50% probability of illness (ID50) for the unadjusted curve is approximately 3 million and that of the adjusted curve is approximately 80 million.
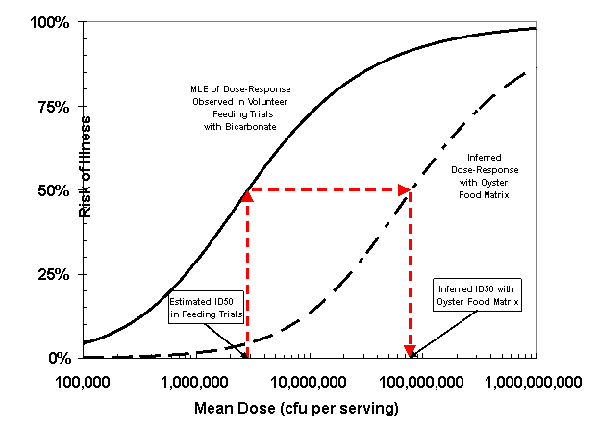
Figure III-3. The Beta-Poisson Dose-Response Model for Vibrio parahaemolyticus Fit to Human Feeding
Trials and Adjusted Using Epidemiological Surveillance Data
[The solid line is the best estimate of the Beta-Poisson Model fit to pooled human feeding studies.
The dashed line shows the shift adjustment so that the model predictions agree with epidemiological
surveillance data. MLE denotes the maximum likelihood estimate. ID50 is the dose corresponding
to a 50% probability of illness.]
Uncertainty in the dose-response relationship was characterized by performing a procedure called non-parametric bootstrapping. This procedure involves hypothetical replication of the observed human feeding study. However, given the limited number of possible outcomes (illness rates), the procedure was conducted as follows. For each possible outcome, the model was refit by the maximum likelihood criteria to obtain a set of parameter estimates, one corresponding to each possible (but unobserved) outcome. Weighting was assigned based on the probabilities of the outcomes. An uncertainty distribution was derived based on the parameter estimates and the weighting. The details of these calculations are provided in Appendix 4.
Figure III-4 shows a graphical representation of the weighted set of dose-response curves from the bootstrapping procedure. The 21 curves in this set were used in the Risk Characterization model. For each simulation (run of the model), a single curve was randomly selected, based on the assigned weight for that curve (the uncertainty distribution). The thick black curve shown in Figure III-4 is the curve that received the most weight (i.e., had the highest probability and would be selected most frequently). The weights for each curve and other supporting information are provided in Appendix 4.
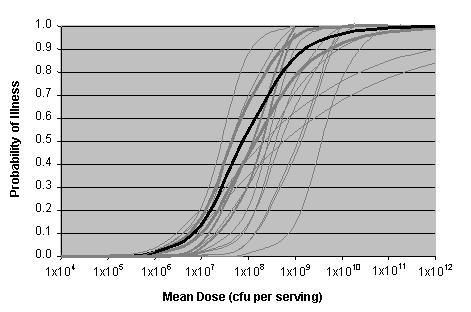
Figure III-4. Vibrio parahaemolyticus Dose-Response Curve and Uncertainty
[The dark line indicates the dose-response curve with the highest weighting (16.5%) and the 20 gray lines
represent the dose-response curves with lower weightings (<1% to 13%).]
We did not apply uncertainty to the dose-response adjustment factor used to bring the model-predicted illnesses in alignment with the reported epidemiological illnesses (i.e., the shift shown in Figure III-3). To incorporate uncertainty in the dose-response shift an effort to assess the uncertainty in the number of illnesses occurring annually (i.e., uncertainty in the number of underreported illnesses) would need to be undertaken. See Appendix 4 for additional information regarding uncertainty in the dose-response model.
The Beta-Poisson Dose-Response model shown in Figure III-4 estimates the probability of the total V. parahaemolyticus risk per serving (gastroenteritis alone and gastroenteritis followed by septicemia) as a function of dose. For example, using the curve with the highest weight (the dark line in Figure III-4), the probability of illness is approximately 0.5 for a dose of approximately 100 million cfu. This means that for every 100 servings at that dose level, approximately 50 individuals will become ill. At exposure levels of approximately 1,000 cfu, the probability of illness is relatively low (<0.001). The probability of illness approaches 1.0 (i.e., 100% certainty of illness) at exposure levels around 1x109 cfu.
For the purpose of this risk assessment, it was assumed that there is no sensitive subpopulation with respect to the occurrence of an infection leading to gastroenteritis. However, given the occurrence of illness, it was estimated that it was more likely that the infection leads to a severe outcome (e.g., septicemia or death) among individuals with an underlying chronic medical condition.
The probability of gastroenteritis progressing to septicemia in healthy and immunocompromised individuals was estimated using an application of Bayes' Theorem (see for example, Fleiss, 1973). The equation below illustrates the relationship between the frequency of a given outcome, health status, and the probability of the outcome.
Pr(illness outcome | health status)
= (Pr(health status | illness outcome) * Pr(illness outcome))/Pr(health status)
where, Pr(illness outcome | health status) denotes the frequency or probability of an illness outcome type within a subpopulation of individuals defined by the existence of a common predisposing health condition ("health status").
All factors on the right hand side of the equation are identifiable based on a set of CDC's epidemiological case series data reported by Angulo and Evans (1999). The statistics of the case series were:
Of the cases with available information:
Substituting the observed data into the above equation provides an estimate of the probability of septicemia occurring. Thus, for the subpopulation identified as having an immunocompromised chronic health condition, the probability of septicemia (given that illness occurs) was estimated as follows:
Pr(septicemia | immunocompromised)
= Pr(immunocompromised | septicemia) * Pr(septicemia)/Pr(immunocompromised)
= ((3/4) * (5/107))/(23/79) = 0.12
The probability of septicemia occurring consequent to culture-confirmed illness in healthy individuals and the total United States population was estimated in a similar fashion (see Appendix 4).
It is important to recognize that the estimated probabilities based on the CDC data pertain to culture-confirmed illnesses; i.e., these are probabilities conditional on both the occurrence of illness and the identification of that illness by a confirmed culture. Analysis of the cases series data (Angula and Evans, 1999) indicates that the rate of reported illnesses that are culture confirmed is higher in individuals with an immunocompromising health condition compared to individuals with no pre-existing health condition. It was assumed that approximately 7% of the United States population has an underlying medical condition (Klontz, 1997). Therefore, the equation was modified to account for the differential reporting rates for culture-confirmed illness for immunocompromised versus healthy subpopulations. For details of this analysis, see Appendix 4.
As shown in Table III-4, the overall estimated risk of progression to septicemia occurring subsequent to V. parahaemolyticus illness is 0.0023, or approximately 2 cases of septicemia per 1,000 illnesses. For immunocompromised individuals, however, the probability of gastroenteritis progressing to septicemia is approximately 10-fold higher, with approximately 25 cases per 1,000 illnesses. This translates to a mean of approximately 7 cases per year of septicemia for the total population, 2 cases per year for the healthy population, and 5 cases per year for the immunocompromised population.
| Population | Probability of Septicemia | Mean Number of Cases (per 1000 Illnesses) | Mean Number of Cases (per Year)a |
|---|---|---|---|
| Total | 0.0023 | 2 | 7 |
| Healthy Individuals | 0.00063 | <1 | 2 |
| Immunocompromised Individuals | 0.025 | 25 | 5 |
a Number of Cases per Year = (total illness/year) × (probability of septicemia) × (percentage of population). Total illness/year assumed to be 2,800 (Painter, 2003); 7% of the population assumed immune compromised (Klontz, 1997) and 93% assumed healthy.