|
June 2005
See also: March 2006 revised report - Approaches to Establish Thresholds for Major Food Allergens and for Gluten in Food and FDA's Responses to Public Comments on the Draft Report
This report summarizes the current state of scientific knowledge regarding food allergy and celiac disease, including information on dose-response relationships for major food allergens and for gluten, respectively. The report presents the biological concepts and data needed to evaluate various approaches to establishing thresholds that would be scientifically sound and efficacious in relation to protection of public health. Each approach has strengths and weaknesses, and the application of each is limited by the availability of appropriate data. It is likely that there will be significant scientific advances in the near future that will address a number of the limitations identified in this report.
The Threshold Working Group expects that any decisions on approaches for establishing thresholds for food allergens or for gluten would require consideration of additional factors not covered in this report. Furthermore, one option that is implicit in the report's discussion of potential approaches is a decision not to establish a threshold at this time.
The report identifies four approaches that could be used to establish thresholds:
Finding 1. The initial approach selected to establish thresholds for major food allergens, the threshold values, and any uncertainty factors used in establishing the threshold values should be reviewed and reconsidered periodically in light of new scientific knowledge and clinical findings.
Finding 2. The analytical methods-based approach can be used to establish thresholds for those major food allergens for which validated analytical methods are available. However, if this approach is used, the thresholds should be replaced by thresholds established using one of the other approaches as quickly as possible.
Finding 3. The safety assessment-based approach, based on currently available clinical data, is a viable way to establish thresholds for the major food allergens. If this approach is employed, the Lowest Observed Adverse Effect Level (LOAEL) or No Observed Adverse Effect Level (NOAEL) determinations used should be based on evidence of the "initial objective symptom." Individual thresholds should be established for each of the major food allergens. If it is not feasible to establish individual thresholds, a single threshold based on the most potent food allergens should be established. In those instances where a LOAEL is used rather than a NOAEL to establish a threshold, an appropriate uncertainty factor should be used.
Finding 4. Of the four approaches described, the quantitative risk assessment-based approach provides the strongest, most transparent scientific analyses to establish thresholds for the major food allergens. However, this approach has only recently been applied to food allergens, and the currently available data are not sufficient to meet the requirements of this approach. A research program should be initiated to develop applicable risk assessment tools and to acquire and evaluate the clinical and epidemiological data needed to support the quantitative risk assessment-based approach. Thresholds established using this approach should be reevaluated periodically as new data and tools become available.
Finding 5.The statutorily-derived approach provides a mechanism for establishing thresholds for allergenic proteins in foods based on a statutory exemption. Potentially, this approach could be used to set a single threshold level for proteins derived from any of the major food allergens. This approach might yield thresholds that are unnecessarily protective of public health as compared with thresholds established using the safety assessment-based approach. However, confirming this would require additional data. If this approach is employed to establish thresholds, it should be used only on an interim basis and should be reevaluated as new knowledge, data, and risk assessment tools become available.
Finding 6.The initial approach selected to establish a threshold for gluten, the threshold value selected, and any uncertainty factors used to establish the threshold should be reviewed and reconsidered periodically in light of new scientific knowledge and clinical findings.
Finding 7. The analytical methods-based approach can be used to establish a threshold for gluten. However, if this approach is used, the threshold should be replaced by a threshold established using one of the other approaches as quickly as possible.
Finding 8.The safety assessment-based approach is a viable approach to establish a threshold for gluten using currently available LOAEL data for celiac disease. An overall uncertainty factor should be estimated from the data and applied to the LOAEL to establish a threshold for gluten. Any threshold derived from this approach should be reevaluated as new research data become available. Available data are insufficient at the current time to use this approach to establish a threshold for oat gluten for those individuals with celiac disease who are also sensitive to oats. However, it is likely that a threshold based on wheat gluten would be protective for individuals susceptible to oat gluten.
Finding 9.Use of the quantitative risk assessment-based approach to establish a threshold for gluten does not appear to be feasible at the present time. However, considering the benefits that could be gained from using the risk assessment-based approach, priority should be given to establishing a research program to acquire the knowledge and data needed.
Finding 10. There appear to be no suitable legal requirements or exemptions that would serve as the rationale using for a statutorily-derived approach to establish a threshold for gluten. This approach is not viable.
Any approach used to establish a threshold to protect consumers with food allergies or susceptible to celiac disease should be used in an iterative manner. The threshold approach should be re-examined periodically to consider new knowledge, data, and approaches.
Threshold Working Group
Robert Buchanan (Lead)
Sherri Dennis (Associate Lead)
Core Writing Team
Steven Gendel (Lead)
David Carlson
Sherri Dennis
Working Group Members
David Acheson
Sue Anne Assimon
Nega Beru
Philip Bolger
Ricardo Carvajal
Catherine Copp
Kenneth Falci
Elizabeth Harden
Rhonda Kane
John Kvenberg
Stefano Luccioli
Douglas Park
Richard Raybourne
Terry Troxell
Katherine Vierk
The Threshold Working Group would also like to acknowledge the assistance provided by:
Lori Pisciotta in preparation of the report,
Eric Garber for information on methods of analysis,
Curtis Barton for information on
statistics and study designs.
Michael Landa for consultations on the FALCPA.
Accurate and informative labeling is critical for allergic consumers, individuals with celiac disease, and their families because they need to rely on strict avoidance to prevent potentially serious reactions. The Food Allergen Labeling and Consumer Protection Act of 2004 (P.L. 108-282) (FALCPA) amends the Federal Food, Drug, and Cosmetic Act (FFDCA) and requires that the label of a food product that is or contains an ingredient that bears or contains a "major food allergen" declare the presence of the allergen as specified by FALCPA. FALCPA defines a "major food allergen" as one of eight foods or a food ingredient that contains protein derived from one of those foods.
An important scientific issue associated with the implementation of FALCPA is the existence of threshold levels below which it is unlikely that a food allergic individual would experience an adverse effect. FALCPA provides two processes by which an ingredient may be exempted from the FALCPA labeling requirements, a petition process (21 U.S.C. 343(w)(6)) and a notification process (21 U.S.C. 343(w)(7).) Under the petition process, an ingredient may be exempt if the petitioner demonstrates that the ingredient "does not cause an allergic reaction that poses a risk to human health." Under the notification process, an ingredient may be exempt if the notification contains scientific evidence that demonstrates that the ingredient "does not contain allergenic protein," or if FDA previously has determined, under section 409 of the FFDCA, that the food ingredient does not cause an allergic response that poses a risk to human health. Thus, understanding food allergen thresholds and developing a sound analytical framework for such thresholds are likely to be centrally important to FDA's analysis of, and response to, FALCPA petitions and notifications.
FALCPA also requires FDA to promulgate a regulation to define and permit the use of the term "gluten free" on the labeling of foods. Such labeling is important to patients suffering from celiac disease, an immune-meditated illness. Strict avoidance of gluten at levels that will elicit an adverse effect is the only means to prevent potentially serious reactions. Thus, consumers susceptible to celiac disease need accurate, complete, and informative labels on food to protect themselves. Understanding thresholds for gluten will help FDA develop a definition of "gluten free" and identify appropriate use of the term. Section 204 of FALCPA directs FDA to prepare and submit a report to Congress. The report is to focus principally on the issue of cross-contact of foods with food allergens, and is to describe the types, current use of, and consumer preferences with respect to advisory labeling. Cross-contact may occur as part of the food production process where residues of an allergenic food are present in the manufacturing environment and are unintentionally incorporated into a food that is not intended to contain the food allergen, and thus, the allergen is not declared as an ingredient on the food's label. In some cases, the possible presence of the food allergen is declared by a voluntary advisory statement. Understanding food allergen thresholds and developing a sound analytical framework for such thresholds is also likely to be useful in addressing food allergen cross-contact and the use of advisory labeling.Both as part of its on-going risk management of food allergens and in response to FALCPA, CFSAN established an internal, interdisciplinary group (the Threshold Working Group) to evaluate the current state of scientific knowledge regarding food allergies and celiac disease, to consider various approaches for establishing thresholds for food allergens and for gluten, and to identify the biological concepts and data needed to evaluate the scientific soundness of each approach. This draft report is the result of the working group's deliberations.
This draft report summarizes the current state of scientific knowledge regarding food allergies and celiac disease, including information on dose-response relationships for major food allergens and for gluten, respectively. The ability to establish a threshold depends on understanding the dose-response relationship between the ingestion of an allergen or gluten and the elicitation of an adverse response. Implicit in establishing such dose-response relationships is the identification of susceptible populations and characterization of any threshold levels below which all, or part, of the susceptible population does not respond. This draft report identifies the biological concepts and data needed to evaluate various approaches for establishing thresholds that would be scientifically sound and efficacious in relation to protection of public health.
The term "threshold" has been used to refer to a variety of different concepts (Table I-1) that apply either to individuals or populations. Thresholds can be measured experimentally in animals or humans [i.e., No Observed Adverse Effect Level (NOAEL) or Lowest Observed Adverse Effect Level (LOAEL)], derived from epidemiological data, estimated by modeling (statistical or simulation), established by statute, or arise as the result of the selection of an analytical method. The ability to measure or determine a threshold may be limited by the sensitivity and specificity of the methods available to measure either the stimulus or the response. Understanding the strengths and limitations of the data underpinning the different approaches is particularly important when dealing with adverse effects that have low probabilities of occurring.
| Type | Description |
|---|---|
| Etymological Definition | "The intensity below which a mental or physical stimulus cannot be perceived and can produce no response." (Webster's Dictionary). |
| Toxicological | The dose at, or below which, an adverse effect is not seen in an experimental setting. |
| Methodological | The limit of detection of an analytical method. |
| Statutory | The establishment of a limit by statute, below which no regulatory action will be taken. |
As noted, FALCPA amends the FFDCA to prescribe the manner in which food labels must disclose that a food is, or contains an ingredient that bears or contains, a major food allergen. The law also requires the FDA to issue a regulation to define and permit use of the term "gluten-free."
FALCPA establishes a petition process through which a food ingredient may be exempt from FALCPA's labeling requirements if the ingredient does not cause an allergic response that poses a risk to human health. FALCPA also establishes a notification
process under which a food ingredient described in section 201(qq)(2) of the FFDCA may be exempt from FALCPA's labeling requirements if the ingredient does not contain allergenic protein, or if FDA previously has determined, under section 409 of the FFDCA, that the food ingredient does not cause an allergic response that poses a risk to human health.
From the perspective of the Working Group, implementation of the FALCPA petition and notification provisions could present several key scientific issues. First, what is an "allergic response?" Second, do all allergic responses pose a risk to human health, or do some allergic responses pose more of a risk than others? Third, can allergens occur in a food either in a form or at a level that is too low to cause harm (i.e., either the allergen does not cause a biological response or the response is too mild to be considered hazardous)?
Under FALCPA, a "highly refined oil" derived from one of eight foods or food groups and "any ingredient derived from such highly refined oil" are exempt from the definition of "major food allergen" and from FALCPA's labeling requirements. As discussed further below, there is evidence that consumption of highly refined oils does not appear to be associated with allergic responses despite the potential presence of low levels of protein in these oils.
Section 206 of FALCPA requires FDA to issue a proposed rule to define and permit use of the term "gluten-free" on labeling of foods. Section 203 of FALCPA recognizes that"the current recommended treatment for celiac disease is avoidance of glutens in foodsthat are associated with" the disease. FALCPA does not directly state how the term "gluten-free" should be defined.
Many consumers consider a wide variety of adverse effects associated with the ingestion of foods to be "food allergies." These adverse effects may occur for a variety of immunologic, toxicologic, or metabolic reasons. The symptoms associated with these effects can range from oral irritation and sensitivity, to enteropathies (gastrointestinal tract injury, pain, and nutrient malabsorption), colitis, and eczema (Jackson, 2003). In some instances, these symptoms can be caused by toxic compounds such as histamine, which is formed by microbial conversion of naturally occurring histidine in foods. In other instances, adverse effects can be caused by metabolic conditions such as lactose intolerance. While these conditions are well documented and in some cases potentially life threatening, they are most appropriately termed food intolerances (Figure II-1) (Johansson et al., 2001; Sampson, 2004).
Immune responses to components of foods can occur that adversely affect portions of the population. These immune responses include: (1) immunoglobulin E (IgE)-mediated hypersensitivity (e.g., oral allergy syndrome, anaphylaxis), (2) cell-mediated hypersensitivity (e.g., celiac disease, food protein-induced enterocolitis), and (3) combined IgE- and cell-mediated immunity (e.g., eosinophilic gastroenteritis, atopic dermatitis). For the purposes of this report, the term "food allergy" will be used to describe IgE-mediated immune responses resulting from the ingestion of specific foods (Johansson et al., 2001; Jackson, 2003; Sampson, 2004). The most severe and immediately life-threatening adverse effects are associated with IgE-mediated hypersensitivity (Johansson et al., 2001; Jackson, 2003; Zarkadas et al., 1999).
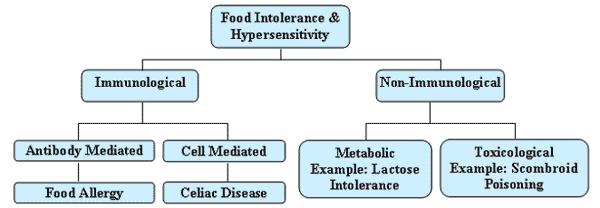
Figure II-1. Food Intolerance and Hypersensitivity
An allergic reaction stems from an abnormal, or exaggerated, immune system response to specific antigens, which in foods are proteins (Sampson, 1999). This immune response occurs in two phases, an initial "sensitization" to an allergen and the "elicitation" of an allergic reaction on subsequent exposure to the same allergen. Sensitization occurs when a susceptible individual produces IgE antibodies against specific proteins in a food. Upon re-exposure to the same food allergen, the allergenic proteins bind to IgE molecules on immune mediator cells (basophiles and mast cells), leading to activation of these mediator cells. This elicitation causes the release of inflammatory molecules (e.g., leukotrienes and histamine). The specific symptoms and severity of an allergic reaction are affected by the concentration of allergen, route of exposure, and the organ systems involved (e.g., skin, GI tract, respiratory tract, and blood) (Taylor and Hefle, 2001).
Sensitization
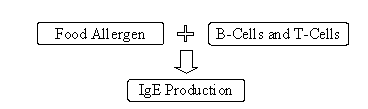
Elicitation
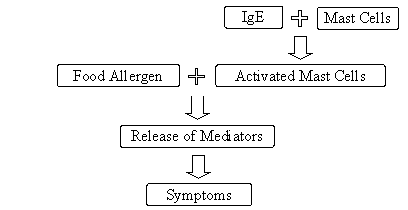
Figure II-2. Mechanism of Allergic Reactions
The clinical manifestations of food allergic reactions range from mild irritation to severe, life-threatening respiratory distress and shock. Specific symptoms may involve the skin (e.g., pruritis, erythema, urticaria, angiodemia, eczema), eyes (e.g., conjunctivitis, periorbital swelling), nose (e.g., rhinitis, sneezing), oral cavity (e.g., swelling and itching of lips, tongue, or palate), or gastrointestinal tract (e.g., reflux, colic, abdominal pain, nausea, vomiting, diarrhea). In more severe reactions, involvement of the respiratory tract (e.g., cough, asthma, difficulty breathing, swelling around the larynx and vocal cords) and cardiovascular system (e.g., faintness, hypotension) can lead to loss of consciousness, asphyxiation, shock, or death. The term "anaphylaxis" is used to describe multisystemic severe reactions to an allergen requiring immediate medical intervention (Jackson, 2003).
Table II-1 provides a summary of subjective and objective symptoms that can be experienced during an allergic reaction. Allergic reactions usually occur within a few minutes to one hour after ingestion of an offending food and often progress from mild to severe, with higher doses causing more severe reactions (Sampson, 2005). Once exposure occurs, individuals may experience immediate numbness or pruritis at the site of contact or experience general uneasiness. These symptoms are characterized as "subjective" since they cannot be observed by others. As the effects progress, "objective" symptoms such as flushed skin, hives, or swelling of the lips and face occur. These symptoms are often mild and short-lived. However, in some cases, they may be associated with more severe symptoms, involving the respiratory and cardiovascular systems. Such symptoms can lead to hospitalization or death, even with appropriate medical intervention. Not all severe, or anaphylactic, reactions are necessarily preceded by milder signs and not all reactions are immediate. In some cases, anaphylactic reactions may be delayed by a few hours after initial symptoms (Sampson, 2005).
The severity of an allergic reaction is affected by several factors that include genetic predisposition (atopy), age, type of food allergen, nature of any food processing, environment, and physiological conditions (Taylor and Hefle, 2001; Sampson, 2003; Maleki, 2004). For example, exercise, medications (e.g., non-steroidal anti-inflammatories), alcohol consumption, and asthma may enhance the severity of an allergic reaction (Sampson, 2005). Most severe and fatal allergic reactions to foods have occurred in adolescents and teens who were highly atopic and had a history of asthma (Sampson, 2003; Pumphrey, 2004).
It is generally assumed that a history of previous serious allergic reaction(s) indicates an increased risk of severe reaction(s). However, a history of mild reactions does not preclude the possibility of a subsequent severe reaction. For example, Sicherer et al. (1998) observed that mild reactions to peanut in childhood tend to become more severe and unpredictable in later childhood and adulthood. This may be due to the fact that these children develop asthma later in life (Sampson, 2005). Also, a recent review of anaphylactic fatalities in the United Kingdom showed that, in 85% of fatal food reactions the patient had previously experienced a non-severe reaction (Pumphrey, 2004). Pumphrey (2004) states that the severity of previous reactions is not a risk factor for a fatal reaction for nut allergic patients. These data imply that any individual with a clinical history of IgE-specific food allergy may be considered to be predisposed to anaphylaxis or severe reaction.
| Subjective Symptoms | Objective Symptomsa | ||
|---|---|---|---|
| CUTANEOUS |
Skin |
Pruritus (Itching) |
Skin flushing or erythema (redness) Pilor erection ("goosebumps") Rash (hives) - acute Eczema (usually delayed, >6 hours) Angioedema (swelling, especially face) |
|
Oral cavity (lips, tongue, palate) |
Pruritus (Itching), numbness, dryness |
Edema (swelling, may also include the uvula) |
|
|
Eyes, conjunctiva |
Pruritus (Itching) |
Periorbital (around eyes) edema, redness of conjunctiva and tearing |
|
|
GASTROINTESTINAL |
Nausea, pain (except infants/young child) |
Vomiting, diarrhea, abdominal pain (infants) |
|
| RESPIRATORY |
Nose |
Pruritus (Itching) |
Nasal congestion or runniness, sneezing |
|
Larynx, throat |
Pruritus (Itching), dryness/tightness |
Swelling around the larynx and vocal cord, voice hoarseness, stridor (inspiratory wheeze), cough |
|
|
Lungs |
Shortness of breath, chest pain/tightness |
Respiratory distress (i.e., ↑ breathing rate, difficulty catching breath, ↓ peak expiratory flow measurement), cough, wheezing |
|
|
HEART and CARDIOVASCULAR |
Chest pain/ tightness, feeling of faintness, dizziness |
Syncope (fainting, loss of consciousness), hypotension (low) or shock (very low blood pressure), dysrhythmia (abnormal heart rhythm) |
|
|
OTHER |
"Sense of impending doom"b |
Uterine contractions (women) |
|
a Anaphylaxis is a poorly defined condition representing a severe or multisystemic allergic reaction. Allergic reactions described by objective symptoms involving the respiratory or cardiovascular systems would be considered severe and managed as an anaphylactic reaction by most clinicians. In some classifications, symptoms in two or more categories above (e.g., cutaneous, gastrointestinal, respiratory), even if relatively mild, would also be classified as anaphylaxis. Anaphylactic "shock" denotes a consequence of anaphylaxis where heart irregularities and leakage of blood vessels leads to extreme blood volume loss (usually greater than 25% of resting blood volume) and extreme hypotension. b A "sense of impending doom" may signal or predict an impending severe reaction. | |||
The most recent information on the prevalence of food allergies in the U.S. suggests that up to 6% of children and 4% of the total population have IgE-mediated food allergies (Sampson, 1997; Sampson, 2004; Sicherer et al., 2003; Sicherer et al., 2004). The estimated prevalence in the U.S. population of allergies to each of the food allergens identified by the FALCPA is given in Table II-2. Severe food-related allergic reactions result in an estimated 30,000 emergency room visits, 2,000 hospitalizations, and 150 deaths per year (Sampson, 2004). Clinical data and surveys indicate that the prevalence of allergy has been rising in recent years, though there are limited historical data to compare to recent numbers (Sicherer et al., 2003; Grundy et al., 2002). Peanut allergy has received the most attention in the U.S., and data indicate an apparent doubling of peanut allergy in children under 5 years old from 1997 to 2002 (Sicherer et al., 2003). A similar increase in peanut allergy has been seen in the United Kingdom (Ewan, 1996; Grundy et al., 2002). Peanuts and tree nuts are the most common cause for fatal reactions in the US (Yunginger et al., 1988; Sampson et al., 1992; Bock et al., 2001).
| Age Group | Percentage of the Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All Allergens | Milk | Egg | Peanut | Tree nuts | Fish | Shellfisha | Wheat | Soy | |
|
Children (<3 years) |
6.0 | 2.5 | 1.3 | 0.8 | 0.2 | 0.1 | 0.1 | UNKb |
0.2 |
|
Adults (>18 years) |
3.7 | 0.3 | 0.2 | 0.6 | 0.5 | 0.4 | 2.0 | UNKb | 0.2 |
|
aShellfish includes both crustaceans and mollusks. bUNK = unknown Sources: Sampson, 1997; Sampson, 2004; Sicherer et al., 2003; Sicherer et al., 2004. | |||||||||
1. Whole foods
The FALCPA identifies eight major food allergens or food groups: milk, eggs, fish (e.g., bass, flounder, cod), crustacean shellfish (e.g., shrimp, crab, lobster), tree nuts (e.g., almonds, walnuts, pecans), peanuts, wheat, and soybeans. These foods account for 90% of all documented IgE-mediated food allergies worldwide and most severe reactions to foods (Bousquet et al., 1998; Hefle et al., 1996). More than 160 other foods are known to cause food allergies; however, these allergies are relatively rare with prevalence rates ranging from a few percent of the allergic population to single cases (Hefle et al., 1996). Each of the eight major food allergens contains multiple allergenic proteins, many of which have not been fully characterized (Gendel, 1998).
2. Food Ingredients
Some food ingredients such as edible oils, hydrolyzed proteins, lecithin, gelatin, starch, lactose, flavors, and incidental additives (e.g., processing aids), may be derived from major food allergens (Taylor and Hefle, 2001). The role that these ingredients play in food allergy has not been fully characterized. For example, lecithin is a common food ingredient which is often derived from soybeans. It is possible that soy lecithin, which contains residual protein, could elicit an allergic reaction in sensitive individuals (Muller et al., 1998; Gu et al., 2001). Another example is protein hydrolysate, which is often made from a major food allergen such as soybeans, wheat, peanuts, whey, or casein. Extensively hydrolyzed proteins present only slight risk to allergic individuals, but partially hydrolyzed protein ingredients can elicit allergic reaction (Bock and Atkins, 1989; Ellis et al., 1991; Saylor and Bahna, 1991; Kelso and Sampson, 1993; Niggemann et al., 1999). For example, hot dogs formulated with partially hydrolyzed casein have elicited allergic reactions in children allergic to cow's milk (Gern, et al., 1991; Kocabas and Sekerel, 2003).
Gelatins are ingredients derived from animals (e.g., cows, pigs) but also from the skin of various species of fish. A study of 10 fish allergic patients and 15 atopic individuals with eczema revealed that 3 and 5 individuals respectively had specific IgE to fish gelatin, suggesting the presence of allergenic protein (Sakaguchi et al., 2000). However, in a recent Double Blind Placebo Controlled Food Challenge (DBPCFC) study, all 30 fish allergic subjects in the study did not respond to a cumulative dose of 3.61 g of fish gelatin (Hansen et al., 2004).
Edible oils can be derived from major food allergens such as soybeans and peanuts, and they may contain variable levels of protein (Taylor and Hefle, 2001). The consumption of highly refined oils derived from major food allergens by individuals who are allergic to the source food does not appear to be associated with allergic reactions. For example, Taylor et al. (1981) and Bush et al. (1985) did not observe any reactions to refined peanut or soy oils in 10 and 7 allergic patients, respectively. This may not be the case for unrefined or cold-pressed oils that contain higher levels of protein residues (Taylor and Hefle, 2001). For example, Hourihane et al. (1997) reported that 6 of 60 peanut allergic individuals reacted to crude peanut oil but none responded to refined peanut oil. Similarly, Kull et al. (1999) reported that 15 of 41 peanut allergic children responded positively to crude peanut oil in skin prick tests, but none responded to refined peanut oil. The actual protein levels reported in various edible oils varies, probably due to differences in the oil, refining process, and the protein detection analytical method used. Crevel et al. (2000) reported that crude peanut and sunflower oils contained 100-300 µg/ml of protein, but that the most highly refined oils contained 0.2-2.2 µg/ml of protein. Intermediate protein concentrations were seen for partially processed oils. Teuber et al. (1997) showed that the amount of protein in both crude and refined gourmet nut oils varied both by type of oil and degree of processing, and reported values of 10-60 µg/ml for various unrefined oils and 3-6 µg/ml for the refined oils. Several other investigators reported undetectable levels of proteins in refined edible oils (Hoffman et al., 1994; Yeung and Collins, 1996; Peeters et al., 2004) using assays with detection sensitivities of <0.3 ng/ml (Peeters et al., 2004) and 0.4 mg/kg (Yeung and Collins, 1996).
Starch, which is a widely used ingredient, is often derived from corn which is not a major food allergen. However, starch can also be derived from wheat, and may contain trace levels of wheat protein. Most of these proteins are from the non-albumin fraction whereas the principal wheat allergenic proteins are albumins. The allergenicity of wheat starch for sensitive individuals has not been clinically evaluated (Taylor and Hefle, 2001).
A wide variety of flavoring substances are used in foods, but only a few are derived from known allergens (Taylor and Dormedy, 1998). As such, IgE-mediated allergic reactions to flavorings are rare, although a few cases have been documented involving hydrolyzed proteins. For example, several milk allergic individuals reacted to either hot dogs or bologna containing partially hydrolyzed casein as part of the natural flavoring used in the formulation of these products (Gern et al., 1991). Two other milk-allergic individuals reacted to milk protein in the natural flavoring used in a dill pickle-flavored potato chip (St. Vincent and Watson, 1994). The presence of peanut flour in the natural flavoring of a packaged soup elicited a reaction in a peanut-allergic individual (McKenna and Klontz, 1997).
3. Cross-Contact
Allergens, or proteins derived from allergenic foods, may be present in foods as the result of cross-contact during processing and handling. The term "cross-contact" describes the inadvertent introduction of an allergen into a product that would not intentionally contain that allergen as an ingredient. Cross-contact is generally the result of environmental exposure during processing or handling, which may occur when multiple foods are produced in the same facility or on the same processing line, through the misuse of re-work, as the result of ineffective cleaning, or from the generation of dust or aerosols containing the allergen. Cross-contact of foods with allergens has been shown to lead to allergic reactions in consumers on numerous occasions (Gern et al., 1991; Jones et al., 1992; Yunginger et al., 1983). Much cross-contact can be avoided by controlling the production environment. Whether all cross-contact can be prevented during food manufacturing is yet to be determined.
1. Design of Food Challenge Studies
A history of clinical reaction to a food and a positive skin prick test or the presence of food-specific IgE antibodies in serum are sufficient to establish that an individual has an allergy to that food. However, none of these reliably predicts the level of patient sensitivity. At present, individual sensitivity can only be determined using food challenge studies (including open, single-blind, and double-blind, placebo-controlled food challenges). The double-blind, placebo-controlled food challenge (DBPCFC) is the "gold standard" for diagnosis of food allergy and for determining clinical reactivity to low concentrations of an allergen. In these studies, neither the subjects nor the researchers know which test foods contain the allergen. Open (where both the subjects and the researchers know which test foods contain the allergen) and single-blinded (where only the researchers know which foods contain the allergen) challenges are used primarily for screening foods of low allergenic importance or for determining tolerance to food allergens. Single-blinded challenges can be placebo-controlled (SBPC). However, in open and SBPC challenges, experimenter bias may play a role in interpreting patient symptoms.
The typical food challenge protocol is a dose escalation study, usually with 15 to 30 minute dose intervals, which proceeds until a clinical effect is observed or the final dose is achieved. The test substance, starting dose and successive incremental doses vary between protocols. Because reactions are assumed to be less severe at lower doses, the starting dose is generally in the milligram range for whole foods (Bindslev-Jensen et al., 2004). Typically, food challenge studies to determine minimal eliciting doses begin in the low microgram range for the whole food or whole food protein. Incremental doses are usually doubled or increased logarithmically, so that a reasonable number of incremental doses (i.e., 6 to 10) separate the starting dose from the end dose. This final dose is usually chosen to be the normal amount in a food serving, usually 8 to 10 gm of dried food or 60 to 100 gm of wet food (Bock et al., 1988; Bindslev-Jensen et al., 2004). The ability to tolerate this amount, followed by a negative open challenge on a different day, is considered to be evidence that the individual is not allergic to that allergen (Taylor et al., 2004).
Most oral challenge studies are designed to establish a diagnosis of food allergy rather than to determine safety (Taylor et al., 2004). Consequently, these studies do not start at doses below a known LOAEL. Thus, individuals who react to the starting dose are not necessarily demonstrating a true LOAEL because it is not possible to know whether these individuals would have reacted to a lower starting dose without further testing. A NOAEL cannot be established as long as one or more study participants reacts to the starting dose.
Most elicited symptoms occur within 3 to 15 minutes after a challenge (Bindslev-Jensen et al., 2004). Thus, an interval of 15 minutes between challenge doses may be sufficient to confirm a negative response. Most challenge studies report the dose that elicits the first objective symptom. Because subjective symptoms may have preceded the first objective symptom at lower doses, it is often difficult to ascertain whether the reported LOAEL truly represents the lowest dose to elicit a reaction. Subjective and objective symptoms, including their measurement and interpretation are discussed below.
2. Inclusion/Exclusion of Sensitive Populations
Individuals with a history of anaphylaxis to foods and infants and children are often excluded from challenge studies for ethical reasons (Taylor et al., 2002). Moreover, individuals with very high food allergen IgE serum titers are often excluded. Thus, food challenge studies may not include key subpopulations of allergic individuals, who may also be the most sensitive to allergen exposure.
Individuals with allergies to a specific food have different genetic backgrounds and express a wide distribution of sensitivity and reactivity. Studies have shown that there may be a range of as much as one-million-fold (106) in eliciting doses from the least sensitive to the most sensitive individuals (Leung et al., 2003; Wensing et al., 2002b; Bindslev-Jensen et al., 2002). Therefore, the inclusion or exclusion of data for highly sensitive individuals can greatly affect the NOAEL determination for the population. To add to this uncertainty, the most sensitive individuals also appear to have more severe reactions (Wensing et al., 2002b; Perry et al., 2004). The thresholds measured for populations that exclude these individuals may not apply to those with severe allergic disease.
3. Testing Materials
Food challenges vary in the type of testing material used (e.g., peanut flour versus ground peanut), oral challenge vehicle (e.g., whole food versus capsules), and in the efficacy of blinding. Differences in these variables could modify the allergen distribution or concentration, affect digestibility and absorption, influence false-positive subjective reactions, and therefore, affect interpretation of the dose-response data.
The nature of the testing material is very important, as this can enhance or diminish the overall immunogenicity of the native allergen (Beyer et al., 2001; Maleki et al., 2003). The matrix used (e.g., fatty substances) can delay absorption, thus affecting the time interval to a reaction, or may affect the intrinsic allergenic properties of the food. Also, gustatory differences in the challenge doses (because of the food matrix used) may influence subjective reactions due to poor taste or fear of consuming the allergen. The use of capsules eliminates problems caused by taste, but bypasses the oral cavity. Because the oral cavity plays an important role in the initial contact and metabolism of food allergens, this may affect the subsequent severity or character of response to the challenge dose.
4. Subjective Versus Objective Reactions
There are two types of physiologic reactions or effects that can occur during a food challenge - subjective symptoms, those reported by the subject, and objective symptoms, those observed by the researcher. Because subjective symptoms may be the result of non-immunological mechanisms, elicitation of objective symptoms is believed to be the more reliable indicator of clinical reactivity to the food allergen (Taylor et al., 2004).
The symptoms of a severe allergic reaction are associated with life-threatening conditions, e.g., anaphylaxis. However, there is no consensus as to which of the less serious symptoms should be considered adverse effects. For example, can eczema be seen as a "safer" reaction than angioedema? Unlike well-defined toxicity endpoints, reactions to allergenic food ingredients are part of a wide spectrum of severity that includes trivial injury, objective systemic reactions, anaphylaxis, and death.
Subjective symptoms may be good indicators of a subsequent objective reaction, i.e, subjective symptoms may precede or signal objective symptoms in a dose-dependent manner (3rd FAARP Threshold Conference, 2004). However, most challenge studies base their LOAEL determinations on the first objective symptom rather than a subjective symptom. For example, although the Hourihane et al. (1997) study reported a threshold for peanut proteins in the milligram range, mild subjective reactions were noted in two individuals at doses of 100 μg of peanut protein. Other studies do not report specific types of reactions but rather combine symptoms into categories of mild, moderate, or severe. For example, a retrospective review of 253 failed challenges at one clinic showed that the initial reaction was severe in 72 (28%) and moderate in 88 (33%) of the challenges (Perry et al., 2004).
Currently, there is no universally accepted endpoint or response that can be used to predict significant harm from an allergic reaction. Anaphylaxis, a clearly significant endpoint, is a syndrome which is poorly described and subject to variable interpretation. Moreover, anaphylactic reactions are at one extreme of a continuum of severity. There are a number of additional factors (e.g., use of medicine, alcohol consumption, anxiety) that can significantly reduce or potentiate the impact of exposure to an allergen. Given this combination of factors, a particular dose could result in mild symptoms one day and life-threatening reactions the next.
5. Anecdotal Evidence
Although a great deal of attention has been focused on the use of challenge studies to determine threshold doses or reaction patterns for food allergens, anecdotal reports of individuals suffering life-threatening allergic reactions from minute exposures to food allergens challenge the notion that an allergen threshold truly exists, especially for sensitive individuals. For example, literature reports have linked kissing (Hallett et al., 2002; Steensma, 2003; Eriksson et al., 2003) and exposure to airborne particles (Crespo et al., 1995; Casimir et al., 1997; Sackesen and Adalioglu, 2003) to allergic reactions. Although in many of these cases the amount of allergen exposure cannot be assessed, it is conceivable that the whole food exposure level needed to elicit a harmful reaction is extremely low. In this context, it should be noted that the statistical model developed by Bindslev-Jensen et al. (2002) suggested that concentrations as low as 700 ng for peanut and in the low microgram ranges for egg, soy flour, and cow's milk may elicit a reaction in one in a million allergic individuals. Although this model suggests that a majority of allergic individuals would likely tolerate food allergen concentrations in the milligram range, it supports the anecdotal evidence that very low concentrations of allergen may, at some low but finite probability, elicit harm in highly sensitive individuals.
1. Matrix Effects
Food allergens often occur as components of processed foods, and many allergic reactions occur following exposure to such allergens (Bock et al., 2001). Therefore, it is important to understand how the nature or composition of the food (the food matrix) affects the reaction elicitation threshold.
Very little information exists on matrix effects for the majority of allergens. It has been reported that fat content can modify the reactions in a peanut DBPCFC (Grimshaw et al., 2003). Three of 4 subjects challenged with peanut flour in a matrix containing 31.5% fat reacted at a higher than expected dose, and had reactions that were more severe than expected, based on previous exposures to a standard recipe containing 22.9% fat. Upon re-challenge with the 22.9% recipe, their reactions returned to expected levels with respect to dose and severity. The cumulative dose of peanut protein required to elicit symptoms was 12 to 31 times higher when using the higher fat recipe. The authors suggested that the peanut allergens in the higher fat recipe were not readily available to react with IgE on mast cells in the mouth. This was based on the observation that radioallergosorbent test (RAST) inhibition assays and enzyme linked immonosorbent assay (ELISA) detection tests showed that peanut allergens in the higher fat mixture were less available in vitro. In addition, these 3 patients all had histories of an initial oral challenge response. The lack of an oral early warning with a high-fat food may have caused these patients to consume more allergen prior to the onset of other symptoms. By the time digestion of the fat took place in the stomach and intestine, the total dose consumed was higher, resulting in a more severe reaction.
Grimshaw et al. (2003) further reported that the slopes of RAST-inhibition curves did not change for peanut allergens in high-fat versus low-fat mixtures, indicating that there was no change in antibody-binding properties. Thus, it appears that the antigenic properties of the peanut flour were not altered by the higher fat matrix, and that the changes in apparent threshold may have resulted from a combination of physiological and behavioral factors.
Kato et al. (2001) also observed a matrix effect with the major egg allergen ovomucoid. The ability of ovomucoid to bind IgE was reduced in a model pasta compound composed of durum wheat and egg white. This decrease was attributed to changes in antigenicity associated with formation of disulfide bonds between the ovomucoid and wheat gliadins.
2. Processing Effects
Numerous studies have described alterations in allergens as a result of processing or cooking. Various types of processing (heating, milling, fermentation, etc.) may alter the antigenic properties of allergens because these processes can affect the 3-dimensional structure of proteins and thus the IgE binding epitopes. The type and extent of structural alterations may vary depending on the processing method. This is especially true for conformational epitopes because they are dependant on tertiary structure (Cooke and Sampson, 1997; Vila et al., 2001). For many food allergens, processing effects are inherent in the data used to characterize thresholds because the test articles used in DBPCFCs are processed. For practical reasons, the test material must be concealed in some way for the study to be "blinded." For example, the taste of peanut butter or peanut flour must be disguised in DBPCFCs for peanut allergies. Preparation of the test material typically involves cooking or processing of the allergenic food. In addition to altering existing epitopes, processing might also induce chemical or structural changes that result in the formation of new antigenic epitopes, or neoantigens (Maleki, 2004).
Altered antigenic reactivity is most commonly assessed by measuring changes in the binding of antibodies to extracts of raw and processed foods. Reduced or enhanced IgE binding in such studies would suggest that the threshold for an allergic reaction could be affected by processing. However, definitive proof of an altered threshold requires DBPCFC testing.
The effects of processing on some major allergens have recently been reviewed, and are discussed below (Besler et al., 2001; Poms and Anklam, 2004). Variable patient responses make it difficult to conclude that a particular processing or cooking procedure affects allergenicity in all cases.
Peanuts. Extracts of roasted peanuts have been shown to bind IgE from patients at 90-fold higher levels than do similar extracts of raw peanuts in competitive, IgE-based ELISAs (Maleki et al., 2000). Using immunoblot techniques, two of the major allergenic proteins in peanut, Ara h 1 and Ara h 2, were shown to be highly resistant to heat and gastrointestinal digestion following treatment in the Maillard Reaction (which occurs during the processing or browning of foods in the presence of heat and sugars). Earlier studies also observed increased IgE binding and altered IgE epitopes in roasted versus raw peanuts (Nordlee et al., 1981). The allergenic proteins Ara h 1, Ara h 2, and Ara h 3 from fried or boiled peanuts bound significantly less IgE than the same proteins from roasted peanuts (Beyer et al., 2001), even though there were similar amounts of the allergenic proteins in peanuts processed by each method. These studies suggest that thresholds for boiled or fried peanuts may be higher than for roasted or raw peanuts, at least for the three major peanut allergens. In practical terms, the vast majority of peanuts consumed whole or in processed foods in the U.S. are roasted. Boiled or fried peanuts are an ethnic or regional specialty and are usually eaten whole, rather than as a component of processed foods.
Milk. Pasteurization and homogenization did not reduce allergenicity in skin prick tests or DBPCFC (Host and Samuelsson, 1988). However, boiling milk for 10 minutes reduced IgE binding of the allergenic proteins alpha-lactoglobulin and casein by 50 to 66% and eliminated beta-lactoglobulin and serum albumin reactivity in skin prick tests (Besler et al., 2001; Norgaard et al., 1996). Hypoallergenic infant formulas produced from heat denatured or enzymatically hydrolyzed caseins or whey proteins showed reduced allergic reactivity by immunoblot, RAST, and DBPCFC in most milk-allergic children. However, some severe reactions have been reported (Sampson et al., 1991; Saylor and Bahna, 1991). Maillard reaction products in milk are reported to have increased allergenicity in skin tests (Maleki, 2004). Allergic reactions have also been reported involving both hard and soft cheeses (Besler et al., 2001).
Egg. Both soft and hard boiling of eggs decreased, but not eliminate, antigen binding of rabbit antiserum to ovomucoid and ovalbumin (Besler et al., 2001). Heated egg white showed a 58% decrease in IgE binding in RAST (Anet et al., 1985). A decrease in positive reactions was seen with heated egg white in 55% of egg allergic patients using DBPCFC (Urisu et al., 1997). There are reports of allergic reaction to egg contained in cooked meatballs or hamburger (Sampson et al., 1992; Besler et al., 2001).
Fish. Boiling of ten species of fish failed to eliminate allergenicity in DBPCFC (Bernhisel-Bradbent et al., 1992). IgE binding to fish proteins in immunoblots was reduced, but not eliminated. Canning appears to reduce allergic reactions to tuna and salmon in allergic patients tested by DBPCFC (Bernhisel-Broadbent et al., 1992). IgE binding of allergenic proteins from canned fish was reduced by 98 to 99% compared to boiled fish. IgE binding studies indicate that fish allergens are present in surimi.
Shellfish. Boiling does not reduce reactivity of shrimp allergens (Daul et al., 1988; Naqpal et al., 1989).
Soy. Heating soybeans at 100ºC for 60 minutes does not completely eliminate IgE binding to allergenic soy proteins (Burks et al., 1992). Various soybean products including sprouts, soy sauce, hydrolyzed soy protein tofu, miso, and lecithin all retained IgE-binding activity (Besler et al., 2001). IgE binding proteins have been found in soy lecithin (Gu et al., 2001; Porras et al., 1985; Paschke et al., 2001). Allergic reactions to soy lecithin have also been reported (Renaud, 1996; Palm, 1999). The protein content of soy lecithin has been reported to vary between 2.8-202 mg per 100 g (Besler et al., 2001; Paschke et al., 2001). IgE binding proteins have been detected in unrefined soybean oils (Paschke et. al., 2001), but inconsistently in refined oil (Awazuhara et al., 1998; Paschke et al., Errahali et al., 2002)
Tree nuts. Protein extracts of several hazelnut-containing products demonstrated less IgE binding than raw hazelnut extracts, suggesting that processing reduced allergenicity. However, some IgE binding capacity remained (Wigotzki et al., 2001). Several cases of anaphylaxis have been described for other processed nut-containing products, suggesting that processed tree nuts in general retain allergenic activity (Besler et al., 2001). Roasting, blanching, autoclaving, or microwaving did not change the ability of animal antisera to bind almond proteins (Venkatachalam et al., 2002).
3. Detecting and Measuring Allergens
There are several factors that make it difficult to detect and measure food allergens. These include sampling problems and difficulties in quantifying proteins, particularly allergenic proteins, in a wide variety of foods. Further, an allergen may be a minor component of a highly complex, heterogeneous food. The food matrix can sequester allergens, hindering detection, while not significantly affecting allergenicity. It is also difficult to estimate the amount of a food allergen that may be present from the result of an assay that only measures protein, particularly when there is more than one allergenic protein.
The only commercial methods that have been shown to detect food allergens reliably use immunological techniques such as ELISA (Poms et al., 2004; Krska et al., 2003; Popping et al., 2004). In many cases, these methods were designed to detect representative biomarkers, not necessarily a specific allergenic protein. Many kits contain polyclonal antibodies that detect both non-allergenic and allergenic proteins. For example, the peanut ELISA assays that have completed Multiple Laboratory Performance Tested validation are designed to detect multiple proteins indicative of the presence of the food (e.g., peanuts), not to detect or quantify specific allergenic proteins (Park et al., 2005). There are no validated detection methods or commercially available kits for most food allergens or for specific allergenic proteins.
The FDA and AOAC investigated the ability of three commercial peanut test kits [BioKits Peanut Testing Kit (Tepnel), Veratox for Peanut Allergens (Neogen Corp.), and RiDASCREEN Peanut (R-Biopharm GmbH)] to measure accurately peanuts in four food matrices (cookies, ice cream, milk chocolate, and breakfast cereal) (Park et al., 2005). The validation study, requiring 60 analyses of test samples at the target level of 5µg peanut/g of food and 60 analyses of "peanut-free" controls, was designed to ensure that the lower 95% confidence limit on the true sensitivity and specificity rates exceeded 90% (Park et al., 2005). The results from this study showed that all the test kits correctly allocated the test samples at the target level. No comparable studies have been completed for any other food allergen.
Scientific practice is to calibrate, standardize, and validate assays and commercial test kits for each food product because minor differences in the matrix change the recovery and detection of specific food proteins. Standardization requires the preparation of samples identical to the test sample and containing known amounts of a specific food allergen. Nevertheless, because different antibody-based assays recognize different protein epitopes, variable results may be obtained using different test systems. This variability was evident in results obtained in the Food Analysis Performance Assessment Scheme (FAPAS®) supervised proficiency studies of wheat, peanut, egg, and milk test kits (FAPAS Reports 2705 and 2705 Supplement (wheat), 2708 (peanuts), 2710 (egg and milk).
Highly variable food matrices and the nature of food production also create sampling challenges. The distribution of allergenic proteins within whole foods is not necessarily homogenous, and allergenic ingredients may not be evenly distributed throughout processed foods. In addition, cross-contact may result in a heterogeneous distribution of allergens within or on a food. For example, nuts may be introduced into chocolate on a production line where nut-containing and nut-free products are processed sequentially. In this case, cross-contact is most likely to occur at the beginning of a production run for the nut-free product. Thus, allergen testing using chocolate taken from the end of a production run might not adequately characterize the risk.
For a food product, development of a scientifically sound sampling plan that includes a statistical analysis of the probability that all allergens are detected, ensures that any allergens present are accurately measured. Important sampling questions that need to be considered include whether the allergen is likely to be heterogeneously distributed within the batch; the number of samples per batch that should be tested; which batches should be tested; which portion of a run should be tested; and how to obtain a specific degree of confidence (e.g., 95% confidence) that no allergen is present.
The currently available commercial assays are designed for the detection of food allergens, not specific allergenic proteins. Tests for specific allergenic proteins (e.g., Ara h 1 in peanut) may provide useful supplemental information, but these tests are research tools and are not currently viewed as practical for routine use.
Three of the major food allergens identified in the FALCPA are actually groups of foods: crustaceans, fish, and tree nuts. It is possible that proteins from two or more species within each of these "collective allergens" might be present in a food and the available analytical methods are unable to distinguish between species in a group. Therefore, it may be necessary to consider total protein levels from all species in a group rather than the level of protein from each species. In addition, an individual allergic to one species is likely to also be allergic to other species in the group.
The ability of available test methods to distinguish different species within each group of "collective allergens" varies. To date, there are no commercially available test kits for finfish proteins and only one for crustacean tropomyosin. Ben Rejeb et al. (2003) reported the development of an ELISA for shrimp that showed significant cross-reactivity with other crustaceans. There are three commercially available tree nut test kits (two for hazel nut, one for almond), but the species specificity of these kits is not clear. Hlywka et al. (2000) showed that an almond ELISA detected protein from seven other tree nuts. The hazel nut ELISA developed by Holzhauser et al. (2002) showed cross-reactivity with other nuts, and the walnut assay developed by Niemann and Hefle (2003) reacted with three other nut species. Wei et al. (2003) developed an ELISA for cashew that showed cross-reactivity with several other nuts. Ben Rejeb et al. (2003) developed a hazel nut-specific ELISA that did not cross-react with other nuts, and Clemente et al. (2004) developed a Brazil nut assay with "negligible" cross reactivity to five other nut species.
Although not likely to be useful for routine screening or testing, techniques such as LC/MS are being used to identify specific allergenic proteins in complex food matrices (Shefcheck and Musser, 2004). These approaches may be useful either as confirmatory tests or for characterization of foods containing several allergens.
Crustacean Shellfish. Allergenic cross-reactivity among crustaceans is considered to be common. Sicherer (2001) estimated that there is a 75% probability that a shrimp-allergic individual will also react to at least one other crustacean. Waring et al. (1985) reported that 11 of 12 (92%) patients with skin prick reactions to shrimp also had positive skin prick reactions to at least one other crustacean. Similarly, Daul et al. (1987) showed that between 73 and 82% of shrimp allergic patients had positive skin prick tests to another crustacean. Chiou et al. (2003) showed that sera from 20 of 32 individuals with either shrimp- or crab-reactive IgE were reactive to both species. Further, inhibition studies with 15 of these cross-reactive sera showed relatively high affinity for both allergens. The basis for this high rate of cross-reactivity appears to be sensitivity to the highly conserved protein tropomyosin, which is considered to be a panallergen (Daul et al., 1993; Leung et al., 1999; Sicherer, 2001).
Fish. Allergenic cross-reactivity among fish species has been described in the clinical literature, but appears to be less common than among species of crustacea. Both Sicherer (2001) and Sampson (1999) estimate that there is a 50% probability that an individual allergic to one fish species will also react to at least one other fish species. Helbling et al. (1999) reported that 4 of 14 (29%) fish allergic patients reacted to two or more species in DBPCFC tests. Bernhisel-Broadbent et al. (1992) reported that 3 of 10 (30%) fish allergic patients responded to more than one fish species in oral challenges, but that skin prick tests were positive to multiple species for all of these patients. Similarly, Hansen et al. (1997) showed that eight cod allergic patients all had positive skin prick tests with two other fish species. The data presented in Pascual et al. (1992) suggest that at least 80% of a group of 79 fish allergic children had IgE antibodies to two or more fish species. In some cases, cross-reactivity has been shown to reflect the presence of one of more closely related allergenic proteins in different species (Pascual, 1992; Hansen et al., 1997; Leung et al., 1999; Hamada et al., 2003).
Tree Nuts. The prevalence of cross-reactivity among tree nuts is difficult to determine accurately for several reasons: the high proportion of severe reactions among nut-allergic patients makes it dangerous to carry out oral challenge studies, many published works test for reactivity to a small number (and variable assortment) of tree nuts, and studies often combine tests for tree nuts and peanuts. Nevertheless, Sicherer (2001) estimates that a tree nut allergic patient has a 37% chance of being allergic to two or more species of tree nut, and Sampson (1999) estimates that the probability of multiple tree nut sensitivities at greater than 50%. Ewan (1996) reported that 12 of 22 (55%) of tree nut allergic patients responded to multiple tree nuts by skin prick tests. Sicherer et al. (1998) and Pumphrey et al. (1999) both used in vitro IgE testing and found multiple sensitivities in 37% and 61% of tree nut allergic patients, respectively. There are a number of studies that report cross-reactions in one or a few patients (e.g., Teuber and Peterson, 1999; Ibanez et al., 2003; de Leon et al., 2003; Asero et al., 2004). The complex pattern of cross-reactivity among the tree nuts may reflect the fact that several different panallergens (lipid transfer proteins, profilins, Bet v1-related proteins) and evolutionarily conserved proteins (seed storage proteins) occur in various tree nuts (Roux et al., 2003).
An extensive literature review was conducted from November 2004 through April 2005 that included key word, author, and "related article" searches of the PubMed database and analysis of citations found in the published literature. Sixteen publications with quantitative dose-response data from DBPCFC testing were reviewed to identify those that contained data that could be used to estimate LOAEL levels for the major food allergens. These studies are described in more detail in Appendix 2. Thirteen (80%) of these report results from testing adults; the remaining three tested infants and children. In four cases, the population being studied was not specifically chosen to be food allergic, and a large fraction of the individuals in these populations did not respond to the highest doses tested. In seven studies (44%), patients reacted to the lowest dose tested, and in three studies there was insufficient information to determine either the lowest dose used or the number of patients who responded to that dose. The most sensitive population was seen by Hourihane et al. (1997b), who reported that 67% of the patients tested reacted to "peanut rubbed on the lip," including one severe reaction.
Peanut. Hourihane et al. (1997) observed the lowest measured dose of an allergen that provoked a reaction (i.e., a LOAEL), 0.1 mg of peanut protein provoked subjective reactions in two patients and 2 mg of peanut protein provoked an objective reaction in one patient. Objective reactions were observed in two other patients on exposure to 5 mg of peanut protein. Wensing et al. (2002a) also reported a LOAEL of 0.1mg for subjective reactions in two of 26 peanut allergic individuals tested. The LOAEL for the intial objective symtom was 10 mg. Several other papers reported LOAELs of 25-100 mg of peanut protein for objective reactions (May, 1976; Hourihane et al., 1997; Bock et al., 1978).
Egg. A wide range of LOAELs have been observed for egg. Caffarelli et al. (1995) reported a LOAEL of 0.5 mg of dried whole egg (approximately 0.45 mg protein). Bock et al. (1978) reported observing an objective reaction with 25 mg of whole egg (approximately 1 mg protein), although the data are difficult to interpret as presented. In contrast, Eggesbo et al. (2001) report a LOAEL of 1 g of whole egg (approximately 250 mg of protein) for an objective reaction.
Milk. Relatively consistent LOAELs have been reported for milk. Bellioni-Businco et al. (1999) found a LOAEL of 1 ml of whole milk (approximately 350 mg of protein) with children, and Pastorello et al. (1989) found a LOAEL of 0.5 g of freeze-dried milk (approximately 185 mg of protein) with adults.
Soy. LOAELs of approximately 50 and 88 mg protein have been reported for soy (Zeiger et al., 1999; Magnolfi et al., 1996).
Tree Nut. Hazel nut is the most commonly studied tree nut. Wensing et al. (2002b) observed reactions to 1 mg of hazel nut protein in 4 of 29 patients, which was the lowest dose tested. Hansen et al. (2003) found a LOAEL of approximately 30 mg of hazel nut protein, although it is not clear whether this was the lowest dose tested.
Fish. Hebling et al. (1999) reported a LOAEL of 50 mg for catfish protein.
The best example of food products that are processed to render them less allergenic are hydrolyzed infant formulas. Enzymatic hydrolysis of cow's milk protein or derivatives (i.e., casein, whey) has been shown to significantly reduce the levels of both total and allergenic (e.g., b-lactoglobulin) protein (Host and Halken, 2004). The degree of protein reduction depends on the method of hydrolysis. There is ample clinical evidence to suggest that hydrolyzed formulas have reduced allergenicity in comparison to intact milk formulas (Amer. Acad. Ped., 2000; Host and Halken, 2004). Furthermore, there is evidence that the use of certain hydrolyzed formulas may also delay or prevent the development of cow's milk allergy (CMA) in high-risk infants (Host and Halken, 2004).
Hydrolyzed formulas contain varying amounts of residual protein, including allergenic proteins, which can be detected using either in vitro or in vivo methods (Giampietro et al., 2001; Docena et al., 2002). Both extensively and partially-hydrolyzed formulas can cause allergic reactions, including anaphylaxis, in sensitive infants (Saylor and Bahna, 1991; Schwartz and Amonette, 1991; Tarim et al., 1994; Ammar et al., 1999; Giampietro et al., 2001; Host and Halken, 2004). Thus, the residual milk proteins (or peptides resulting from their partial hydrolysis) in these formulas still retain immunologic activity. In general, the higher the level of residual protein, the higher the risk for a reaction. Although partially hydrolyzed formulas tend to show higher residual protein levels, the degree of hydrolysis cannot always be used as a predictor of the degree of allergenicity. Hydrolysis methods are not standardized, and formulas undergoing similar treatments may vary considerably in their residual protein levels. Additional processing, such as heat treatment and ultrafiltration, may further reduce residual protein levels in certain products (Host and Halken, 2004).
In 1989, the American Academy of Pediatrics (AAP) determined that a formula could be considered "hypoallergenic" if challenge studies showed, at a minimum, 95% confidence that 90% of allergic infants would not react adversely to the formula (Amer. Acad. Ped., 1989). Since this time, a number of DBPCFC studies using various infant formula preparations have been performed in infants with CMA (Sampson et al., 1991; Sampson et al., 1992; Giampietro et al., 2001; Sicherer et al., 2001), and a substantial number of infant formulas have met this criterion for hypoallergenicity. Even though they note that extensively hydrolyzed hypoallergenic formulas contain residual proteins and may provoke allergic reactions in infants with CMA, the AAP currently recommends these formulas as alternatives for infants with CMA stating that at least 90% of these infants will tolerate the formula (Amer. Acad. Ped., 2000).
Newer technologies, such as genetic modification, are being developed to reduce allergenicity by removing, silencing, or modifying the genes for specific allergenic proteins within foods (Tada et al., 1996; Herman et al., 2003; Dodo et al., 2005; Gilissen, 2005). To date, however, there is no example of a food allergen that has been rendered completely devoid of allergenic activity using these methods. This is due to the fact that each food contains a number of allergenic proteins, each with multiple allergenic epitopes. Unless these methods can eliminate all of these proteins, or modify all allergenic epitopes, the remaining proteins or epitopes could still elicit a reaction in sensitive individuals.
Celiac disease (also known as celiac sprue) is a chronic inflammatory disorder characterized by mucosal damage of the small intestine leading to gastrointestinal illness, nutrient malabsorption, and a wide range of clinical manifestations. There is a consensus opinion that celiac disease is caused by an aberrant (T lymphocyte) immune response to dietary glutens predominantly found in wheat, barley, and rye (NIH, 2004). However, there is evidence that at least some persons who have celiac disease also cannot tolerate oats (Lundin et al., 2003; Arentz-Hansen et al., 2004). Those individuals who have a genetic predisposition to celiac disease react to peptides within the proline- and glutamine-rich protein fractions of the grains. For affected individuals, celiac disease is a lifelong condition and, if not treated, is associated with significant morbidity and increased mortality (Fasano, 2003; Corrao et al., 2001; Dewar et al., 2004). There is no cure for celiac disease. Strict avoidance of potentially harmful concentrations of glutens in the diet is the only known means of completely preventing the clinical and pathological complications of celiac disease.
Celiac disease is characterized by injury to the mucosa of the small intestine and specifically targets the fingerlike projections, called villi, where absorption of key nutrients takes place (Figure III-1). This injury is believed to be due to an autoimmune disorder involving modification of the antigenic presentation of gluten in the intestinal tract of genetically predisposed individuals expressing the major histocompatibility haplotypes HLA-DQ2 or HLA-DQ8 (Farrell and Kelly, 2002; Fasano, 2003). In these individuals, binding of the enzyme tissue transglutaminase (tTG) to wheat gluten (a glutamine rich protein) potentiates uptake and presentation by antigen-presenting cells in the lamina propria, triggering a vigorous T-cell response (Schuppan and Hahn, 2002), leading to production of IgG and IgA directed to wheat gluten peptides (i.e., gliadins and glutenins) and IgA to tissue transglutaminase (tTG). The activated T-cells are responsible for the self-perpetuating mucosal damage seen in celiac disease (Fasano and Catrassi, 2001). This immune-mediated damage occurs in two compartments, the epithelium and the lamina propria (Green and Jabri, 2003). Early intestinal disease is characterized by an increased number of intestinal intraepithelial lymphocytes (IELs). As the disease progresses, increasing numbers of lymphocytes and plasma cells infiltrate the lamina propria. This increase in the numbers of cells leads to elongation of intestinal crypts and shortening of villi, which eventually results in partial or total villous atrophy (James, 2005). Elimination of intestinal gluten results in modification of T lymphocyte and antibody responses and, in most cases, full mucosal recovery (Kaukinen et al., 1999; Fasano and Catassi, 2001).
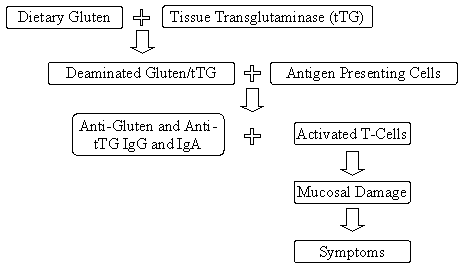
Figure III-1 Mechanism of Celiac Disease
The clinical manifestations of celiac disease are highly variable in character and severity and depend largely on the age and immunological status of the individual, the amount, duration, or timing of exposure to gluten, and the specific area and extent of the gastrointestinal tract involved by disease (Dewar et al., 2004). These clinical manifestations can be divided into gastrointestinal, or "classic," and non-gastrointestinal manifestations. Gastrointestinal manifestations usually present in children 4 to 24 months old and include acute symptoms of abdominal pain and cramping, bloating, recurrent or chronic diarrhea in association with weight loss, poor growth and symptoms consistent with nutrient deficiency, and (in rare cases) a life-threatening metabolic emergency termed celiac crisis, characterized by hypokalemia and acidosis secondary to profuse diarrhea (Farrell and Kelly, 2002; Baranwal et al., 2003). Non-gastrointestinal manifestations are more insidious and highly variable and are the common presenting symptoms in older children and adults. These manifestations are frequently the result of long-term nutrient malabsorption, including iron deficiency anemia, short stature, delayed puberty, infertility, and osteoporosis or osteopenia (Fasano, 2003). In children, progressive malabsorption of nutrients may lead to growth, developmental, or neurological delays (Catassi and Fasano, 2004). Extra-intestinal manifestations such as dermatitis herpetiformis, hepatitis, peripheral neuropathy, ataxia, and epilepsy have also been associated with celiac disease. Individuals with untreated celiac disease are at increased risk for potentially serious medical conditions, such as other autoimmune diseases (e.g., Type I diabetes mellitus) and cancers associated with high mortality (Farrell and Kelly, 2002; Peters et al., 2003; Catassi et al., 2002). For example, individuals with celiac disease have an 80-fold greater risk of developing adenocarcinoma of the small intestine and a greater than two-fold increased risk for intestinal or extraintestinal lymphomas (Green and Jabri, 2003). The latter complications are responsible for nearly two thirds of deaths due to celiac disease and are a major reason for the nearly two-fold increase in overall mortality of adult patients with celiac disease compared to the general population (Corrao et al., 2001).
Currently, individuals with clinical manifestations, or "symptomatic" celiac disease, are believed to represent a small portion of the total celiac population. A much larger number of individuals have "silent" celiac disease, characterized by positive serology and limited involvement of the GI tract. There is an even larger population with "latent" celiac disease, individuals who are positive for serological markers or genetic susceptibility to disease but show no intestinal mucosal involvement. Individuals in these latter two categories may have atypical disease manifestations or, in most cases, be entirely asymptomatic. It is generally accepted that individuals with silent or latent disease have aberrant immune responses following exposure to dietary glutens and are, therefore, at increased risk for both acute and long-term complications of celiac disease (Fasano, 2003; Schuppan, 2000). The long-term benefit of strict gluten avoidance for these individuals is unproven (Green and Jabri, 2003).
Until recently, celiac disease was considered to be a rare disorder in the U.S., with an estimated prevalence rate of 1:10,000 (Talley, 1994). However, a large epidemiological study screened more than 13,000 people in 23 states and estimated a prevalence rate of 1:133 within the general U.S. population (Fasano, 2003). The National Institutes of Health Consensus Development Conference Statement on Celiac Disease currently estimates that 3 million Americans, a little less than 1 percent of the population, may have celiac disease (NIH, 2004). Celiac disease occurs widely among North American and European populations, where wheat is a staple food, but is infrequent among native descendents of China and Japan and those with an African-Caribbean background, where wheat is not as widely consumed (Farrell and Kelly, 2002).
Precise prevalence data for celiac disease are not available. This disease is often misdiagnosed as another gastrointestinal malabsorptive disorder (e.g., Crohn's disease, irritable bowel syndrome) due to similarities in their symptoms. Due to the existence of silent or latent cases, it is assumed that the incidence of celiac disease is underreported. These forms of celiac disease may go undetected in individuals for years before they develop symptoms causing them to seek medical attention (Green and Jabri, 2003). Mäki and Collin (1997) postulated that there are many more currently healthy individuals who are genetically predisposed to developing celiac disease in future years than there are individuals who are now affected by celiac disease. Only recently has the medical community become more aware of the need to screen for celiac disease when patients experience health problems that may be associated with the disease or when patients have family members, especially first- and second-degree relatives, who have celiac disease (NIH, 2004).
Celiac disease is caused by an immune response in genetically predisposed individuals to specific storage proteins, commonly referred to as "glutens," that occur naturally in cereal grains (Shan et al., 2002). Technically, "gluten" is a term applied specifically to the combination of the prolamin proteins called "gliadins" and the glutelin proteins called "glutenins" found in wheat. However, the term "gluten" has been used generically to refer to prolamin and glutelin protein mixtures found in other cereal grains (Kasarda, 2005, personal communication). Although all cereal grains contain prolamin and glutelin proteins, these proteins are not identical in different grains. These proteins differ in their amino acid sequences in different grains, and not all have been shown to evoke an abnormal immune response that affects the intestinal lining of persons genetically susceptible to celiac disease (Kasarda, 2003). The term "gluten" will be used in this report in the more general sense of the combination of both prolamin and glutelin proteins found in cereal grains.
The grains considered to be capable of producing adverse effects in individuals with celiac disease include the different species of wheat (e.g., durum, spelt, kamut), barley, rye, and their cross-bred hybrids (e.g., triticale, which is a cross between wheat and rye) (Kasarda, 1994; Kasarda, 2004). There is also evidence that some individuals with celiac disease may react adversely to oats (Lundin et al., 2003; Arentz-Hansen, 2004). These grains are all members of the grass family (Gramineae, also known as Poaceae) and are closely related taxonomically. The cereal grains assumed to be safe for persons with celiac disease include amaranth, buckwheat, corn, Indian rice grass, Job's tears, millet, quinoa, ragi, rice, sorghum, and teff (or tef) (Kasarda, 2001; Kasarda, 2004b; Kupper, 2004).
The grain prolamins of concern include gliadin in wheat, secalin in rye, hordein in barley (Thompson, 2001; Green and Jabri, 2003; Kagnoff, 2005) and possibly avenin in oats (Arentz-Hansen, et al. 2004; Lundin, et al., 2003). There is substantial evidence that both prolamin proteins (i.e., gliadins) and glutelin proteins (i.e., glutenins) in wheat affect individuals with celiac disease (Shan et al., 2002; Hausch et al., 2002; Vader et al., 2002; van de Wal et al., 1999; Molberg et al., 2003).
Wheat gliadin subtypes alpha, gamma, and omega, have been shown to affect individuals with celiac disease (EFSA, 2004). Rye, barley and triticale are also considered to affect individuals with celiac disease because they are taxonomically related to wheat, express peptides structurally similar to those found in wheat, and have been shown to affect individuals with celiac disease (Vader et al., 2002; Kasarda, 2001; Kasarda, 2004b). In contrast, the prolamins in other cereal grains (e.g., zein in corn and orzenin in rice) have been shown not to affect individuals with celiac disease (EFSA, 2004; Kasarda, 2004b). However, much is still unknown about which proteins in the different grains can affect individuals with celiac disease (Kasarda, 2001).
Analytical information is not available on the actual amount of gluten proteins in different grain-derived food ingredients or finished foods. For single ingredient foods made from wheat, rye, barley, triticale, and oats, the simple presence of "protein" in that food may be used as an indicator that gluten proteins are present. The USDA National Nutrient Database for Standard Reference, Release 17 (USDA, 2004), the major source of composition data for foods in the U.S., includes hundreds of food items that contain wheat, rye, barley, triticale or oats as an ingredient. Wheat, in particular, is used to manufacture a wide range of food ingredients and finished foods. Rye, barley, triticale, and oats are used to make substantially fewer food products.
Koehler and FDA (2005) estimated the average amount of total grain and individual types of grain available for consumption per person in the U.S., and the total exposure to gluten-forming proteins that would result from this grain consumption. The estimated mean daily consumption rate was approximately 250 grams of grain per capita. Wheat provided 180 of the 187 grams per person per day of grains that are of concern for individuals with celiac disease.
There is no consensus as to whether oats present a hazard for all individuals with celiac disease. Several studies, including one that lasted 5 years, have reported that most celiac study participants both preferred and tolerated moderate amounts (e.g., 50-70 grams daily) of oats (Janatuinen et al., 1995; Janatuinen et al., 2000; Janatuinen et al., 2002). However, two smaller, more recent studies that investigated the effects of daily consumption of 50 grams of oats by individuals with celiac disease suggest that oat proteins can elicit symptoms in some sensitive celiacs (Arentz-Hansen et al., 2004; Lundin et al., 2003). The oats used in these studies were tested to ensure that they did not contain any gluten proteins from wheat, rye, or barley.
In the U.S., most commercially available oat products are believed to contain some gluten proteins from wheat, rye, or barley due to cross-contact with these grains during growth, harvest, transport, storage, or processing (Kasarda, 2001; Kasarda, 2005 personal communication; AGA, 2001; Thompson, 2003). In a recent study, Thompson (2004) analyzed four lots of three brands of rolled or steel-cut oats commercially available in the U.S. for prolamins from wheat, barley, or rye. For one brand, all samples contained 338 to 1807 ppm gliadin (expressed as µg per mg of food product). For each of the other two brands, the level of gliadin detected in all but one lot ranged from 12-725 ppm in one brand and 120-131 ppm in the other brand. Thus, only one lot of these two brands was negative for gliadin. Thompson (2004) concluded that none of these three brands could be considered a reliable source of oats free of potentially harmful gluten proteins.
Grains that do not contain gluten can become contaminated with grains that contain gluten at any step in the farm-to-table continuum, particularly if shared equipment is not thoroughly cleaned between uses. It is difficult, if not impossible, to prevent all cross-contact situations, considering the tons of grain handled by farm equipment, bulk storage, and transport containers on a daily basis. In fact, the Official United States Standards for Grains (USDA, 1999) assume that most grains that have an established U.S. standard will contain a small percentage of other grains.
There is little information in the literature on minimal disease-eliciting doses of gluten for sensitive individuals. Gluten challenges have generally been performed in individuals where diagnosis is uncertain (e.g., infants, Laurin et al., 2002) or in individuals with unclear intestinal pathology results (Wahab et al., 2001). Challenges have also been performed to determine the time of disease relapse after a prolonged period of gluten avoidance (Mayer et al., 1989). In most cases, gluten challenges have been performed to elicit or confirm disease rather than to measure sensitivity (Farrell and Kelly, 2002).
There is no standard protocol for gluten challenges, and challenge studies have varied greatly in amount and duration of gluten exposure. Although some studies have been designed to determine the acute effects (i.e., after 4 hours) of exposure to gluten (Sturgess et al., 1994; Ciclitira et al., 1984), most challenges consist of an open challenge to a fixed or incremental dose of daily gluten over a minimum period of 4 weeks. Many challenge studies use a high exposure (> 10 gm/daily) to gluten, because this is believed to shorten time to disease confirmation or relapse and, therefore, to minimize discomfort to subjects (Rolles and McNeish, 1976). However, some studies have shown that low daily exposures to gluten can elicit a disease response (Catassi et al., 1993; Laurin et al., 2002; Hamilton and McNeill, 1972).
Catassi et al. (1993) reported that children, whose celiac disease had previously been controlled on gluten-free diet, had evidence of intestinal mucosal or immunological changes (changes in intraepithelial lymphocyte counts and the villous height to crypt depth ratio) following 100 mg or 500 mg of daily gliadin over 4 weeks; this corresponds to 200 mg and 1000 mg of daily gluten respectively (Collin et al., 2004). The degree of inflammation was dose-dependent. However, this study had several important limitations, which include the short term follow up (4 weeks), testing in young children, the small number of subjects (n=20), and the lack of control groups. In addition, although gliadin is believed to be the major immunogenic portion of gluten, T cells from the small intestine of CD patients have been shown to be responsive to peptides from the glutenin portion as well (Van de Wal et al., 1999). Thus, the Castissi et al. (1993) study was also limited by the use of gliadin rather than gluten. Estimating potential harm by extrapolating from gliadin levels may not be representative of the harm from total gluten exposure.
A study currently in progress [The Italian Microchallenge Study] has extended the scope of these earlier findings by evaluating the effects of exposure to either 10 or 50 mg of purified gluten per day for 3 months with a population of 36 celiac disease individuals in a double-blind, placebo-controlled study (Catassi et al., 2005). Preliminary unpublished results suggest that minimal mucosal abnormalities occur with a strict gluten-free diet, that both 10 mg and 50 mg daily gluten are well-tolerated, but that there is a trend for mucosal changes to occur at the 50 mg dose. These results can be compared to estimated gluten exposures from gluten-free diets containing various levels of gluten contamination (Table III-1, from Collin et al., 2004, reproduced below). Fasano (2005 personal communication) used these values to suggest that a conservative threshold for gluten exposure for sensitive individuals would lie between 20 and 100 ppm.
| Gluten Content in Food (ppm) | Daily Amount of Gluten-Free Food Consumed (g) | |||
|---|---|---|---|---|
| 50 | 100 | 200 | 300 | |
| ----------------Daily Amount of Gluten Consumed (mg)----------------- | ||||
| 200 | 10 | 20 | 40 | 60 |
| 100 | 5 | 10 | 20 | 30 |
| 50 | 2.5 | 5 | 10 | 15 |
| 20 | 1 | 2 | 4 | 6 |
| Source: Collin et al.,
2004. Note: Gluten content in food multiplied by food consumed equals gluten consumed. Six slices of bread is equivalent to approximately 100 g baking mix. ppm=mg/kg | ||||
In an alternate approach, Collin et al. (2004) analyzed gluten levels in a number of different types of wheat starch (n=24) and naturally gluten-free (n=59) flours consumed by 76 individuals with celiac disease who had been on gluten-free diets for 1 to 10 years. These individuals had no reported evidence of mucosal deterioration or significant provocation of symptoms while on this diet. The range of gluten found in these products was 0 to 200 ppm. Collin et al. (2004) then estimated that the total daily flour consumption for these individuals to be 10-300 gm (median 80 gm). Based on this estimate and the gluten content of the flour, a chart depicting estimated daily gluten exposures was devised (Collin et al., 2004). Collin et al. (2004) used this chart and data from low dose gluten challenge studies to suggest the use of a threshold of 100 ppm gluten. The main limitations of this study include lack of a prospective study design (for actual dose-response information) and the lack of information detailing diagnostic assessment (i.e., minimal mucosal involvement) for characterizing mucosal relapse in these individuals.
Currently, commercial immunology-based ELISA test kits for the detection of gluten in foods are manufactured by Immunotech (Czech Republic), Ingenasa (Spain), Morinaga (Japan), Diffchamb (Sweden), Neogen Corporation (U.S.), R-Biopharm (Germany), and Tepnel BioSystems (U.K.). All of these detect prolamins, the proteins found in soluble aqueous-alcohol extracts from cereals. None is designed to detect all proteins associated with celiac disease. Five of the assays have separately undergone multi-laboratory validation studies (Skerritt and Hill, 1991; Akiyama et al., 2004; Gabrovská et al., 2004; Immer et al., 2003). Each of these studies employed different target levels and matrices. The Tepnel kit was validated by AOAC at 100 ppm (Skerritt and Hill, 1991). All the ELISA kits rely on the preparation of an aqueous-alcohol extracts as analytical samples, and four of the manufacturers include the use of reducing-denaturing conditions for the analysis of baked goods. During the 25th session of the Codex Committee on Nutrition and Foods for Special Dietary Uses in 2003, the R5-Mende zELISA method, which entails the use of reducing - denaturing conditions, was forwarded to the Codex Committee on Methods of Analysis and Sampling for endorsement (Codex Alimentarius Commision, 2003). These ELISA test kits cross react, to differing degrees, with prolamins derived from wheat, rye, and barley. None of the test kits cross-reacts with protein extracts from oats (Gabrovská et al., 2004; Nonaka, 2004; Abouzied, 2004; Brewer et al., 2004). As such, the ELISA test kits do not provide protection to individuals with celiac disease who are sensitive to oats (Peraaho et al., 2004; Storsrud et al., 2003; Arentz-Hansen et al., 2004; Lundin et al., 2003). Proficiency testing studies conducted by the Food Analysis Performance Assessment Scheme (FAPAS®) have shown variability between the prolamin ELISA test kits (FAPAS Series 27 Round 05, Report No. 2705, 2003), indicating that further validation studies for these kits need to be carried out under comparable conditions. In addition to ELISA test kits, two of the manufactures, Tepnel BioSystems and R-Biopharm, market lateral flow devices for the detection of gluten. To date, neither of these have been validated.
At this time there is no correlative information on the efficacy of using these tests to predict or help prevent adverse effects in individuals with celiac disease.
Although gluten-free diets are considered the only effective treatment for individuals with celiac disease, it has been recognized that it is difficult, if not impossible, to maintain a diet that is completely devoid of gluten (Collin et al., 2004). Therefore, several attempts have been made to define gluten-free in regulatory contexts. Efforts by the Codex Alimentarious to define a standard for gluten-free date back to 1981. At that time, due to the lack of sensitive, specific analytical methods, a threshold value of 0.05 g nitrogen per 100 g dry matter was set for wheat starch, on the assumption that wheat protein would be the only source of nitrogen in starch (Codex Standard 118-1981). The Codex Committee on Nutrition and Foods for Special Dietary Uses is developing a revised standard. The current draft proposal would define three categories of gluten-free foods: naturally "gluten free" (≤ 20 ppm of gluten), products that had been rendered gluten-free by processing (≤ 200 ppm), and any mixture of the two (≤ 200 ppm). The Australia New Zealand Food Agency (ANZFA) defines gluten to mean "the main protein in wheat, rye, oats, barley, triticale and spelt relevant to the medical conditions, Coeliac disease and dermatitis hepetiformis." ANZFA recognizes two classes of foods, gluten-free foods ("...no detectable gluten" and low-gluten foods ("...no more than 20 mg gluten per 100 gm of the food") (ANZFA Food Code Standard 1.2.8). The Canadian standard for gluten-free is more general, simply stating that "No person shall label, package, sell or advertise a food in a manner likely to create an impression that it is a "gluten-free" food unless the food does not contain wheat, including spelt and kamut, or oats, barley, rye, triticale or any part thereof" (Canadian Food and Drugs Act Regulation B.24.018).
Four general approaches were identified that could be used to establish thresholds for allergens and glutens: analytical methods-based, safety assessment-based, risk assessment-based, and statutorily-derived. With any of these approaches, planned iterative re-evaluation of threshold values should be carried out as new knowledge becomes available. These approaches are summarized in Table IV-1 and described in detail below.
|
Type of Approach |
Examples |
|---|---|
|
Analytical methods-based |
Labeling of sulfiting agents "Zero" tolerance policy for Listeria monocytogenes in ready-to-eat foods |
|
Safety assessment-based |
Evaluation of food additive petitions |
|
Risk assessment-based |
Guidance levels for Vibrio parahaemolyticus in raw oysters |
|
Statutorily-derived |
Labeling exemption for highly refined oil in the FALCPA |
1. Analytical Methods-Based Approach. In an analytical methods-based approach, thresholds are determined by the sensitivity of the analytical method(s) that can be used to verify compliance. This effectively establishes a "regulatory threshold," although this threshold is not necessarily correlated to biological effects. This approach has been used in food labeling. For example, the requirement to declare sulfiting agents on product labels when foods contain 10 ppm or greater is based on the limit of sensitivity of the analytical method used to measure these agents.
The issues that need to be considered when using an analytical methods-based approach to establish a threshold include:
The strength of this approach is that it is relatively simple, straightforward, and easy to implement. However, it is appropriate to use an analytical methods-based approach to establish thresholds for allergens or gluten only if analytical techniques are available for all major food allergens and celiac-associated glutens.
2. Safety Assessment-Based Approach. Safety assessments are routinely applied to public health issues related to substances in foods, such as chemical contaminants or food additives, particularly when a biological threshold can be justified scientifically. The definition of "safe" varies according to the applicable legal provision. For example, for contaminants, the statutory definitions of safety are proscribed in section 402(a)(1). Food is considered adulterated if an added contaminant is in the food in a quantity "...which may render it [the food] injurious to health", or, if the substance in an inherent natural constituent of the food (i.e. "not an added substance"), is in the food in a quantity that would "ordinarily render it [the food] injurious to health". As another example, the phrase "reasonable certainty that no harm will result" is used in section 408 (a)(4) regarding the safety of tolerances for a pesticide chemical residue in or on a food.
For a safety assessment, the term "safety" has connotations involving both the degree of certainty and an assumption of "negligible risk." The prototype chemical safety assessment is the Acceptable Daily Intake (ADI) method which was first articulated by Fitzhugh and Lehman (1954) for use in considering the significance of available animal data. This approach or variations of it are used throughout the world (WHO, 1987). The ADI for a chemical is calculated from the No Observed Adverse Effect Level (NOAEL) and Uncertainty Factor (UF) using the following equation:
ADI = NOAEL / UF.
The same basic methodology can be used to derive other regulatory standards such as Tolerable Daily Intake (TDI), Reference Dose (RfD), and Minimal Risk Level (MRL). These values are derived from controlled animal studies, human clinical studies, or epidemiological studies that provide the exposure level for which there is not apparent or the lowest observable adverse effect (i.e., NOAEL, LOAEL). These adverse effect levels are also considered in conjunction with one or more uncertainty factor(s). Uncertainty factors are applied to account for inter-species and inter-individual differences and other uncertainties in the data (WHO, 2004).
There have been consistent efforts to improve this process to make better use of scientific knowledge. These efforts have focused on both replacing the NOAEL approach and refining the development of uncertainty factors. One example is the development of the benchmark dose (BMD) concept (Crump, 1984; Kimmel and Gaylor, 1988). The BMD concept involves fitting a dose-response model to all the available data and to determine the statistical lower bound of the BMD (i.e., the BMDL). The major advange of the approach is that the BMDL is not constrained to one of the experimental doses from a controlled study, as is the case with the NOAEL (Crump, 1994). EPA uses the BMD method in health risk assessments (Filipsson et al., 2003).
3. Risk Assessment-Based Approach. A risk assessment is a systematic, scientific examination of known or potential adverse heath effects resulting from human exposure to a hazard. The generally accepted paradigm separates risk assessment into four components: hazard identification, exposure assessment, hazard characterization (dose-response), and risk characterization. This framework allows for organization of information, definition of uncertainties, and identification of data gaps. Risk assessments can describe the likelihood of adverse health effects either quantitatively or qualitatively depending on the extent of the knowledge available, the complexity of the problem, and the time available to conduct the assessment. In quantitative risk assessments, risk is expressed as a numerical estimate of the chance of illness or death after exposure to a specific hazard. This estimate represents the cumulative probabilities of certain events happening and the uncertainty associated with those events. A qualitative risk assessment, on the other hand, uses verbal descriptors of the risk and uncertainties, and often involves the aggregation of expert opinions.
Of the four approaches, the quantitative risk assessment-based approach is the most scientifically rigorous and provides insight into the level of risk associated with specific exposures and the degree of uncertainty inherent in the risk estimate. An example of the use of a risk estimate and associated uncertainty is the current standard for hypoallergenic infant formulas, where there is 95% certainty that 90% of the sensitive population will not react (Amer. Acad. Pediatrics, 2000). The risk assessment-based approach is preferred when a biological threshold cannot be justified scientifically. Several recent papers have discussed the application of the risk assessment-based approach to food allergens (Bindslev-Jensen et al., 2002; Moneret-Vautrin and Kanny, 2004; Cordle, 2004; Wensing et al., 2002a).
The issues that need to be considered when using a risk assessment-based approach include:
Other issues that should be considered in regard to understanding the relationship between the exposure level and nature of the response include:
It is not clear whether the data and modeling techniques available at the present time are sufficient to allow use of the risk assessment-based approach to establish thresholds for food allergens and for gluten. As an example of the complexity of this approach, the following describes the process of developing a dose-response model that can be used in a quantitative risk assessment:
Steps in Developing a Dose-Response Model
4. Statutorily-Derived Approach. The statutorily-derived approach establishes a threshold by extrapolating from an exemption established by Congress for another purpose. For example, the FALCPA defines "major food allergen" to include a food ingredient "that contains protein derived" from one of eight foods or food groups, "except... any highly refined oil" derived from one of those foods. If consumption of highly refined oils is not associated with allergic reactions, and if there is nothing unique about the proteins in highly refined oils, then consumption of another food containing levels of protein that results in an exposure that is equal to less than the level in a typical serving of highly refined oils should not be associated with allergic reactions. Thus, a threshold could be established for all food allergen proteins based on the level of protein in highly refined oils. There is no comparable statutory standard for gluten.
The general criteria used to evaluate the four approaches to establish thresholds for allergens and gluten are shown in Table IV-2. Specific criteria related to food allergens are given in Section IV-C and gluten in section IV-D. The specific criteria should be weighted appropriately when implementing a particular approach. The general criteria focus on data availability and data quality. The Threshold Working Group recognizes that scientific knowledge is the product of a process which is inherently imperfect and often incomplete. As such, the degree of uncertainty in the data is a key consideration. It is expected that any decisions on approaches for establishing thresholds for food allergens or for gluten would require consideration of additional factors not covered in the current report. For example, ease of compliance and enforcement, stakeholder concerns (i.e., industry, consumers, and other interested parties), economics (e.g., cost/benefit analysis), trade issues, and legal authorities are all significant factors that are likely to influence the practicality of implementing any approach. One option that is implicit in the following discussion of potential approaches is a decision not to establish thresholds at this time, at least for food allergens.
|
Criteria |
Description |
|---|---|
|
Data Availability |
Identification and review of currently available data that can be used in any of the four approaches to establish a specific threshold. |
|
Data Quality |
Evaluation of the available data for utility, completeness, and scientific soundness. Evaluation of the degree of uncertainty associated with the data. |
1. Feasibility. The published and unpublished literature summarized in Sections II and III of this report were reviewed to determine the availability of the specific types of data needed for each of the approaches to establish thresholds. When necessary information was not available, the following questions were used to evaluate the existing information:
2. Uncertainty. Uncertainty is typically thought to arise from the lack of data or information. Other sources of uncertainty are often considered to be relevant to scientific evaluations such as subjective judgment, statistical variation, sampling errors, and inherent randomness (Byrd and Cothern, 2000). Techniques are available to account for or measure some of these uncertainties. For example, the uncertainty in a dose-response model can be characterized using advanced techniques, such as model weighting, that measure the degree of credibility associated with the model results (Carrington, 1997). State-of-the-art food safety risk assessment models, such as the HHS/FSIS Listeria monocytogenes risk assessment for ready-to-eat foods (HHS/FSIS, 2003) also used techniques that separate uncertainty from biological variability. It is important to note that uncertainty is different from variability. Uncertainty reflects incomplete knowledge about a system or population which can be reduced with additional study. Variability reflects the fact that all systems or populations have inherent, biological heterogeneity that is not reducible through further measurement or study (Voysey et al., 2002). Sufficient knowledge is needed to account for both variability and uncertainty in order to evaluate the four approaches for establishing thresholds.
As described above, uncertainty factors are used in safety assessment calculations. Fitzhugh and Lehman (1954) originally proposed a single safety factor of 100-fold applied to animal data. The justification for this factor included both scientific issues and social values. The scientific issues included the possibility that humans may be more sensitive to chemicals than the rodents used in laboratory tests and that there may be substantial variability among individuals in a population. In general, as uncertainty increases, the uncertainty factor employed in a safety assessment should increase proportionally. As a matter of practice, uncertainty is not characterized in a safety assessment, either formally or subjectively, as is done in a quantitative risk assessment. A minimum uncertainty factor of 10 is generally used to account for variation within the population when relying on human data and additional uncertainty factors may be included as appropriate. For example, the Food Quality and Protection Act (FQPA) of 1996 requires, in certain cases, a 10-fold factor in addition to any other uncertainty factors to protect infants and children from exposure to pesticides . The assignment of uncertainty factors should be based on science but typically will include the application of expert judgment.
3. Data Quality. The FDA Information Quality Guidelines were used in evaluating the scientific data contained in this report. These guidelines describe policies and procedures for ensuring the quality of the information disseminated by FDA. In these guidelines, data quality is defined in terms of utility, objectivity, and integrity. Utility is defined as the usefulness of the information to its intended users; objectivity as presentation of the data in an accurate, clear, complete, and unbiased manner; and integrity as protecting the information from unauthorized access or revision. In particular, the guidelines provide transparency standards and ensure clarity.
This section provides an evaluation of the data needed to establish thresholds for the major food allergens. Based on the availability and quality of the data, the Threshold Working Group provides findings that can be applied to establish such thresholds.
1. Evaluation of Data Availability and Data Quality
a. Sensitive Populations. Most clinical studies exclude patients who have had previous anaphylactic reactions or who have very high IgE titers. This suggests that the most sensitive individuals within the allergic population may be systematically excluded from these studies. Therefore, it is possible that the distribution of doses reported to elicit the "initial objective symptoms" is higher than would be expected if the entire allergic population was considered. The observed dose distribution may also not be representative of the allergic population in studies that use patient populations that are not known to be allergic to the food being tested (e.g., testing milk allergic patients for sensitivity to soy). In addition, individual sensitivity varies over time. This means that "high sensitivity" may be a transient condition for an individual.
There are a number of reports in the scientific literature documenting unusual allergic reactions in individuals. These case studies include reactions to incidental exposures to allergens. These reports are difficult to interpret because the level of exposure and potential influence of other factors (e.g., medications, exercise) are not known. Nevertheless, if these reports document true allergic reactions, this suggests that these individuals could be considered to be highly sensitive when compared to the general population of food allergic individuals.
Based on currently available data, the Threshold Working Group was unable to identify any scientifically-based studies that indicate that the standard 10-fold uncertainty factor used in safety assessments for inter-individual variability is not adequate to account for variation within the sensitive population. However, because of the limitations in the clinical studies and the case reports discussed above, this assumption should be reexamined as more data on the distribution of sensitivities within the population become available.
b. Biomarkers. Because a number of different symptoms are associated with allergic reactions, it is important to identify the most appropriate biological markers for establishing thresholds. The symptoms of an allergic reaction can be either subjective (reported by the patient but not overtly measurable) or objective (overt reactions that are observed or measured by another person). Objective symptoms vary on a continuum of severity from mild rashes to fatal anaphylaxis. Although each of these symptoms is an "adverse effect," there is no consensus where on this continuum the symptoms become "serious adverse effect." This makes it difficult to apply either risk assessment- or safety assessment-based approaches to establish thresholds for food allergens because both approaches require that the adverse end point be well defined.
Most clinical studies expose patients to increasing doses of an allergen until the first objective symptom is observed. This is often, but not always, a relatively mild symptom. For ethical and technical reasons, few studies measure dose-response relationships for individual patients beyond the initial objective symptom. Therefore, the currently available literature provides data based on the "initial objective symptom." Although the "initial objective symptom" is the biomarker measured in most available allergen clinical studies, it is unclear whether these symptoms are consistently considered across these studies. It is also not clear whether and when subjective reactions should be considered "adverse effects," or should influence the selection of a NOAEL or LOAEL for safety assessments.
Normally, the use of the "initial objective symptom" would lead to threshold values that are "protective" in relation to the overall risk to food allergic consumers. However, it should be noted that severe reactions have been reported as the initial objective symptom in some cases. For example, Perry et al. (2004) reported that "reaction severity did not increase as the amount of challenge food ingested increased." Likewise, the only severe reaction observed by Hourihane et al. (1997) in a population of 100 patients occurred at the lowest dose tested. However, considering that the use of the "initial objective symptom" does appear to be generally protective, and that such data would be used in conjunction with appropriate uncertainty factors, it may not be necessary to differentiate among "mild," "serious," or "life-threatening" symptoms when establishing a safety assessment-based threshold from existing clinical data.
c. Analytical Methods for Food Allergens. The criteria used to evaluate the available analytical methods for the major food allergens are shown in Table IV-3 and are applied in Appendix 1.
| Criteria | Comments |
|---|---|
| 1. Has the method been validated? | Methods that have been validated (such as by AOAC) are preferred. Alternatively, the sensitivity, precision, and reproducibility of the method has been demonstrated in a peer reviewed publication. |
| 2. Is the method sufficiently sensitive? | The limit of detection and the limit of quantitation should be below the levels that appear to cause biological reactions. |
| 3. Does the method detect both raw and processed food allergens? | The relevant processing methods (e.g., boiling, roasting, retorting) will depend on the food. |
| 4. Has the species specificity of the method been determined? | This is most relevant to methods for allergens such as fish and tree nuts. |
| 5. Has the protein target (or targets) for the method been determined? | This is relevant to determining whether the assay detects specific allergenic proteins or general biomarkers. |
| 6. Is the method practical? | The method should use common laboratory equipment and supplies. |
The response of sensitive consumers to exposure to an allergen is dependent on the levels of the allergen in the food and the amount of food consumed, two factors for which there is both variability and uncertainty. The levels of allergen in foods may not be known for a number of reasons, particularly when the presence of the allergen is the result of cross-contact. Even in highly controlled clinical studies, questions regarding the level of allergen arise due to differences in the methods used to process and prepare the test material, incomplete characterization of this material, variability in allergen levels among different sources of the food, lack of standardized reference materials, and differences in the analytical methods used to quantify the levels of the allergen.
The methods used to quantify and express the doses received during clinical studies and adverse event investigations are not consistent, and this increases the uncertainty associated with the available data. The amount of an allergen consumed has been described in terms of total weight of a food consumed, total protein from an allergenic ingredient, or amount of specific allergenic proteins. Although the last description is scientifically the most accurate, it is also the most difficult to use because not all individuals are allergic to the same proteins in a food allergen and all the allergenic proteins may not have been identified for a particular food. Measurements based on the whole foods are simple, but increase the level of uncertainty because the composition of the food may vary. For example, changes in water content of a food would change the relative amount of allergenic protein present in serving sizes of a specified mass. Further, the amount of protein present as a percent of the total weight of the food may vary due to environmental factors, seasonal factors, production variability, or between different cultivars or strains. The Threshold Working Group recognized that the scientifically most accurate means of assessing exposure would be to quantify individual allergenic proteins, but concluded that the most practical approach for evaluating the currently available data is to measure exposure in terms of the total protein from a food allergen. This is also consistent with current technology for detecting food allergens.
It should also be noted that, while clinical exposures are expressed in terms of doses (i.e., g, mg, or μg), allergen levels in foods are actually measured as concentrations (i.e., ppm, percent, or mg/kg). These values can be related by defining a standard serving size, usually 100 g. However, it is well documented that the actual serving eaten by consumers should be treated as a variable and a source of uncertainty when assessing food related exposures.
d. Challenge Studies. Clinical food challenge studies are recognized to be the most accurate way to diagnose allergies and to measure sensitivity to an allergen (Sampson, 2005). Unfortunately, the design of these food challenge studies varies widely. The lack of standard protocols, variations in the dosing regimes (including number of doses, the interval between doses, and the relative size of the doses), and differences in the food sources (including differences in preparation and presentation) result in uncertainties when comparing the results of different studies. Double blind placebo controlled food challenges (DBPCFC) are considered the most robust clinical studies and data from these studies should be given preference whenever they are available. Food challenge studies are generally not designed to determine a lack of reaction (i.e., NOAEL). Instead, the doses that produce positive allergic reactions are generally reported, providing an estimate of the LOAEL for the population being studied. Despite the uncertainties associated with food challenge data from the literature, LOAELs from human clinical trials currently provide the best data for estimating population-based reactions to food allergens. In a safety assessment-based approach, the use of LOAELs instead of NOAELs would introduce additional uncertainty. A standard DBPCFC protocol has been proposed to identify NOAELs for various food allergens, but few publicly available, peer-reviewed data of this nature are available at this time. The specific criteria used to evaluate food challenge studies are shown in Table IV-4, and applied in Appendix 2.
| Criteria | Comments |
|---|---|
| 1. Has the study been published in a peer-reviewed journal? | Published, peer-reviewed studies are preferred although unpublished studies may be considered. |
| 2. Were the criteria for selecting the test population clearly and completely described, and are they appropriate? | This information is needed to evaluate how the study results apply to at-risk populations (i.e., was the tested population allergic to the tested food?). |
| 3. Was the test material clearly and completely described? | This information is needed to determine the amount of allergenic protein in the test material. |
| 4. Was the lowest tested dose of allergen described, or can it be calculated? | This information is needed to determine a NOAEL or LOAEL. |
| 5. Were the total number and progression of dose levels described, or can they be calculated? (i.e., can the entire dose series be explicitly determined?) | This information is not needed for a safety assessment, but is needed for a risk assessment. |
| 6. Did some of the test population respond to the lowest dose? | NOAELs and LOAELs cannot be determined in studies in which reactions occurred at the lowest dose tested. |
| 7. Were the allergic reactions observed clearly described? | Objective reactions are preferred for both safety and risk assessments |
| 8. Were the data sufficient to describe the dose-response pattern for the population tested (e.g. for determining a cumulative dose-response curve)? | This information is needed for a risk assessment. |
e. Differences Among Food Allergens. Allergens differ widely both in their potential to elicit allergic reactions and in the severity of these reactions. The simplest approach to dealing with these differences would be to establish a single threshold based on sensitivities to the most potent allergens. This threshold is likely to be unduly restrictive for many allergic consumers. Alternatively, separate thresholds could be established for each food allergen. However, the data needed for the separate threshold approach are not available for many allergens. The Threshold Working Group concluded that, to the extent possible, each food allergen should be treated independently but that a single threshold should be established if independent treatment is not possible. If a single threshold is established, it could be based on the allergenic food that elicits an allergenic reaction at the lowest total protein level.
Some of the major allergens identified in the FALCPA consist of multiple species (i.e., tree nuts, fish, crustacean shell fish). Because consumers who are sensitive to one species in a group are also likely to be sensitive to other members of the group, the Threshold Working Group concluded that any thresholds established for these allergens should be based on the combined amount of these species present.
f. Processing Effects. Most of the food allergens identified in the FALCPA are eaten in a processed form. The existing data show that processing can increase, decrease, modify, or have no affect on allergenicity depending on the allergen, the process, and the matrix involved. A process that modifies the structure of an allergenic protein could reduce allergenicity for one population of susceptible individuals while simultaneously increasing allergenicity for a separate susceptible population.
Most clinical studies are conducted using test materials that have been processed, such as peanut butter prepared from roasted peanuts. Therefore, these studies are likely to mimic actual consumer exposure to the allergen. However, some uncertainty remains because consumers are exposed to food allergens processed in many different ways. It would not be practical to conduct the large number of clinical studies that would be necessary to reduce this uncertainty. Fish appears to be an important exception because raw fish is often used as a test material. Most people eat cooked fish and this should be taken into account when evaluating the results of these studies.
2. Options and Findings
There are four general approaches that could be used to establish thresholds for food allergens - analytical methods-based, safety assessment-based, risk assessment-based, and statutorily-derived. Each approach has strengths and weaknesses, and the application of each is limited by the availability of appropriate data. It is likely that that there will be significant scientific advances in the near future that will address a number of the limitations identified in this report. The Threshold Working Group was aware of several potentially important studies that are currently in progress, but was unable to evaluate them because the data or analyses were incomplete.
Finding 1. The initial approach selected to establish thresholds for major food allergens, the threshold values, and any uncertainty factors used in establishing the threshold values should be reviewed and reconsidered periodically in light of new scientific knowledge and clinical findings.
a. Analytical Methods-Based Approach. The analytical methods-based approach could be used to establish thresholds if the available data are insufficient to establish thresholds using one of the other approaches. This approach requires that analytical methods be available to detect all allergens. Thresholds would be defined by the limits of detection of the available analytical methods, but there would be no relationship between these thresholds and the biological response thresholds. Currently, the lower detection limits for commercially available allergen ELISA or immunoassay test kits are in the range of 0.1 to 1.0 µg protein/g of food, but such kits are not available for all food allergens. Establishing thresholds at levels higher than the lower detection limits of the analytical methods would require the use of assumptions about the biological response thresholds. In that case, the thresholds are actually based on using one of the other three approaches and should not be considered an analytical methods-based threshold.
Advantages. When accurate, validated methods are available to measure food allergens, determining a threshold based on these methods can be a straightforward way to establish that products are in compliance with this defined level.
Limitations. There are several disadvantages to using this approach in determining thresholds for food allergens:
Presumably, the analytical methods used to establish thresholds in this approach could also be used to evaluate compliance with any applicable legal requirements. However, the ability to use these methods to help prevent the introduction of unlawful product into the market place would require that the methods be applied in a scientifically supportable manner. This would require the establishment of a statistically supportable sampling plan. The cost of the sampling to a degree sufficient to provide reasonable statistical confidence is potentially an issue.
Finding 2. The analytical methods-based approach could be used to establish thresholds for those food allergens for which validated analytical methods are available. However, if this approach is used, the thresholds should be replaced by thresholds established using one of the other approaches as quickly as possible.
b. Safety Assessment-Based Approach. The safety assessment-based approach could be used to establish thresholds based on NOAELs or LOAELs reported in the literature in combination with appropriate uncertainty factors. Because very few publications report NOAELs or present results in a form that allows NOAELs to be calculated, this type of analysis would, for most food allergens, be based on LOAELs. NOAELs should be used when they are available or can be calculated (see Appendix 2).
As discussed previously, there are substantial differences in the relative potency of different food allergens (e.g., peanut vs. soy). As noted in Appendix 2 and summarized in Table IV-5, the reported LOAELs for peanuts are considerably lower (maximum of 10 mg protein) compared to soy (maximum 522 mg protein). A single threshold for food allergens, based on the most potent food allergens, could be employed if, as a matter of risk management policy, a single threshold is considered desirable. However, this could be considered overly protective, particularly in the case of soy.
| Food | Range of LOAEL (mg protein) |
|---|---|
| Egg | 0.13 to 1.0 |
| Peanut | 0.25 to 10 |
| Milk | 0.36-3.6 |
| Tree Nuts | 0.02-7.5 |
| Soy | 88-522 |
| Fish | 1-100 |
Advantages. Calculation of threshold levels based on NOAELs or LOAELs and the application of appropriate uncertainty factors to estimate exposure is relatively straightforward. When there are limited data in the literature, the application of appropriate uncertainty factors provides confidence that the majority of the sensitive populations will be protected. For a number of the major food allergens, there is reasonably good agreement among the reported LOAEL values. Establishing thresholds using the safety assessment-based approach and currently available clinical data has the advantage of being directly linked to biological effects.
Limitations. There are limited clinical trial data for most allergens and most available clinical food challenge studies have not been designed to identify a NOAEL. Furthermore, an inherent, but unexamined, assumption in all clinical studies is that the reactions seen in a clinical setting are representative of the reactions to food allergen exposure that occur in the real world. Most available clinical data are primarily limited to identifying LOAELs, and there is no way to know whether doses below the observed LOAEL would still elicit a reaction. Thus, the selection of appropriate factors to account for uncertainty and inherent variability is critical in using the safety assessment-based approach. Until there is a consensus as to whether subjective symptoms are acceptable biomarkers or which objective symptoms are considered harmful, it appears prudent to consider as adverse any objective reaction observed in a clinical trial.
We have identified several data gaps for allergens that add to the uncertainty associated with setting thresholds. Critical areas of uncertainty and variability include:
The Threshold Working Group acknowledges that it is difficult to estimate uncertainty factors that apply in all situations for all allergen threshold determinations when using a safety assessment-based approach. We can, however, assume that a standard uncertainty factor of 10-fold should be applied for intraspecies differences in humans. Additional uncertainty factors could be added if justified from data gaps. In Table IV-6 we use peanuts, widely considered to be among the most potent food allergens, to illustrate how specific uncertainty factors may be developed for use in a safety assessment-based approach to set a threshold if that approach is adopted.
|
Description |
Uncertainty Factor |
Justification |
|---|---|---|
|
Intraspecies difference1 |
10 |
Standard factor for intraspecies variability |
|
Estimation of NOAEL2 |
Not applicable |
Two studies were identified that report NOAELs |
|
Sensitive population3 |
10 |
Used to account for additional margin of protection for more susceptible populations not included in clinical trials |
|
Overall Uncertainty Factor for Peanuts = 100 |
||
1 This includes both between- and within-individual variability. 2 This includes both a factor for converting the LOAEL to a NOAEL and an additional factor for the uncertainty associated with that conversion. In this example for peanuts, there are data on both subjective and objective NOAELs and LOAELs. If the NOAEL values are used, the uncertainty factor is 1-fold (i.e., not applicable). If the LOAELs had been used, this value would have been higher. If subjective symptoms observed at lower levels are used, a different uncertainty factor may be considered. 3 This includes uncertainty associated with an additional margin of protection to account for the potential severity of reaction (e.g., lethality) for the highly sensitive subpopulation. | ||
Finding 3. The safety assessment-based approach, based on currently available clinical data, is a viable way to establish thresholds for food allergens. If this approach is employed, the LOAEL or NOAEL determinations used should be based on evidence of the "initial objective symptom." Individual thresholds should be established for each of the major food allergens. If it is not feasible to establish individual thresholds, a single threshold based on the most potent food allergens should be established. In those instances where a LOAEL is used rather than a NOAEL to establish a threshold, an appropriate uncertainty factor should be used.
c. Risk Assessment-Based Approach. The use of the risk-assessment-based approach requires analysis of the population distributions of allergic sensitivities for each of the major food allergens. These distributions would then be used in conjunction with data on exposures to assess the probability of an adverse effect. These distributions could also be used to evaluate the likely efficacy of different risk reduction strategies.
Advantages. The quantitative risk assessment-based approach is the most scientifically rigorous approach and provides the most insight into both the level of protection and the degree of uncertainty associated with an exposure level. Several recent publications that present preliminary quantitative risk assessments based on data from clinical trials suggest that this approach shows promise (Bindslev-Jensen et al., 2002; Moneret-Vautrin and Kanny, 2004; Cordle, 2004; Wensing et al., 2002a).
Limitations. Quantitative risk assessments require the most data of any approach to establish thresholds for food allergens, because they are based on determining the entire dose-response curve, not simply a NOAEL or LOAEL. The data currently available in the literature for food allergens are generally not detailed enough to be useful for quantitative risk assessment. Further, the underlying mathematical procedures and assumptions have not been fully described for the models that have been published. No consensus has been reached regarding the most appropriate mathematical model to use for analyzing allergen reaction data.
Finding 4. Of the four approaches described, the quantitative risk assessment-based approach provides the strongest, most transparent scientific analyses to establish thresholds for the major food allergens. However, this approach has only recently been applied to food allergens, and the currently available data are not sufficient to meet the requirements of this approach. A research program should be initiated to develop applicable risk assessment tools and to acquire and evaluate the clinical and epidemiological data needed to support the quantitative risk assessment-based approach. Thresholds established using this approach should be reevaluated periodically as new data and tools become available.
d. Statutorily-Derived Approach. As discussed above, an allergen threshold could be extrapolated from a statutory exemption established by Congress for another purpose, such as the FALCPA exemption for "highly refined oils." Thus, a threshold could be established for all food allergen proteins based on the level of protein in highly refined oils.
There are surprisingly few data available in the published scientific literature reporting on the levels of proteins in highly refined oils. The criteria used to evaluate studies measuring protein levels in food oils are shown in Table IV-7 and applied in Appendix 3.
|
Criteria |
Comments |
|---|---|
|
1. Has the study been published in a peer-reviewed journal? |
Published, peer-reviewed studies are preferred, although unpublished studies can be considered. |
|
2. Was the oil completely described, including all refining and treatment steps? |
The level of processing must be known both to compare values among studies and because each processing step may change the level of protein in oil. |
|
3. Was the method used to extract the protein completely described? |
Extraction procedures should be described in sufficient detail to allow the extraction to be reproduced and, ideally, extraction efficiencies should be measured and reported. |
|
4. Was the method used to quantify protein levels completely described? |
The lack of these data increases the level of uncertainty |
|
5. Were replicate samples or batches tested, and was there a statistical analysis of these data? |
The lack of these data and statistical analysis increase the level of uncertainty. |
Based on the data presented in those studies that reported levels other than "not detected", the overall range of protein concentrations for highly refined oils was 0.014 to 16.7 µg protein/ml oil, with a mean of 2.35 µg/ml. The combined mean protein concentration for the two most widely used oils derived from food allergens, soy and peanut, is 0.74 µg/ml with a standard deviation (std) of 1.3 µg/ml. A threshold could be based on the mean protein concentrations or on the mean plus some multiple of the standard deviation. For example, using the mean protein concentrations for peanut and soy oils, protein levels for the mean, mean + 1 std, mean + 2 std, or mean + 3 std would be the 0.74, 2.05, 3.36, and 4.67 ug/ml, respectively.
Advantages. The primary advantage to the statutorily-derived approach is that it is derived from FALCPA's exemption for highly refined oils from labeling provisions in the FALCPA.
Limitations. The primary limitation of this approach is that it is based on an extrapolation of a level derived from a statutory exemption rather than a rigorous, systematic evaluation of all the available scientific data. Because not all the eight major food allergens are used to produce highly refined oil, the use of a statutorily-derived threshold for all food allergens would be based primarily on the protein levels in highly refined soy or peanut oil. Another current significant limitation is the lack of data on the levels of protein in highly refined oils. Based on the data that are currently available and estimates of the amount of oil consumed as a food or food ingredient, it is likely that a threshold based on this approach would be unnecessarily protective of public health.
Finding 5. The statutorily-derived approach provides a mechanism for establishing thresholds for allergenic proteins in foods based on a statutory exemption. Potentially, this approach could be used to set a single threshold level for proteins derived from any of the major food allergens. This approach might yield thresholds that are unnecessarily protective of public health compared to thresholds established using the safety assessment-based approach. However, confirming this would require additional data. If this approach is employed to establish thresholds, it should be used only on an interim basis and should be reevaluated as new knowledge, data, and risk assessment tools become available.
Section 206 of the FALCPA requires that the term "gluten-free" be defined for use on food labels. The law neither describes how gluten-free should be defined nor states whether there is a safe level of gluten.
This section provides an evaluation of the available data to support various approaches for establishing a threshold for gluten. A threshold, if established, could be the basis for decisions on whether to use the term "gluten-free" on product labels.
1. Evaluation of Data Availability and Data Quality
a. Sensitive Populations. Like food allergies, celiac disease affects only a small part of the U.S. population (estimated at 1%). Susceptibility to celiac disease is genetically determined and is linked to the presence of the DQ2 or DQ8 HLA alleles. However, carrying these alleles does not necessarily lead to celiac disease. Both acute and chronic morbidity have been well documented for individuals with symptomatic celiac disease. A gluten-free diet has been shown to greatly reduce the risk for cancer and overall mortality for these individuals. The potential benefit of a gluten-free diet has not been established for individuals with silent or latent celiac disease.
b. Biomarkers. Biomarkers of genetic susceptibility and gluten exposure which allow for non-invasive diagnosis of individuals with celiac disease have been defined. Examples of these biomarkers include circulating antibodies for gliadin (AGA), endomysial (EMA), and tissue transglutaminase (tTG), each of which can be monitored in individuals carrying the DQ2 and/or DQ8 HLA alleles. In asymptomatic individuals with these genetic markers, the presence of anti-EMA or anti-tTG antibodies indicates the presence of latent celiac disease. However, intestinal mucosal inflammation may occur long before the development of clinical symptoms or a rise in antibody titers following a gluten challenge. Further, antibody titers have not been shown to correlate with disease severity. Therefore, for asymptomatic individuals, intestinal biopsies are required to evaluate disease activity or severity. Biopsies are invasive, associated with false negatives, and impractical for frequent monitoring of disease activity or severity.
c. Foods of Concern. The foods of concern for individuals with, or susceptible to, celiac disease are the cereal grains that contain the storage proteins prolamin, gliadin and glutelin (commonly referred to as glutens in wheat), including all varieties of wheat (e.g., durum, spelt, kamut), barley (where the storage proteins are called hordiens), rye (where the storage proteins are called secalins), and their cross-bred hybrids (such as triticale). A small percentage (5% to 10%) of individuals with celiac disease is also sensitive to the storage proteins in oats (avenins).
d. Methods of Analysis. The criteria used to evaluate the available methods of analysis for gluten in food are shown in Table IV-8 and are applied in Appendix 4. A number of commercial immunology-based ELISA test kits for the detection of gluten in foods are available, and one has been validated by AOAC (the Tepnel kit, validated at 160 ppm). One limitation of these kits is that they only detect prolamins. This is not likely to limit the detection of gluten in foods because prolamins and glutelin occur together. However, it may lead to an underestimate of the level of gluten present. Also, none of the test kits cross-reacts with protein extracts from oats, which limits their efficacy for the small portion of celiac patients who are also sensitive to oats. This would require the development of test kits suitable for the detection of oats.
|
Criteria |
Comments |
|---|---|
|
1. Has the method been validated? |
Methods that have been validated (such as by AOAC) are preferred. Alternatively, the sensitivity, precision, and reproducibility of the method should have been demonstrated in a peer reviewed publication. |
|
2. Is the method sufficiently sensitive? |
The limit of detection and the limit of quantitation should be below the levels that appear to cause biological responses in most patients with celiac disease. |
|
3. Are extraction methods available for both raw and baked foods? |
Different methods may be needed; each should be validated. |
|
4. Does the method measure proteins from all relevant foods? |
The cereal grains associated with celiac disease include wheat, barley, rye, and their cross-bred hybrids. Oats may be of concern for some celiac patients. |
|
5. Does the method measure both gliadins and glutenins? |
The storage proteins in cereal grains (generally referred to as gluten) include both prolamin proteins (gliadins) and glutelin proteins (glutenins). Ideally, both of these should be measured. |
|
6. Is the method practical? |
The method should use common laboratory equipment and be reasonably priced. |
e. Oral Challenge Studies. The criteria used to evaluate the available gluten oral challenge studies are provided in Table IV-9 and applied in Appendix 5. Only a limited number of gluten or gliadin challenge studies have been conducted. Of these, most have monitored the subjects' acute responses to a single high dose of gluten or gliadin. These acute studies were not designed to establish a NOAEL or (in most cases) a LOAEL, and the results are not directly applicable to the chronic, low level exposures that may lead to celiac disease. Most clinical studies only test one or two doses. In addition, many studies monitor only biomarkers of gluten exposure (humoral and cell-mediated immune system activity) instead of using biopsies to measure intestinal damage in response to gluten in the diet. Based on the criteria in Table IV-9, two currently available studies are considered to be of high utility. The data in these studies can be used to calculate LOAELs for acute high dose exposures. There are essentially no data available on the impact of chronic consumption of lower gluten levels.
|
Criteria |
Comments |
|---|---|
|
1. Has the study been published in a peer-reviewed journal? |
Published, peer-reviewed studies are preferred although unpublished studies may be considered. |
|
2. Were the criteria for selecting the test population clearly described in the study? |
This information is needed to evaluate how the study results apply to the at-risk population. |
|
3. Was the tested food material clearly and completely described? |
It is important to know the level of gluten in the test material. |
|
4. Was the dose regime clearly and completely described? |
A study designed to measure chronic exposure (low doses over a long period of time) is preferable. Extrapolation of long-term effects from short term studies increases the level of uncertainty. |
|
5. Were the criteria for characterizing responses clearly described? |
This information is needed to evaluate the relevance of the response measured. A definitive diagnostic assessment showing clinical symptoms or intestinal mucosal changes compared to controls is preferred. |
|
6. Are the raw data (individual dose and responses) available for each individual tested? |
These data are needed to develop a risk assessment-based dose-response model. |
2. Options and Findings
The feasibility of using each of the four methods to establish a threshold for gluten was evaluated in light of the available data. As with food allergens, it is likely there will be significant scientific advances in the near future that will address a number of the limitations identified in this report. The Threshold Working Group is aware of several potentially important studies that are currently in progress, but we are unable to evaluate them because the data or analyses are incomplete.
In particular, the Threshold Working Group is aware of unpublished data from an ongoing clinical trial of the subchronic effects of gluten on celiac patients. The "Italian Microchallenge Study" is utilizing intestinal biopsies to relate changes in the intestinal mucosa to antibody biomarkers (Fasano, 2005 personal communication). Preliminary results indicate that daily consumption of both 10 mg and 50 mg of dietary gluten were well tolerated after three months of continuous consumption, but that minimal histological changes were seen in patients consuming 50 mg of gluten daily. Because these data have not yet been published, these results were not considered further.
Finding 6. The initial approach selected to establish a threshold for gluten, the threshold value selected, and any uncertainty factors that were used to establish the threshold should be reviewed and reconsidered periodically in light of new scientific knowledge and clinical findings.
a. Analytical Methods-Based Approach. As with food allergens, an analytical methods-based approach could be used to establish a threshold for gluten if the available clinical and epidemiological data are insufficient to use one of the other approaches. This approach requires that analytical methods be available to detect all relevant glutens. Thresholds are defined by the limits of detection of the available analytical methods, but there is no relationship between these thresholds and the biological response thresholds. Currently, the lower limit of detection for the commercially available gluten test kits are in the range of 10 µg gluten/g of food. Establishing thresholds at levels higher than the lower detection limits of the analytical methods requires the use of assumptions about the biological response thresholds. In that case, the thresholds are actually based on using one of the other three approaches and should not be considered an analytical methods-based threshold.
Advantages. A threshold established using the analytical methods-based approach can easily be incorporated into any applicable FDA compliance programs that combine a specific standard method with a standardized sampling scheme.
Limitations. Several factors limit the applicability of the analytical methods-based approach to establish a threshold for gluten. At this time, only one commercially available analytical method has been validated, and that method was validated for detection at a relatively high concentration of gluten. In addition, there are limited data on the performance of the available methods in the wide variety of food matrices that could potentially contain gluten. Therefore, further characterization of available methods would be necessary before an analytical methods-based threshold could be established. Appropriate methods would need to be developed for the detection of oat gluten.
Finding 7. The analytical methods-based approach could be used to establish a threshold for gluten. However, if this approach is used, the threshold should be replaced by a threshold established using one of the other approaches as quickly as possible.
b. Safety Assessment-Based Approach. The safety assessment-based approach could be used to establish a threshold for gluten based on NOAELs or LOAELs reported in the literature in combination with appropriate uncertainty factors. Clinical data in the literature are limited, but a few studies are available that meet the Threshold Working Group's data quality criteria. The currently available clinical studies do not report NOAELs. However, studies are available that could be used to establish a LOAEL from which a threshold could be derived.
Advantages. Establishing a threshold based on NOAELs or LOAELs and the application of appropriate uncertainty factors to estimated exposure levels is fairly straightforward. When there are limited data in the literature, the application of appropriate uncertainty factors can provide confidence that the majority of the sensitive populations will be protected. Establishing thresholds using the safety assessment-based approach and currently available clinical data has the advantage of being directly linked to biological effects.
Limitations. The primary limitation of this approach is the dearth of available clinical data and the general lack of information about the impact of chronic low level consumption of gluten on the emergence of symptomatic disease in individuals with latent or silent celiac disease. At the current time, the size of the combined uncertainty factors needed would be substantial due to the general lack of data; applying large uncertainty factors to the available data could lead to a gluten threshold that is not achievable, as a practical matter, in foods.
We have identified several data gaps for gluten that contribute to current uncertainty about setting gluten thresholds. The critical areas of uncertainty and variability are:
The uncertainty associated with gluten thresholds arises primarily from the limited amount of clinical data. The critical knowledge gap about individuals with celiac disease is whether chronic, low-level exposure to gluten in a gluten-free diet will cause any harm over a lifetime. We are not aware of any prospective clinical trials that have examined the health of individuals with celiac disease on a gluten-free diet, and there is uncertainty about whether data from short term clinical trials will accurately predict symptoms following chronic, low-level gluten exposure. Conversely, there appears to be only a small degree of uncertainty as to whether the most sensitive celiac disease populations were included in the available clinical trials.
As discussed in Section III, there may be an oat-sensitive subpopulation. The possible existence of this oat sensitive subpopulation raises questions related to the definition of "gluten." Because there are limited clinical data on the sensitivity of this subpopulation of individuals with celiac disease, the uncertainty related to the LOAELs or NOAELs for these individuals is high. Nevertheless, it is unlikely that theses individuals are substantially more sensitive to oat gluten than they are to wheat gluten.
Table IV-10 presents an example of how an overall uncertainty factor could be derived when estimating a threshold for gluten using the safety assessment-based approach. A standard uncertainty factor of 10 should be applied for intraspecies differences in human responses to gluten.
| Description | Uncertainty Factor | Justification |
|---|---|---|
| Intraspecies difference1 | 10 |
Standard for intraspecies variability |
| Extrapolation from LOAEL 2 | 10 | Standard if NOAEL data not available. Supported by clinical trial data |
| Chronic, low level gluten exposure3 | 6 | Estimate using data from gluten clinical trials. |
| Overall Uncertainty Factor4 = 600 | ||
|
1 This includes both between- and within-individual variability. 2 This includes both a factor for converting the LOAEL to a NOAEL and an additional factor for the uncertainty associated with that conversion factor. Preliminary NOAEL data from an unpublished clinical trial (Fasano, 2005 personal communication) supports an approximate 10-fold difference between a NOAEL and published LOAELs (Catassi et al., 1993). 3 Estimated by comparing published LOAELs in an acute, single dose exposure (Ciclitira et al., 1984) with repeated exposure over four weeks (Catassi et al., 1993). 4 Uncertainty is likely to decrease as clinical trial data becomes available. | ||
Finding 8. The safety assessment-based approach is a viable approach to establish a threshold for gluten using currently available LOAEL data for celiac disease. An overall uncertainty factor should be estimated from the data and applied to the LOAEL to establish a threshold for gluten. Any threshold derived from this approach should be reevaluated as new research data become available. Available data are insufficient at the current time to use this approach to establish a threshold for oat gluten for those individuals with celiac disease who are also sensitive to oats. However, it is likely that a threshold based on wheat gluten would be protective for individuals susceptible to oat gluten.
c. Risk Assessment-Based Approach. There are few data from human clinical trials that can be used to develop a dose-response model for gluten and celiac disease. In addition, limited data are available on exposure; for example there are limited data on the actual levels of gluten in the diet of individuals on "gluten-free diets" and on the effects of low level, chronic gluten exposure in individuals with silent or latent celiac disease. These limitations would lead to a very high level of uncertainty associated with models designed to predict the health effects of gluten in the diet. Therefore, a scientifically defensible hazard characterization and exposure assessment are not possible at the current time.
Finding 9. Use of the quantitative risk assessment-based approach to establish a threshold for gluten does not appear to be feasible at the present time. However, considering the benefits that could be gained from using the risk assessment-based approach, priority should be given to establishing a research program to acquire the knowledge and data needed.
d. Statutorily-Derived Approach. The FALCPA does not include requirements or exemptions that could be used to establish a statutorily-derived threshold for gluten. Also, the law does not define the term "gluten-free." Potentially, a threshold could be established using the international standards currently under review by Codex (Codex Alimentarius Commision, 2004 ). However, the proposed Codex standards do not appear to be based on either a scientific rationale for a distinction between naturally gluten-free foods and foods processed to be free of gluten, or a systematic evaluation of clinical data related to the effect of gluten on acute or chronic celiac disease etiology. The levels being considered by Codex seem to be based on anecdotal evidence and on the levels of gluten that are presumed to be historically present in foods that have been called "gluten-free."
Finding 10. There appear to be no suitable statutory requirements or exemptions that would serve as the rationale for using for a statutorily-derived approach to establish a threshold for gluten. This approach is not viable.
Although the FALCPA requires establishing a definition for the term "gluten-free" for food labeling, the quantity and quality of the data needed to accomplish this on a scientific basis are severely limited at the current time relative to all three of the potentially viable approaches. This was aptly summarized by the consensus statement published after a conference of experts convened by the National Institutes of Health which noted that "The strict definition of a gluten-free diet remains controversial due to the lack of an accurate method to detect gluten in food products and the lack of scientific evidence for what constitutes a safe amount of gluten ingestion" (NIH, 2004). These experts concluded that additional research is needed to "Define the minimum safe exposure threshold of gluten in the diet relative to celiac disease" (NIH, 2004).
In view of the consensus opinion stated in the NIH report, the Threshold Working Group concluded that Finding 6 should be re-emphasized. Any approach used to establish a threshold for gluten to protect consumers with, or susceptible to, celiac disease should be used in an iterative manner. Any such the threshold and approach should be re-examined periodically to consider new scientific knowledge and data.
| Allergen | Manufacturera | Method | Validationb | Sensitivity (LOD) (ppm) | Quantitation (LOQ) (ppm) | Raw and Processed Foods? | Species Specificity | Protein(s) Detected | Practicality |
|---|---|---|---|---|---|---|---|---|---|
| Peanut | Abkem Iberia | Peanut DiagnoKit | No | ? | ? | Yes (For specified foods) |
? | ? | Yes |
| Elisa Systems | Peanut | No | ? | ? | ? | ? | ? | ? | |
| Neogen | Alert for Peanut | No | 5 | No | ? | ? | ? | Yes | |
| Neogen | Reveal for Peanut | No | 5 | No | ? | ? | ? | Yes | |
| Neogen | Veratox for Peanut | AOAC MLPT | ? | 2.5 | Yes (For specified foods) |
Peanut | ? | Yes | |
| R-BioPharm | RiDASCREEN Peanut | No | 2.5 | ? | Yes | ? | Total | Yes | |
| R-BioPharm | RIDASCREEN FAST Peanut | AOAC MLPT | 1.5 | 2.5 | Yes (For specified foods) |
Peanut | Total | Yes | |
| Tecra | Peanut Visual Immunoassay | No | 0.5 | 2.5 | Yes | ? | ? | Yes | |
| Tepnel | BioKits Peanut | AOAC MLPT | 0.1 | 1 | Yes (For specified foods) |
Peanut | Conarachin (Ara h 1) | Yes | |
| Tepnel | BioKits Rapid Peanut | No | ? | No | ? | ? | ? | Yes | |
| Milk | Abkem Iberia | Casein DiagnoKit | No | ? | 0.16 | Yes (For specified foods) |
? | Casein | Yes |
| Elisa Systems | Beta-lactoglogulin | No | ? | ? | ? | ? | Beta- Lactoglobulin | ? | |
| Elisa Systems | Casein | No | ? | ? | ? | ? | Casein | ? | |
| Neogen | Alert for Total Milk | No | 5 whole milk 10 dry non-fat milk |
No | Yes | ? | ? | Yes | |
| Neogen | Veratox for Total Milk | No | ? | 2.5 | Yes (For specified foods) |
? | ? | Yes | |
| R-BioPharm | RIDASCREEN Beta-Lactoglobulin | No | 5 | ? | ? | ? | Beta-Lactoglobulin | Yes | |
| SafePath | Milk Residue | No | ? | ? | ? | ? | Beta-Lactoglobulin | Yes | |
| Tepnel | BioKits BetaLG | No | 7.5 | 25 | Yes | ? | Primarily Beta-Lactoglobulin | Yes | |
| Tepnel | BioKits BSA | No | 10 | 25 | Yes | ? | Bovine Serum Albumin | Yes | |
| Tepnel | BioKits Casein | No | 1 | 2 | Yes | ? | Primarily Alpha-Casein | Yes | |
| Egg | Elisa Systems | Egg | No | ? | ? | ? | ? | ? | ? |
| Neogen | Alert for Egg | No | 5 | N | Yes (For specified foods) |
? | ? | Yes | |
| Neogen | Veratox for Egg | No | ? | 2.5 | Yes (For specified foods) |
? | ? | Yes | |
| R-BioPharn | RIDASCREEN Egg Protein | No | 2 | ? | Yes (for specified foods ?) |
? | White | Yes | |
| SafePath | Egg Residue | No | ? | ? | ? | ? | Ovomucoid | Yes | |
| Tecra | Egg Visual Immunoassay | No | 0.5 | 0.6 | Yes | Total | Yes | ||
| Tepnel | BioKits Egg | No | 0.1 | 0.5 | Yes | ? | Ovomucoid | Yes | |
| Tree Nuts | Abkem Iberia | Almond DiagnoKit | No | ? | 0.06 | ? | ? | ? | Yes |
| Abkem Iberia | Hazelnut DiagnoKit | No | ? | 0.08 | ? | ? | ? | Yes | |
| Elisa System | Almond | No | ? | ? | ? | ? | ? | ? | |
| Elisa System | Hazelnut | No | ? | ? | ? | ? | ? | ? | |
| Neogen | Alert for Almond | No | 5 | No | ? | ? | ? | Yes | |
| Neogen | Veratox for Almond | No | ? | 2.5 | ? | ? | ? | Yes | |
| R-BioPharm | RIDASCREEN Hazelnut | No | 3.3 | ? | ? | ? | Total | Yes | |
| R-BioPharm | RIDASCREEN FAST Almond | No | 1.7 | 2.5 | ? | ? | Total | Yes | |
| Soy | Elisa System | Soy | No | ? | ? | ? | ? | ? | ? |
| SafePath | Soy Residues | No | ? | ? | ? | ? | Trypsin Inhibitor | ? | |
| Tepnel | Soya Protein | No | 0.5% soy protein in food sample |
0.5% soy protein in food sample |
? | ? | ? | Yes | |
| Crustaceans | Abkem Iberia | Crustacean DiagnoKit | No | ? | 0.005 | ? | shrimp, crab, lobster and scampi. |
Tropomyosin | Yes |
| Elisa Systems | Crustacean | No | ? | ? | ? | ? | Tropomyosin | ? | |
|
* Information from manufacturers web sites. + MLPT - Multiple Laboratory Performance Tested |
|||||||||
| Study | Published | Test Population | Food Allergen Tested | Test Material | Lowest Dose Tested (mg proteina) |
Dose Progression | Responses at lowest dose tested? | LOAEL Observed (mg proteina) |
Symptom(s) used to determine LOAEL | Population Dose/Response Data |
|---|---|---|---|---|---|---|---|---|---|---|
| May, 1976 | Y | Asthmatic children | Peanut | Raw peanut | 25 | 2 -10 fold increase | Y | 25 | Objective | ? |
| Milk | Whole | ? | N | ? | Objective | ? | ||||
| Egg | Whole | ? | N | ? | Objective | ? | ||||
| Dried | ? | N | 250 + | Objective | ? | |||||
| Bock et al., 1978 | Y | Children with suspected allergy | Peanut | Unroasted | ? | ? | ? | 25 | Objective | N |
| Milk | Dried nonfat | ? | ? | ? | 280 | Objective | N | |||
| Egg | Dried whole | ? | ? | ? | 1 | Objective | N | |||
| Soy | Protein isolate | ? | ? | ? | ? | Objective | N | |||
| Cashew | ? | ? | ? | ? | ? | Objective | N | |||
| Pecan | ? | ? | ? | ? | ? | Objective | N | |||
| Filbert | ? | ? | ? | ? | ? | Objective | N | |||
| Cashew | ? | ? | ? | ? | ? | Objective | N | |||
| Pistaschio | ? | ? | ? | ? | ? | Objective | N | |||
| Pasterello et al., 1989 | Y | Adults with suspected allergy | Milk | Dried | Not clear - differed for different foods | Dose doubling | ? | 187 | Objective | N |
| Egg white | Dried | N | 1500 | Objective | N | |||||
| Hazelnut | Ground | N | 2775 | Objective | N | |||||
| Bernhisel-Broadbent et al., 1992b | Y | Fish allergic children and adults | Fish | Raw and cooked extracts of 9 species | ? | ? | ? | ? | ? | N |
| Caffarelli et al., 1995 | Y | Infants and children with no previous egg exposure | Egg | Dried egg | 0.042 | ? | N | 0.42 | Objective | N |
| Magnolfi et al., 1996 | Y | Atopic children | Soy | Formula | "1 drop" for infants | 6 defined doses | N | 360 | Objective | N |
| 88 mg soy protein for older children | 7 defined doses | Y | 88 | Objective | N | |||||
| Hourihane et al., 1997a | Y | Peanut allergic adults | Peanut | Peanut flour | 0.01 | 12 defined doses | N | 0.1 | Subjective | Y |
| 2 | Objective | |||||||||
| Hourihane et al., 1997b | Y | Peanut allergic adults | Peanut | Whole peanut | ? (Labial challenge) | 4 defined doses | Y | ? | Objective | N |
| Nelson et al., 1997 | Y | Peanut allergic adults | Peanut | Defatted peanut | 0.45 | 12 defined doses | ? | ? | Subjective | N |
| Bellioni-Businco et al., 1999 | Y | Milk allergic children | Milk | "Fresh" whole milk | "1 drop" | ? | 3.65 + | Objective | N | |
| Hebling et al., 1999 | Y | Fish allergic adults | Fish | Cooked meat from 3 species of fish | 50 | 4 specified levels | Y | 50 | Subjective and Objective | Y |
| Zeiger et al., 1999 | Y | Milk allergic infants and children | Soy | Formula | 1 drop to 5 ml | 6 to 7 doublings | N | 52.2 | Objective | N |
| Otolani et al., 2000 | Y | Hazelnut allergic adults | Hazelnut | Ground nuts | 224 | Dose doubling, possibly 4 levels | ? | ? | ? | N |
| Sicherer et al., 2000 | Y | Children with a variety of food allergiesb | Peanut | ? | 400 or 500 mg of food | 6 or 7 specified levels | Y | ? | ? | N |
| Milk | ? | ? | ? | N | ||||||
| Egg | ? | ? | ? | N | ||||||
| Soy | ? | ? | ? | N | ||||||
| Fish | ? | ? | ? | N | ||||||
| Wheat | ? | ? | ? | N | ||||||
| Eggesbo et al., 2001 | Y | Children | Egg | Pancakes | 260 | Dose doubling until reaction or maximum dose | Y | 260 | Objective | N |
| Bindslev-Jensen and Hansen in in Taylor et al., 2002 | N | ? | Fish | Cod | ? | ? | ? | 5mg of fish | ||
| Mackerel | ? | ? | ? | 500 mg of fish | ||||||
| Herring | ? | ? | ? | 5 mg of fish | ||||||
| Plaice | ? | ? | ? | 6000mg of fish | ||||||
| Bindslev-Jensen and Mortz in Taylor et al., 2002 | N | ? | Peanut | Ground | ? | ? | ? | 40 | ? | N |
| Bindslev-Jensen and Norgaard in Taylor et al., 2002 | N | ? | Milk | Whole | ? | ? | ? | 180 | ? | N |
| Egg | Whole raw | ? | ? | ? | 0.65 | ? | N | |||
| Bock in Taylor et al., 2002 Bock | N | ? | Peanut | Ground | ? | ? | ? | 1.25 | ? | N |
| Milk | Nonfat dried | ? | ? | ? | 67 | ? | N | |||
| Egg | Whole or dried | ? | ? | ? | ? | ? | N | |||
| Fish | Minced | ? | ? | ? | 200 mg of fish | ? | N | |||
| Burks and Christie in Taylor et al., 2002 | N | ? | Peanut | Peanut butter | ? | ? | ? | 100 | ? | N |
| Milk | Nonfat dried | ? | ? | ? | 140 | ? | N | |||
| Egg | Whole dried | ? | ? | ? | 200 | ? | N | |||
| Hill in Taylor et al., 2002 | N | ? | Peanut | Peanut butter | ? | ? | ? | 6 | ? | N |
| Milk | Whole | ? | ? | ? | 0.6 | ? | N | |||
| Egg | Raw white | ? | ? | ? | 2 | ? | N | |||
| Host in Taylor et al., 2002 | N | ? | Milk | Formula | ? | ? | ? | 75 | ? | N |
| Lack in Taylor et al., 2002 | N | ? | Peanut | Ground | ? | ? | ? | 125 | ? | N |
| Milk | Whole | ? | ? | ? | 150 | ? | N | |||
| Egg | Cooked white | ? | ? | ? | 10 | ? | N | |||
| Raw white | ? | ? | ? | 20 | ? | N | ||||
| Moneret-Vautrin #1 in Taylor et al., 2002 | N | ? | Peanut | Ground | ? | ? | ? | 1.25 (single blind) 2.5 (double blind) |
? | N |
| Milk | Whole | ? | ? | ? | 30 (double blind 150 (single blind) |
? | N | |||
| Egg | White | ? | ? | ? | 0.2 | ? | N | |||
| Fish | Minced | ? | ? | ? | 15 mg of fish (single blind) 65 mg of fish double blind |
? | N | |||
| Moneret-Vautrin #2 in Taylor et al., 2002 | N | ? | Peanut | Ground | ? | ? | ? | 66 | ? | N |
| Egg | White | ? | ? | ? | 26.5 | ? | N | |||
| "National Jewish" in Taylor et al., 2002 | N | ? | Peanut | Ground | ? | ? | ? | 2 | ? | N |
| Rance in Taylor et al., 2002 | N | ? | Peanut | Ground | ? | ? | ? | 0.25 | ? | N |
| Milk | Whole | ? | ? | ? | 15 | ? | N | |||
| Egg | Whole raw | ? | ? | ? | 0.13 | ? | N | |||
| Fish | Minced | ? | ? | ? | 16 mg of fish | ? | N | |||
| Zeiger in Taylor et al., 2002 | N | ? | Milk | Formula | ? | ? | ? | 1.5 | ? | N |
| Wensing et al., 2002a | Y | Hazelnut allergic adults | Hazelnut | Raw nuts | 1 | 7 specified doses | Y | 1 | Subjective and Objective | Y |
| Wensing et al., 2002b | Y | Peanut allergic adults | Peanut | Roasted peanut meal | 0.030 | 10 specified doses | N | 0.1 | Subjective and Objective | Y |
| Fiocchi et al., 2003 | Y | Children with allergy to both milk and soy | Milk | Whole | 43.2 | 4 specified doses | Y | 43.2 | Objective | Y |
| Soy | Formula | 21.8 | 21.8 | Objective | ||||||
| Hansen et al., 2003 | Y | Hazelnut allergic adults | Hazelnut | Raw and roasted nuts | ? | ? | ? | 32 - roasted 16 - raw + |
Oral allergy syndrome | N |
| Morisset et al., 2003 | Y | Undefined | Peanut | Crushed | 1.25 | 5 specified levels | Y | 1.25 | Objective | N |
| Oil | ? | N | ? | Objective | N | |||||
| Milk | Lactose free | 0.36 | Y | 0.36 | Objective | N | ||||
| Egg | Raw white | 0.2 | Y | 0.2 | Objective | N | ||||
| Soy | Oil | ? | N | ? | Objective | N | ||||
| Osterballe and Bindslev-Jensen, 2003 | Y | Egg allergic children | Egg | Pasteurized whole egg | 2.9 | 8 specified doses | Y | 2.9 | Objective | N |
| Perry et al., 2004 | Y | ? | Milk, egg, peanut, soy, wheat | ? | ? | ? | ? | ? | ? | N |
|
Note: Question marks (?) in the table indicate either that the information was not given or could not be determined. | ||||||||||
| Oil Type | Reference a | Protein Concentration (µg/g) |
Description of Oil | Published | Protein Separation Method c | Protein Quantitation Method |
|---|---|---|---|---|---|---|
| Soy | Tattrie and Yaguchi, 1973 | 0.96 | Refined, deodorized | Yes | Chromatography | Amino Acid Analysis |
| Klurfeld and Kritchevsky, 1987 | 1.93 | Crude | Yes | Aqueous Extraction | Commercial Bradford Assay | |
| 0.72 | "Processed" | |||||
| Awazuhara et al., 1998 | 0.014 0.017 0.018 0.023 0.027 0.040 |
Uncharacterized, commercial | Yes | Aqueous Extraction | Lowery Assay | |
| Reeves, 1999 | 0.033 0.042 0.049 0.057 0.082 0.114 0.222 |
Fully refined, commercial | No | Unknown | Amino Acid Analysis | |
| Paschke et al., 2001 | 0.0332 0.0353 |
Refined | Yes | Acetone Precipitation | Bradford Assay | |
| 0.0898 0.1010 0.1380 |
Unrefined | |||||
| Errahali et al., 2002 | 0.32 | Deodorized | Yes | Aqueous Extraction | Unknown | |
| 1.80 | Cold pressed | |||||
| Nordlee et al., 2002 | 0.16 - 20.8 | Degummed | No | Aqueous Extraction | Amino Acid Analysis | |
| 0.043 - 6.8 | Refined | |||||
| 0.033 - 3.1 | Bleached | |||||
| 0.021 - 0.443 | Deodorized | |||||
| Peanut | Klurfeld and Kritchevsky, 1987 | 0.120 0.154 0.204 0.206 0.580 |
Processed | Yes | Aqueous Extraction | Bradford Assay |
| Hoffman and Collins-Williams, 1994 | 0.2 0.6 3.3 3.3 |
Cold pressed | Yes | Aqueous Extraction | Commercial Coomassie Dye Assay | |
| Teuber et al., 1997 | 3.0 ± 0.3 5.7 ± 1.2 |
Refined, bleached, deodorized | Yes | Aqueous Extraction | Commercial Bradford Assay | |
| 10.5 ± 0.4 10.7 ± 0.8 |
Unrefined | |||||
| Olszewski et al., 1998 | 0.10 0.13 0.15 0.16 0.20 |
Refined, commercial | Yes | Aqueous Extraction | Commercial Bicinchoninic Acid (BCA) Assay | |
| Skinner and Haynes, 1998 | 187 | Crude | No | Aqueous Extraction | Lowery and Commercial BCA Assay | |
| 60 | Alkali refined, neutralized | |||||
| 15 | Alkali refined, neutralized, bleached | |||||
| 2.2 | Alkali refined, neutralized, bleached, deodorized | |||||
| ISEO, 1999 | 0.047 0.049 0.063 |
Fully refined, commercial | No | Unknown | Amino Acid Analysis | |
| 0.828 | Partially refined, commercial | |||||
| Crevel et al., 2000b | 48 | Refined, neutralized, bleached, deodorized | No | Aqueous Extraction | Commercial BCA Assay | |
| 91 220 |
Crude | |||||
| Peeters et al., 2004 | 0.09 6.4 |
Crude, noncommercial | Yes | Unknown | ELISA (not described) | |
| 2.55 | Cold pressed | |||||
| Tree Nut | Teuber et al., 1997 (Almond) | 2.2 ± 0.7 16.7 ± 0.8 |
Refined, bleached, deodorized | Yes | Aqueous Extraction | Commercial Bradford Assay |
| 12.7 ± 2.8 | Blend | |||||
| 62.2 ± 2.2 | Unrefined | |||||
| Teuber et al., 1997 (Walnut) | 7.0 ± 2.5 7.0 ± 0.8 9.2 ± 3.1 |
Refined, bleached, deodorized | Yes | Aqueous Extraction | Commercial Bradford Assay | |
| 16.5 ± 2.4 | Unrefined | |||||
| 20.4 ± 1.8 | Blend | |||||
|
Note: Protein levels too low to detect or measure were reported by Tattrie and Yaguchi (1973),
Hoffman and Collins-Williams (1994), Yeung and Collins (1996), Peeters et al. (2004)
for peanut oils and by Tattrie and Yaguchi (1973), Porras et al. (1985) for soy oils.
These values were not included due to the lack of methodological information. aNone of the publications provide sufficient information to evaluate the overall extraction efficiency, accuracy, reproducibility, or precision of the method used. In addition, in most cases, it was not clear whether replicate samples were tested or whether replicate measurements were carried out for individual samples. bCrevel et al. (2000) is a review paper that includes previously unpublished data. These data are given here, but are considered unpublished because the research that generated these values has not specifically been peer reviewed. |
||||||
| Methoda | Validation | Sensitivity (LOD) (ppmb) | Quantitation (LOQ) (ppmb) | Raw and Baked Foods? | Species Specificity | Protein(s) Detected | Practicality |
|---|---|---|---|---|---|---|---|
| Diffchamb Transia Plate Gluten | No | 10 | ? | ? | Wheat, triticale, rye, barley | Gliadin | Yes |
| Diffchamb Transia Plate Prolamins | Working Group on Prolamin Analysis and Toxicity | 3 | ? | Yes | Wheat, triticale, rye, barley | Gliadin | Yes |
| Ingensa Gluten EIA | No | 3 | ? | ? | Wheat, rye, barley | Gliadin | ? |
| Neogen Alert for Gliadin | No | 20 | No | ? | Wheat, rye, barley | Gliadin | Yes |
| Neogen Veratox for Gliadin | No | ? | 5 | ? | Wheat, rye, barley | Gliadin | Yes |
| R-BioPharm RIDASCREEN Gliadin | Prolamin Working Group Ring Studyc | 3 | 5 | Yes | Wheat, rye, barley | Gliadins | Yes |
| R-BioPharm RIDASCREEN FAST Gliadin | No | 4 | 10 | Yes | Wheat, rye, barley | Gliadin | Yes |
| R-BioPharm RIDAQUICK Gliadin | No | 5 | No | Yes | Wheat, rye, barley | Gliadin | Yes |
| Tepnel BioSystems Wheat Gluten | AOAC | 160 2 - not validated | 16 | Yes | Wheat, triticale, rye | Omega gliadin | Yes |
| Tepnel BioSystems Gluten Rapid Test Kit | No | 50 - breads, etc 200 - "highly processed flour" | No | Yes | Wheat, triticale, rye | Omega gliadin | Yes |
|
aInformation from manufacturers web sites: Ingensa Neogen R-BioPharm Tepnel bExpressed as ppm of gluten. cImmer et al., 2003. |
|||||||
| Study | Published Peer-reviewed | Population Tested | Diagnostic Assessment (Biomarker) | Test Dose characterized | Dose Level | Dose Duration | Raw Data Available |
|---|---|---|---|---|---|---|---|
| Fasano, 2005 | No; study is ongoing | 36 CD individuals | Intestinal biopsy, symptoms | 10 or 50 mg gluten/day | 3 months | ||
| Catassi et al., 1993 | Yes | 10 CD children each dose | Intestinal biopsy, symptoms | 100 mg or 500 mg gliadin/d | 200 mg or 1 gm gluten/d | 4 weeks | |
| Ciclitera et al., 1984 | Yes | 1 CD adults each dose | Intestinal biopsy | 10 mg or 600 mg gliadin | 20 mg or 1.2gm gluten | 4 hr | |
| Ciclitera et al., 1984 | Yes | 7 CD adults | Intestinal biopsy, symptoms | 1.2 -2.4 mg gliadin/d | 2.8 -- 4.8mg gluten/d | 1 week | |
| Ciclitera et al., 1985 | Yes | 10 CD adults | Intestinal biopsy, symptoms | 1.2 -2.4 mg gliadin/d | 2.8 -- 4.8mg gluten/d | 6 weeks | |
| Montgomery et al, 1988 | Yes | 8 CD adults | Intestinal biopsy, symptoms, anti-gluten Ab | 2.5 -- 5 gm gluten/d | 6 months (median) | ||
| Sturgess et al, 1994 | Yes | 4 CD adults | Intestinal biopsy | 1 gm gliadin by intraduodenal infusion | 2 gm gluten | 6 hrs |
Notice of Availability; Request for Comments and for Scientific Data and Information June 17, 2005