U.S. Food & Drug Administration
Center for
Food
Safety &
Applied
Nutrition
Office of Premarket Approval
May 2000
(Effective June 18, 2001, Office of Premarket Approval is now
Office of Food Additive Safety. See
updated contact information)
|
The latest version of this guidance issued on
April 10, 2002. Below is an earlier version.
Guidance for Industry
Preparation of
Premarket Notifications for Food Contact Substances:
Chemistry
Recommendations
Additional copies are available from:
Office of Food Additive Safety (OFAS), HFS-200
Center for Food Safety & Applied Nutrition (CFSAN)
Food and Drug Administration (FDA)
5100 Paint Branch Parkway.
College Park, MD 20740
(Tel) 301-436-1200
Contact information updated since original publication.
(See
additional contact information.)
U. S. Department of Health and Human Services
Food and Drug Administration
Center for Food Safety and Applied Nutrition (CFSAN)
September, 1999 (revision 1.1, May, 2000)
 HIGHLIGHTS OF THE 1999
GUIDANCE
HIGHLIGHTS OF THE 1999
GUIDANCE
This document addresses FDA's recommendations for chemistry information that should be
submitted in a premarket notification (PMN) for a
food-contact substance (FCS). These recommendations are based on an adaptation and revision
of the June, 1995 document for indirect food additive petitions
entitled "Recommendations for Chemistry Data for Indirect Food Additive Petitions."
Highlights of this document include:
Alternate approaches to estimating migration to food, such as migration
modeling, are presented.
Consumption factors (CFs) for several specific polymer packaging categories, such as
polyolefins, cellophane, and nylons, have been
updated.
Testing for "wet-end" additives used in the manufacture of paper and paperboard is
discussed.
FDA Form No. 3480 "Notification for New Use of a
Food Contact Substance" is available (PDF).
CF for poly(ethylene terephthalate) (PET) updated to 0.21
(5/2000).

Note: If your browser does not display the table of contents and sections
using Roman numerals and alphabetic characters, you
may request a hard copy of the document as described above.
TABLE OF
CONTENTS
- INTRODUCTION
- CHEMISTRY
INFORMATION FOR PREMARKET NOTIFICATIONS
- Identity
- Use
- Intended Technical
Effect
- Migration Testing &
Analytical Methods
- Design of the Migration Experiment
- Migration Cell
- Test Sample
- Food Simulants
- Temperature and Time
of Test
- End
Tests
- Characterization of Test Solutions & Data Reporting
- Analytical
Methods
- Description of the Method
- Standard Curves
- Examples of Spectra or
Chromatograms
- Example
Calculations
- Validation of
Analytical Methods
- Migration
Database
- Migration
Modeling
Consumer Exposure
- Calculation of Exposure
- Consumption Factor
- Food-type Distribution
Factor
- Concentration in the
Daily Diet and EDI
- Cumulative
Exposure
Exposure
Refinement
APPENDIX I.
FATTY-FOOD SIMULANTS FOR SPECIFIC POLYMERS
APPENDIX II. SELECTED
MIGRATION TESTING PROTOCOLS
- General Protocols (Single-Use Applications) Corresponding to
Condition of
Use
- Adjuvants for
Polyolefins
- Adjuvants for Polymers
(other than Polyolefins)
Adjuvants for More than One Polymer
- Articles Intended for
Repeated Use
- Coatings for Cans
- Uncoated &
Clay-Coated Papers with Latex Binders
- Specially Treated
Papers
- Adhesives (Room
temperature or below)
- Laminates &
Coextrusions
- Boil-In-Bags
- Special High-Temperature
Applications
- Dual-Ovenable Trays
- Microwaveable
Containers
- Microwave
Heat-Susceptor Packaging
Colorants for Plastics
Dry Foods with Surface
Containing No Free Fat or Oil
Wet-End Additives used in
the Manufacture of Paper and
Paperboard
APPENDIX
III. ILLUSTRATIVE EXAMPLE OF VALIDATION OF ANALYSES
APPENDIX IV. CONSUMPTION
FACTORS, FOOD-TYPE DISTRIBUTION FACTORS, AND EXAMPLE OF EXPOSURE
ESTIMATE CALCULATIONS
APPENDIX V.
REFERENCES

- INTRODUCTION
Section 309 of the Food and Drug Administration Modernization Act of 1997 (FDAMA),
amends Section 409 of the Federal Food, Drug, and Cosmetic Act
(FFDCA) (21 U.S.C. 348) to establish a premarket notification (PMN) procedure as the primary
method by which the Food and Drug Administration (FDA)
regulates food additives that are food contact substances (FCS). A
food contact substance is any substance that is intended for use
as a component of
materials used in manufacturing, packing, packaging, transporting, or holding food if the use is
not intended to have any technical effect in the
food.
Notifications for food contact substances must contain sufficient scientific information to
demonstrate that the substance that is the subject of the
notification is safe for the intended use (21 U.S.C 348(h)(1)). Because the safety standard is the
same for all food additives, whether subject to the
petition process or the PMN process, information in a PMN should be comparable to that
recommended for inclusion in a food additive petition.
This guidance has been prepared by the Office of Premarket Approval of
the Center for Food Safety and Applied Nutrition (CFSAN) at the Food
and Drug Administration in
accordance with FDA's "Good Guidance Practices"
(62 FR 8961; Feb. 27, 1997). The purpose of this document is to provide
general guidance for the chemistry information that should be included
in a PMN for an FCS.
This guidance
represents FDA's current thinking on the chemistry information
for a PMN.
It does not create or confer any rights for or on any person and does not operate to bind the
Agency or
the public. An alternative approach may be used if such approach satisfies the requirements of
the applicable statute and regulations. For situations
not addressed in this guidance, notifiers are advised to consult FDA.
Periodically, FDA will update this guidance in light of new information.
CHEMISTRY
INFORMATION FOR PREMARKET NOTIFICATIONS
The chemistry information that FDA
recommends to support the proposed use of a FCS is
described in Section III., items C. through F.,
of the guidance document entitled "Preparation of Premarket Notifications for Food-contact
Substances: Administrative." This information is reproduced below in
italics, followed by a detailed discussion on each recommendation. A clear and concise
presentation of the information in the format described
below will facilitate review of the PMN.
- Identity
Item C: Notifications should include detailed information on the chemical identity of the
FCS and the impurities and residual reactants from the
production of the FCS including, where possible, the chemical and structural formula and the
Chemical Abstract Service (CAS) Registry Number.
Identity information is used to describe the FCS that is the subject of a PMN and to identify
substances that may migrate into food from
use of the FCS. Migrating substances may include not only the FCS itself, but also degradation
products and impurities in the FCS that may have no
functional effect.
Information identifying the FCS should be as complete as possible with respect to its name,
composition, and method of manufacture. These items
include:
-
Chemical Name
.
The IUPAC or Chemical Abstracts name is acceptable.
Common or Trade Names
.
These should not be the only means of identification. FDA does not maintain a compilation of
common or trade names.
Chemical Abstracts Service
.
Registry Number(1).
Composition
.
A full description of the composition of the FCS is used to compile a list of potential migrants to
food. This should include: chemical formulae,
structures, molecular or formula weights for single compounds or components of commercial
mixtures. For polymers, notifiers should also submit the
weight average (Mw) and number average (Mn) molecular
weight, the molecular weight distribution, and the methods used for their
determination. If the molecular weight is not readily obtainable, the notifier should furnish other
properties of the polymer that are functions of the
molecular weight, These as intrinsic or relative viscosity or melt flow index.
In addition, the notifier should provide the following information:
- A complete description of the manufacturing process, including
purification procedures, and the chemical
equations for all steps of the synthesis.
- A list of reagents, solvents, catalysts, purification aids, etc., used in the
manufacturing process, the amounts or
concentrations used, their specifications, and their CAS Reg. Nos.
- Chemical equations for known or likely
side reactions occurring during manufacture of the FCS, including catalyst
degradation reactions, if known.
- Concentrations of all major impurities
(e.g., residual starting materials, including all reactants, solvents, and
catalysts, in addition to byproducts and degradation products) together with supporting analytical
data and calculations. In the case of polymers,
concentrations of residual monomers should be included.
- Spectroscopic data to characterize the FCS. In some cases an infrared (IR) spectrum is
sufficient, but occasionally other
information, such as visible and ultraviolet absorption spectra or nuclear magnetic resonance
(NMR) spectra, are more useful.
Those data and information not intended for public disclosure, such as trade secret or
confidential commercial information, should be so
identified.
Properties.
Notifiers should submit the physical and chemical specifications of the
FCS (e.g., melting point, impurity specifications). Notifiers should
report properties that can affect migration potential, such as solubilities in food simulants. In the
case of new polymers, notifiers should provide
glass transition temperatures, ranges for densities and melt flow indices, and information on
morphology (e.g., degree of crystallinity) and
stereochemistry. For new adjuvants in regulated polymers, notifiers should submit
information on the properties of the polymer (e.g.,
Tg) used in migration testing.
Analyses
.
If the FCS is a component of an otherwise regulated material (e.g., an antioxidant in a regulated
polymer), notifiers should provide analytical
methods for determining the FCS in the material. Notifiers should submit supporting analytical
data (refer to
Section D.3.).
Use
Item D: Notifications should include detailed information on the intended conditions of
use of the food contact material manufactured with the food
contact substance (e.g., maximum use temperature, type of food with which the substance
is intended to come into contact, duration of the contact,
and whether the food contact material is intended for repeat or single use application.)
Notifiers should examine general use limitations in notifications and regulations for similar
food contact substances and propose a comprehensive set
of limitations on the notified use. Certain of these limitations may be the basis for assumptions
made in deriving exposure estimates for the FCS. Any
applicable limitations should be included in the description of the notified use. In the absence of
appropriate limitations, FDA may be
required
to use assumptions in estimating exposure that would result in more conservative values for
certain classes of FCS.
- Notifiers should provide the maximum use level of the FCS and the types of
food-contact articles in which it may be used. "Use level" refers to
the concentration of a substance in the food-contact article, not in the food. Notifiers should state
the range of possible uses, such as films, molded
articles, coatings, etc., and report the anticipated maximum thickness and/or weight per unit area
of these articles.
- Notifiers should list the types of food (with examples) expected to be used in contact
with the FCS and the maximum temperature and time conditions
of food contact(2). Classifications that
may be helpful are given in 21 CFR 176.170(c), Table 1 (Types of Raw and Processed
Foods) and Table 2, which lists various conditions of use. These tables are not all-inclusive,
however.
Intended Technical
Effect
Item E: Notifications should include a statement of the intended technical effect of the
food contact substance, and data that demonstrate the
minimum amount of the substance that will achieve the intended technical effect.
Notifiers should present data to show that the FCS will achieve the intended technical effect
and that the proposed use level is the minimum level
required to accomplish the intended technical effect. In these circumstances, "technical effect"
refers to the effect on the food-contact article, not on
the food. An example would be the effect of an antioxidant in preventing oxidative degradation
of a particular polymer. In the case of a
new polymer, notifiers should present data that demonstrate the specific properties of
the polymer that make it useful for food-contact
applications. This information is frequently available in product technical bulletins.
In cases where the use level of an FCS is self-limiting, notifiers should provide supporting
data.
Migration Testing &
Analytical Methods
Item F: Notifications should include sufficient data to enable FDA to calculate the
estimated daily intake (EDI) resulting from the intended use of
the substance, including levels of residual reactants and impurities.
A notifier should provide information sufficient to permit estimation of the daily dietary
concentration of the FCS, i.e., consumer exposure.
FDA will calculate the concentration of the FCS expected in the daily diet from analyzed or
estimated levels of an FCS in food or food simulants. A more
complete discussion of this topic is given in Section II.E.
and Appendix IV.
The concentration of an FCS in the daily diet may be determined from measured levels in
food or in food simulants, or estimated using information on
formulation or residual levels of the substance in the food contact article and the assumption of
100% migration of the FCS to food. Although FDA has
always accepted reliable analyses of FCS in real foods, in practice, many analytes are difficult to
measure in food. As an alternative, notifiers may
submit migration data obtained with food simulants that can reproduce the nature and amount of
migration of the FCS into food. Because an FCS may be used
in contact with many foods with different processing conditions and shelf lives, the submitted
migration data should reflect the most severe
temperature/time conditions to which the food-contact article containing the FCS will be
exposed.
Before undertaking migration studies, the notifier should carefully consider the potential uses
of the FCS. If, for example, use at temperatures no
higher than room temperature is anticipated, it makes little sense to conduct migration
experiments that simulate high temperature food contact. Such
experiments would lead to elevated levels of the FCS in the food simulants that might require a
more extensive toxicological data package to support the
exaggerated exposure. In some cases where the use level of the FCS is low, it may be possible to
dispense with migration studies altogether by assuming
100% migration of the FCS to food. The following example illustrates this approach:
Consider an adjuvant added prior to the sheet-forming operation in the manufacture of
paper. If analysis or calculation shows that the final
adjuvant concentration in paper cannot exceed 1 mg/kg
and the basis weight of the finished paper is 50 pounds/3000 ft2, or 50
mg/in2,
then the maximum weight of adjuvant per unit area of paper is 1 x 10-6 g
adjuvant/g paper x 50 mg/in2 = 0.000050 mg/in2. If
all the adjuvant migrates into food and 10 grams of food contacts 1 square inch of paper (FDA's
default assumption), the maximum concentration in food
would be 5 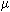 g/kg. It may be
expected that this low concentration in food would lead to a
commensurately low EDI
for the FCS. Therefore, although
migration studies which could result in further lowering of the estimate of daily intake, such
studies might be unnecessary.
g/kg. It may be
expected that this low concentration in food would lead to a
commensurately low EDI
for the FCS. Therefore, although
migration studies which could result in further lowering of the estimate of daily intake, such
studies might be unnecessary.
Levels in food should be based on the results of migration testing or other methods as
applicable, so as to reflect as closely as possible the
actual use conditions of the food contact article containing the FCS. In general, migration values
determined using the assumption of 100% migration to
food should be avoided to reduce conservatisms to the greatest extent possible.
- Design of the
Migration Experiment
- MIGRATION
CELL.
When use of an FCS is anticipated with one particular type of package, such as a beverage bottle,
packages may be filled with food simulants and tested.
For more general uses or when the package surface area does not produce sufficient extractives
for adequate characterization, a migration cell
should be used in which a specimen of known surface area is extracted by a known volume of
simulant. The two-sided migration cell described in an article
by Snyder and Breder (Snyder and Breder, 1985) is recommended. Although this
specific cell may
not be universally applicable, FDA recommends that two of its essential features be incorporated
in modified designs. These are:
- (1)
Polymer plaques of known surface area and thickness (see Section II.D.1.b. for further discussion)
are separated by inert spacers (such as glass beads) so that simulant flows freely around each
plaque. Migration from the plaque is considered to be two-sided.
- (2) The headspace is
minimized, and gas-tight and liquid-tight seals are maintained. (Minimum headspace and gas
tightness are of lesser importance if
the migrant of interest is non-volatile.)
Additionally, and importantly, the cell should be subjected to mild agitation to minimize any
localized solubility limitation that might result in
mass-transfer resistance in the food simulant.
For applications in which a two-sided cell design is not suitable, such as laminate
constructions, the notifier may refer to the references in
Appendix V. for applications describing other cell
designs. The notifier may also devise an alternative cell. FDA is
willing to comment on any such design prior to performance of the migration experiment.
TEST SAMPLE.
Some important considerations are the following:
- (1) Formulation:
Use the highest proposed concentration of the FCS in the food-contact article in preparing
samples for migration testing. Notifiers
should provide information that characterizes resin samples used in testing, including the
concentrations and identities of other components that may be
present, the chemical composition of the resin (including co-monomer content where
appropriate), molecular weight range, density, and melt flow index.
If the formulation is plasticized, the most highly plasticized formulation should be used
for testing.
- (2) Sample Thickness & Surface Area:
Report both the thickness of the test plaque
and surface area of the sample tested. If a plaque is tested by immersion and is of sufficient
thickness to
ensure that the initial FCS concentration at its center is unaltered by migration that occurs from
both sides during the test period, the surface area of
both sides can be used to calculate migration (units of mg/in2).
Migration may be considered to be independent from both sides of the sample if the sample
plaque thickness is at least 0.05 cm (20 mil or 0.020 in) and not
more than 25 percent of the FCS has migrated by the end of the experiment. If these
conditions are not met, the surface area of only one side should
be used in the calculation and consideration should be given in the PMN to proposing a
limitation on film thickness.
- (3) Polymer properties:
If the FCS is a polymer adjuvant, notifiers should perform migration testing on the
polymer with the lowest average molecular weight. If the
FCS is a new polymer, notifiers should test the polymer that would be expected to give
the highest levels of extractives, i.e., the polymer
with the lowest average molecular weight, percent crystallinity, and degree of
cross-linking.
FOOD SIMULANTS
.
The following food simulants are recommended. Additional discussion on this subject is found in
Appendix I.
|
Food-Type as defined in 21 CFR 176.170(c) Table
1 |
Recommended Simulant |
| Aqueous & Acidic Foods (Food Types I, II, IVB, VIB, and
VIIB) |
10% Ethanol(a) |
| Low- and High-alcoholic Foods (Food Types VIA, VIC) |
10 or 50% Ethanol(b) |
| Fatty Foods (Food Types III, IVA, V, VIIA, IX). |
Food oil (e.g., corn oil), HB307, or Miglyol
812(c) |
afor exceptions, see main
text.
bactual ethanol concentration may be substituted
(see main text and
Appendix II.).
cHB307 is a mixture of synthetic triglycerides,
primarily C10, C12, and
C14. Miglyol 812 is derived from coconut oil (see main
text and
Appendix
I.). |
When food acidity is expected to lead to significantly higher levels of migration than with
10% ethanol, or if the polymer or adjuvant is
acid-sensitive, or if trans-esterification occurs in ethanol solutions, separate extractions in water
and 3% acetic acid in lieu of 10% ethanol should be
conducted(3).
10% Ethanol is intermediate in alcohol concentration between wine
and beer. Migration levels to wine and beer are not expected to be very different
from 10% ethanol values. Therefore, test results developed with 10% ethanol can generally be
used to evaluate exposures and support clearances for contact
with alcoholic beverages with up to 15 volume % ethanol.
Unsaturated food oils (like corn and olive oils) can at times be difficult matrices for the
analysis of a migrant because these oils are susceptible to
oxidation, especially at high temperature. Miglyol 812, a fractionated coconut oil having a
boiling point range of 240° to 270°C and composed of
saturated C8 (50-65%) and C10 (30-45%) triglycerides, is an
acceptable alternative fatty-food simulant for migration
testing.(4) HB 307, a mixture of synthetic
triglycerides, primarily C10, C12, and
C14, is also useful as a fatty-food simulant.(5)
In some cases, analysis of a migrant in a food oil will not be practical and a simple solvent
must be used. There does not appear to be one solvent
that will effectively simulate a food oil for all polymers. A list of various polymers and their
recommended fatty-food simulants appears in
Appendix I. For other polymers, a notifier should
consult with the FDA concerning use of an appropriate
fatty-food simulant before performing migration experiments.
The simulant volume should ideally reflect the volume-to-specimen surface area
ratio expected to be encountered in actual food packaging. A ratio
of 10 mL/in2 is acceptable. Other ratios may be acceptable if migration levels do
not approach concentrations reflecting the
partition limit (i.e., the solubility of the FCS in the food simulant). Precipitation of the FCS from
solution or a cloudy solution is an indication
that this limit has been reached. The volume-to-surface area
ratio should be reported.
TEMPERATURE AND TIME OF TEST.
Notifiers should conduct migration testing under the most severe conditions of temperature and
time anticipated for the proposed use. If the intended
use of the food contact article involves contact with food at temperatures higher than room
temperature, tests should be conducted at the highest
use temperature for the maximum time period. In many instances, short time periods of elevated
temperature-food contact are immediately followed by
extended periods of storage at ambient temperatures. For such applications, FDA's recommended
migration protocols call for short-term accelerated testing
designed to simulate FCS migration that may occur during the entire food-contact scenario.
Recommended protocols for selected situations are given in
Appendix II.; however, depending on the particular
food-contact application, a specific protocol may be devised.
For room-temperature applications, a test temperature of 40°C (104°F) for 10 days
is recommended. This accelerated testing protocol is based on
studies showing that experimental migration levels were roughly equivalent to levels obtained
after extended storage (6-12 months) at 20°C (68°F)
(6).
For refrigerated or frozen food applications, the recommended test temperature is 20°C
(68°F).
For polymers, such as polyolefins, that are used with food at temperatures above their
glass transition temperatures (i.e., the polymer is in
the rubbery state), the highest migration values (typically, but not always, the ten day
values) are used by FDA to calculate the concentration of
migrants in food.
Polymers such as polyethylene terephthalate (PET) and polystyrene (PS), however, are used
with food at temperatures below their glass transition
temperatures (i.e., the polymer is in the glassy state). At a fixed temperature, the rate of
diffusion of migrants through a polymer in the
glassy state is lower than if the polymer were in the rubbery state. For this reason, accelerated
testing for 10 days at 40°C might underestimate
migration that would occur during the entire food-contact scenario. Therefore, migration data
obtained over ten days at 40°C should be extrapolated to
30 days in order to better approximate migration levels expected after extended time periods at
ambient conditions. The notifier may carry out
testing for 30 days to avoid uncertainties in extrapolation. If a notifier provides data that
demonstrate that a different extrapolation period
is more appropriate for a given adjuvant/polymer combination, such information would be used
for evaluating exposure.
For restricted uses where the maximum shelf life and food-contact temperature of an article
are known, the notifier is encouraged to carry out
migration studies for the maximum shelf life under temperature conditions approximating
expected use. Notifiers may want to consult FDA before undertaking
such tests.
For each migration experiment, FDA recommends that
portions of the test solutions should be analyzed during at least four time intervals.
Recommended sampling times for a ten-day
test are 2, 24, 96, and 240 hours. FDA recommends that notifiers analyze a blank or control using
a test cell identical to that used for the test
article.
END TESTS.
It is important for potential notifiers to realize that the appropriate migration test conditions for a
new FCS are not those described in
§175.300, §176.170 or other sections in 21 CFR. These published "end-test"
extractions are quality control test methods that are used to
verify whether a particular product is equivalent to the material that served as a basis for the
regulation. End tests bear no relation to the
migration testing recommended for evaluating probable exposure to a new FCS and may
not be used to support notification of an FCS.
Characterization of Test Solutions & Data Reporting
Notifiers should perform migration studies in triplicate and analyze the test solutions for the
migrants. If the PMN is for a polymer, the
notifier should determine the amount and nature of total nonvolatile extractives (TNEs).
Ordinarily, the TNEs are determined gravimetrically. The nature
of the extractives, which may include monomers, oligomers, adjuvants, and catalyst residues, is
determined by suitable chemical or physical
tests, such as NMR, UV-visible, and atomic absorption spectroscopy, mass spectrometry, and gas
or liquid chromatography. The notifier should indicate the
limit of quantitation and selectivity of the methods used. If quantitation of individual
migrants is not possible, the notifier should determine the
distribution of the extractives between organic and inorganic fractions by solvent
fractionation (i.e., the fraction of the TNE residue that is
soluble in chloroform). This serves, as a first step, to focus on the migrants of interest (e.g.,
organic components) in determining exposure
estimates. In these instances, FDA generally will estimate exposure to TNEs from the use of the
FCS assuming that the TNEs (or chloroform-soluble TNEs)
consist solely of low molecular weight oligomers that are chemically equivalent. Because the
degree of toxicological testing depends on the magnitude of
the exposure estimate, it should be to the notifier's advantage to quantitate the components in the
TNEs that are not chemically equivalent
(e.g., differentiate between low molecular weight oligomers and polymer adjuvants).
Test solutions from polymers that are the subject of a PMN should also be
analyzed for constituent monomers. Alternatively, the known residual
monomer level in the polymer may be used to calculate monomer dietary concentrations by using
the density of the polymer, the maximum anticipated
thickness of the food contact article, and by assuming that all of the residual monomer migrates
into food and that ten grams of food contact one square
inch of food-contact article.
If the PMN is for a polymer adjuvant, FDA normally recommends that the test
solutions be analyzed only for the adjuvant. Occasionally, however,
it may be necessary to quantitate, in the test solutions, impurities or decomposition products
present in the adjuvant if they might be expected to become
components of the daily diet in toxicologically significant quantities.
A common
example would be the presence of carcinogenic impurities in the adjuvant.
It may also be necessary to quantitate, in the test solutions, decomposition products produced
(a) as a result of the FCS exhibiting its intended
technical effect in the food contact article, or (b) in the test solutions after migration of the FCS.
An example would be the use of a new antioxidant
for polyolefins. Polymer antioxidants, by their very nature, would be expected to partially
decompose during
thermal processing of the resin or food-contact article containing the substance. Frequently,
decomposition also occurs after migration of the FCS into
food or food simulant, where temperatures may reach 120°C with fatty-food simulants.
Information on decomposition in the food simulants may be obtained
by conducting stability studies on the FCS in parallel with the migration studies.
Notifiers should report results in terms of milligrams of substance extracted per square
inch (mg/in2) of surface area. Migration
amounts are often expressed in terms of mg/dm2. The mixed unit
mg/in2 is preferred, however, to facilitate conversion to
concentration in food. If ten grams of food are in contact with one square inch of packaging
surface, a migration of 0.01 mg/in2 corresponds to
a concentration in food of 1 mg/kg.
For specialized food-contact applications where an assumed ratio of 10 g food per
in2 is not appropriate,
such as in dual-ovenable trays and microwave heat-susceptor applications, notifiers should use
the lowest ratio from the actual food-contact applications
and provide justification for the ratio selected.
Analytical Methods
Notifiers should submit the following for each method:
-
DESCRIPTION OF THE METHOD.
A detailed description that can be followed by an experienced analytical chemist.
The description should include discussions on the procedure's
accuracy, precision, selectivity, limit of quantitation (LOQ), and limit of detection (LOD)(7). If a literature
reference is available, a copy should be included in the PMN.
STANDARD CURVES.
Standard curves or calibration curves obtained by analyzing a prepared medium fortified with
several known amounts of analyte to obtain concentrations
both greater than, and less than, the concentration of migrant in the test solutions. The prepared
medium may be the pure solvent, a solution of known
ionic strength, etc. The data points from which the standard curve is derived should bracket the
concentration of the migrant in the test solution.
An analyte concentration of 1 mg/kg determined from a standard curve obtained from
concentrations of 10, 15 and 20 mg/kg
would be unacceptable. The
correlation coefficient and standard errors of the Y intercept and the slope should be reported
with the standard curve.
EXAMPLES OF SPECTRA OR CHROMATOGRAMS
.
Sample spectra and chromatograms, clearly identifying and labeling all major peaks to avoid
ambiguities in interpretation.
EXAMPLE CALCULATIONS
.
Example calculations relating the data obtained from instrumental methods to the reported levels
(preferably in milligrams migrants per square inch of
sample surface area) provide the reviewer with an internal check on the reported method.
VALIDATION OF ANAYLTICAL METHODS.
Validation of a method's intended use, the determination of accuracy and precision, usually
involves: 1) replicate analyses of appropriate matrices
fortified with known amounts of the analyte, at concentrations similar to those encountered in the
migration studies, and 2) determination of the
percentage recovery of the fortified analyte. In cases where a polymer adjuvant is the
subject of interest, test solutions of the polymer
formulated without the adjuvant may serve as the matrix for fortification and recovery
measurements. Recovery is defined as the difference between
measured analyte levels in the fortified and unfortified matrices. Percent recovery is the recovery
divided by the fortified level times 100, i.e.,
if "a" is the measured level in the unfortified solution, "b" is the measured level in the fortified
solution and "c" is the fortification level, then
percent recovery equals (b-a)/c x 100.
If migration test solutions are fortified, they should be fortified before analytical
workup but after the prescribed test time,
e.g., 240 hours. FDA recommends that the actual test solutions be fortified and
not the pure food simulants. Fortification of pure
simulants instead of the test simulants is probably the most common deficiency in the validation
section of an analytical method.
The notifier should perform fortification and recovery experiments using three (3) sets of
triplicate samples of the test simulants with each set
fortified at a separate level. The fortification levels should be one-half (½), one (1), and two (2)
times the measured concentration of the analyte in
the food simulant. In the event that the FCS is not detected, the notifier should determine the
LOD for the method. For quantifiable levels of the analyte,
acceptable recoveries should meet the following criteria:
| Levels in food or food
simulants(a) |
Acceptable average recovery |
Acceptable relative standard deviation |
| <0.1 mg/kg |
60-110% |
<20% |
| >0.1 ppm |
80-110% |
<10% |
| (a)If 0.001 mg of a
substance is extracted from one square inch of packaging material into 10 grams of
food or food simulant, the estimated concentration in food is 0.1
mg/kg. |
In evaluating the precision of the analytical method, the variability arising from analyses of
individual samples can be eliminated by performing
triplicate analyses on a homogeneous composite (a blend of the triplicate samples) where
practicable.
Other validation procedures may be appropriate depending on the particular analysis. For
example, analysis of the same test solution by two independent
analytical methods would be acceptable validation. Similarly, the method of standard additions is
an acceptable alternative in certain cases, such as metal
analysis by atomic absorption spectroscopy. In this case, the notifier should fortify the matrix at
two separate concentrations (at least) in addition to
the unfortified concentration, and verify the linearity of the standard addition curve by calculation
of the least squares correlation coefficient
(r should be >0.995).
The notifier should submit representative spectra or chromatograms from validation analyses
of fortified and blank samples. Spectra or chromatograms of
the "blank" should be submitted to facilitate the verification of the absence of interferences. An
illustrative example appears in
Appendix III.
Migration Database
Migration data for specific migrant/polymer/food simulant systems at given temperatures
that exhibit a predictable migration-time behavior, e.g.,
Fickian diffusion, may be used to predict migration at other temperatures. Thus, the need for
migration studies for new applications, which in certain
cases such as high temperature applications may be difficult to perform, may be reduced.
For example, migration data obtained over 10 days (240 h) at 40°C that exhibits Fickian
behavior, in combination with migration data obtained at
other temperatures (e.g., 60°C and 80°C), may be extrapolated by means of an
Arrhenius plot to predict migration under retort conditions
(121°C/2 h and 40°C/238 h), if no apparent change in polymer morphology,
such as glass transition or polymer melting, is expected between
30°C and 130°C. Apparent diffusion coefficients, D, at 121°C for each
migrant/polymer/food simulant can be obtained from a plot of ln D vs
1/T(K). Thus, migration for 2 hours at 121°C can be estimated and added to migration after
238 hours at 40°C to obtain total migration expected
for retort and ambient storage conditions. The density and thickness of the polymer sample and
initial concentration of the migrant in the polymer are
also necessary for the calculations.
The FDA migration database is intended as a resource for migration data, including diffusion
coefficients and relevant polymer/additive properties. FDA
continues to compile migration data from various sources for use in estimating migration levels
for FCSs. Reliable migration data, e.g., data
that follow Fickian diffusion, submitted in support of a PMN would be added to the database. In
addition, only migration levels that have been measured at
three or more time intervals for a given temperature will be considered for inclusion in the
migration database. Notifiers may submit suitable data for
inclusion into the database in the form of a letter, as part of a notification, or in a Food Additive
Master File. The FDA migration database (hardcopy)
is available through the Freedom of Information Act (FOIA) (also see the CFSAN website at
http://vm.cfsan.fda.gov/~dms/foia.html).
Migration Modeling
FDA recommends that a notifier submit relevant and reliable data on an FCS for use in
estimating migration levels in food and, in turn, potential
consumer exposure. As discussed above, migration levels in food are typically estimated based
on the results of migration testing under the anticipated
conditions of use or, in certain cases, under the assumption of 100% migration of the FCS to
food. These two approaches are adequate in most instances.
A third alternative involves migration modeling. One simple approach to modeling migration
for specific migrant/polymer/food simulant systems,
based on select experimental data, was discussed above in Section II.D.4. However, the mathematical modeling of
migration
using the basic principles of diffusion has not found widespread application, largely due to the
lack of necessary material constants such as diffusion
coefficients. Diffusion coefficients may often be found in the open literature or the FDA
migration database. In all cases, the source of any material
constants used in migration modeling should be appropriately referenced.
Recently, however, semi-empirical methods have been developed to determine
migration levels with limited or, in certain cases, no migration data
(see, e.g., (Limm and Hollifield,
1996) and (Baner, et al, 1996)). These
diffusion models rely on estimation of diffusion coefficients based on the nature of the migrant
and the physical properties
of the polymer. They may be useful substitutes for, or additions to, experimental data under
limited circumstances. Several caveats should be considered
in the application of such diffusion models. First, distribution of the migrant in the polymer is
considered isotropic. Non-isotropic distribution,
whether intentional or unintentional, would be expected to result in non-Fickian migration. Two,
other aspects of migration, such as partitioning, mass
transfer, polymer morphology, shape/polarity of the migrant, and plastization of the polymer are
not considered in these models. These factors should be
carefully considered when deriving migration levels to food using modeling
techniques.
CONSUMER
EXPOSURE
Migration data developed using the procedures outlined in Section II.D. are intended to provide estimates of the highest
level of migration to food that might result from the anticipated use of the FCS. FDA estimates
probable exposure to the FCS by combining the migration
data with information on uses of food-contact articles that may contain the FCS (i.e., on the
fraction of a person's diet likely to contact
packaging materials containing the FCS).
From a given concentration of the FCS in the daily diet, the estimated daily intake (EDI) is
calculated as the product of that concentration and the
total food intake, assumed to be 3000 grams per person per day (solids and liquids). A
concentration in the daily diet of 1 ppm corresponds to an EDI of
1 x 10-6 g FCS/g food x 3000 g food/person/day or 3 mg/person/day.
Both the concentration in the daily diet and the EDI from the subject PMN and the
cumulative EDI (CEDI) from all regulated uses and effective PMNs are used by
FDA in the safety evaluation of an FCS. The CEDI
of the FCS should be used in determining the types of toxicity studies
used to establish safety under the proposed conditions of use. Toxicological data
recommendations for several tiers of CEDIs
resulting from all proposed and permitted uses of the FCS, including regulated uses, uses that
were the subject of previous PMNs, and the
use in the subject PMN, are described in the document entitled "Preparation of Premarket Notifications for Food Contact
Substances: Toxicology Recommendations".
-
Calculation of Exposure
-
CONSUMPTION FACTOR.
The term "Consumption Factor" (CF) is used to describe the fraction of the daily diet expected to
contact specific packaging materials. The CF
represents the ratio of the weight of all food contacting a specific packaging material to the
weight of all food packaged. CF values for both packaging
categories (e.g., metal, glass, polymer and paper) and specific food-contact polymers are
summarized in Table I of
Appendix IV. These values were derived using information on the types of food consumed,
the types of food contacting each packaging surface, the
number of food packaging units in each food packaging category, the distribution of container
sizes, and the ratio of the weight of food packaged to the
weight of the package. These values may, however, be modified as new packaging information is
received. Several of the values contained in
Table I and Table
II of Appendix IV have been updated since the 1995 document entitled "Recommendations
for Chemistry Data for
Indirect Food Additive Petitions".
When FDA computes exposure to an FCS, the Agency assumes that the FCS will
capture the entire market for which it is intended for use. This
approach reflects both uncertainties about likely market penetration as well as limitations in the
data surveyed. Thus, if a company proposes the use of
an antioxidant in polystyrene, it is assumed that the antioxidant will be used in all polystyrene
manufactured for food contact. In certain cases where an
adjuvant is intended for use in only a part of a packaging or resin category, a lower CF
representing the coverage that is sought may be used. For
example, if a stabilizer is intended for use only in rigid and semirigid
(polyvinyl chloride) (PVC),
a CF of 0.05 rather than 0.1 could be used in
estimating exposure since only about 50% of all food-contact PVC could contain the stabilizer.
Another example is the division of polystyrene into impact
and non-impact categories (see Table I, Appendix IV.).
In order to reduce conservatisms, notifiers are encouraged to
submit as detailed information as possible on the anticipated resin or packaging market(s) that
may be captured by articles manufactured from the FCS.
When new products are introduced, they will initially be treated as replacement items for
existing technology. FDA generally makes estimates based on
the assumption that the new product will capture the entire market. For example, the retortable
pouch was initially treated as a replacement for coated
metal cans and was assigned a CF of 0.17. As additional information on actual use of the
retortable pouch became available, the CF was lowered to 0.05. In
certain cases, the submission of resin or packaging market data may lead to the use of a lower
CF.
FOOD-TYPE DISTRIBUTION FACTOR.
Before migration levels can be combined with CF values to derive estimates of probable
consumption, the nature of the food that will likely contact the
packaging material must be known. Migration into a fatty-food simulant, for example, will be of
little use in estimating probable exposure if the
packaging material is used exclusively to package aqueous food. To account for the variable
nature of food contacting each packaging material, "food-type
distribution factors" (fT) have been calculated for each packaging material to
reflect the fraction of all food contacting each material that
is aqueous, acidic, alcoholic and fatty. Appropriate fT values for both packaging
categories and polymer types appear in
Table II of Appendix IV.
CONCENTRATION IN THE DAILY DIET AND EDI.
FDA recommends the following approach for calculating the concentration of the FCS in the
daily diet. The concentration of the FCS in food contacting
the packaging material, <M>, is derived by multiplying the appropriate fT
values by the migration values, Mi, for simulants
representing the four food types. This, in effect, scales the migration value from each simulant
according to the actual fraction of food of each type
that will contact the packaging material.
<M> = faqueous and acidic(M 10%
Ethanol)+falcohol (M 50%
Ethanol)+ffatty(Mfatty)
where Mfatty refers to migration into a food oil or other appropriate
fatty-food simulant.
The concentration of the FCS in the diet is obtained by multiplying <M> by CF. The
EDI is then determined by multiplying the dietary concentration
by the total weight of food consumed by an individual per day. FDA assumes that an individual
consumes 3kg of food (solid and liquid) per day (see
Appendix IV. for sample calculations):
EDI = 3 kg food/person/day x <M> x CF
CUMULATIVE EXPOSURE.
If the FCS that is the subject of a PMN is already regulated for other uses in 21 CFR 170-199, or
has been the subject of previous effective PMNs, FDA
generally estimates the cumulative exposure to the FCS from the proposed and
permitted uses (see the example in Appendix
IV.). Information on the regulatory status of an FCS may be obtained by inspection of 21
CFR 170-199, searching the CFR on the Government Printing
Office (GPO) World Wide Website at http://www.access.gpo.gov/nara/cfr/index.html, or contacting
FDA directly. Information on effective PMNs for an FCS may be obtained through the FDA
website or by
contacting FDA directly. An estimate of cumulative exposure for the regulated and notified uses
of an
FCS can be obtained by contacting FDA. FDA also maintains a database of
cumulative EDIs for food contact substances on the agency's internet
site.
The approach outlined above is designed to deal with the majority of FCSs intended for
single-use. For estimating dietary exposures to components
of repeat-use items and articles used in or with food processing equipment, exposure
estimates will also consider estimates of the amount of food to
be contacted during the service life of the food-contact article (see Appendix II. Section 4.).
Exposure Refinement
Exposure estimates will, in general, be made using the aforementioned procedures. More
refined exposure estimates may be possible, however, with
additional information provided in a PMN. For instance, subdividing packaging or resin
categories could reduce the calculated exposure by lowering the CF
for the category. The division of PVC into rigid and plasticized categories and PS into impact
and non-impact categories, cited above, are two examples.
Another example is the division of polymer coatings for paper into subcategories, such as
poly(vinyl acetate) coatings, styrene-butadiene coatings,
etc. If an FCS is to be used solely in styrene-butadiene coatings for paper, use of the CF for
polymer-coated paper (0.2,
Appendix IV. Table 1), would be a gross exaggeration.
As noted above, FDA encourages the submission of marketing
information that may be used to subdivide the packaging market(s) anticipated for articles
manufactured from the FCS.
In those cases where the nature of the coverage requested may necessitate more detailed
information or where a notifier believes that exposure will be
overstated by simply selecting CF and fT values presented in Appendix IV., data of the following type may be
submitted to facilitate calculations of CF and fT values for materials likely to
contain the FCS:
- Estimates of the
total amount of food in contact with the packaging material determined using
either:
- (1) package unit data (number of units and their size
distribution), or
- (2) pounds of packaging material produced for food contact, container size
distribution, and ratios of weight of food packaged to weight of
package.
Characterization of the foods that
might contact the food package, along with supporting documentation, and
the likely fT values.
Information that would demonstrate
that only a fraction of a packaging or resin category would be affected by
the coverage sought.
Technological limitations that could
affect the type of food contacted or the fraction of the diet that might
be contacted.

APPENDIX I.
FATTY-FOOD SIMULANTS FOR SPECIFIC POLYMERS
A food oil is the most extreme example of a fatty food. If contact with fatty foods is
anticipated, FDA recommends that a notifier conduct migration
studies using a food oil as the food simulant. In addition to food oils such as corn and olive oil
for which extensive migration data already exist, the
use of HB307 (a mixture of synthetic triglycerides, primarily C10,
C12, and C14) as a fatty-food simulant has previously
been recommended. Studies in FDA laboratories have shown that Miglyol 812, a fractionated
coconut oil having a boiling range of 240-270°C and composed
of saturated C8 (50-65%) and C10 (30-45%) triglycerides, is also
an acceptable alternative. Since use of these oils for FCS migration
may not always be practicable, the use of aqueous-based solvents that simulate the action of these
liquid fats is sometimes necessary. While it seems
unlikely that one solvent will be found that simulates the action of a food oil for all food-contact
polymers, the following list presents polymers for
which adequate data exist to support the use of aqueous-based solvents as fatty-food simulants.
The recommendation of these solvents is based upon studies
done at FDA, at the National Institute of Standards and Technology (formerly The National
Bureau of Standards), and by Arthur D. Little, Inc. under
contract to FDA (a list of general references pertaining to these studies is shown in Appendix V). For
polymers other than those listed below, notifiers should consult FDA before undertaking any
migration experiments.
| 1. Polyolefins complying
with §177.1520 and ethylene - vinyl acetate copolymers
complying with §177.1350 |
95% or absolute ethanol |
| 2. Rigid (polyvinyl chloride) |
50% ethanol |
| 3. Polystyrene and rubber-modified polystyrene |
50% ethanol |
| 4. Poly(ethylene terephthalate) |
50% ethanol |
Absolute or 95% ethanol has been found to be an effective fatty-food simulant for
polyolefins; however, it appears to exaggerate migration for other
food-contact polymers.
Previous test protocols (prior to 1988) recommended the use of heptane as a fatty-food
simulant. To account for the aggressive nature of heptane
relative to a food oil, division of migration values by a factor of five was permitted. Studies have
shown, however, that the exaggerative effect of
heptane relative to a food oil varies over orders of magnitude depending on the polymer
extracted. Thus, heptane is no longer recommended as a
fatty-food simulant. However, we recognize that in cases
where very low migration is anticipated, such as for inorganic adjuvants or certain highly
cross-linked polymers, heptane
can be useful due to the ease of analytical workup.
Because of the known variance in the exaggerative effect of heptane
relative to food oil, if heptane is used,
migration values will generally
not be divided by any factor unless there is adequate justification.

APPENDIX II.
SELECTED MIGRATION TESTING PROTOCOLS
The following migration testing protocols are intended to simulate most anticipated end-use
conditions of food-contact articles. These protocols are
based on the premise that migration to aqueous- and fatty-based foods is typically
diffusion-controlled within the polymer, strongly affected by the
temperatures encountered during food contact, and further modified by the solubility of the FCS
in the foods. Therefore, migration testing with food
simulants at the highest temperatures to be experienced by the package during food contact is
recommended. Testing with actual fatty foods is also an
option, although determination of the analytes of interest is often very difficult. In those instances
where the expected use conditions are not
adequately simulated by these protocols or testing with food simulants at the highest anticipated
food-contact temperature is not practical, alternatives
to those
protocols presented below should be developed in consultation with FDA.
-
General Protocols (Single-Use Applications) Corresponding to Condition
of Use
As noted in Appendix I., migration to fatty foods is
evaluated using a fatty food, a pure liquid fat, or, alternatively,
aqueous ethanol solutions when analytical limitations preclude sensitive analyses. As noted in
Section II.D.1.c.,
migration to aqueous, acidic, and low-alcoholic foods is generally evaluated using 10% ethanol
and migration to high-alcohol foods is generally evaluated
using 50% ethanol.
The migration protocols given below are intended to model thermal treatment and extended
storage conditions for polymers, such as polyolefins, used
with food at temperatures above their glass transition temperatures. The extended storage period
generally involves testing at 40°C for 240 hours (10
days). As
discussed in Section II.D.1.d., migration data obtained
at 10 days for polymers used below their glass transitions
temperature should be extrapolated to 30 days to better approximate migration levels expected
after extended storage at ambient conditions.
FDA recommends the following approaches:
-
High temperature, heat sterilized or retorted above 100° C (212°F)
| 10% Ethanol(a) |
121°C (250°F) for two hours |
| 50% Ethanol |
71°C (160°F) for two hours |
| Food Oil (e.g., corn oil) or HB307 or Miglyol 812 |
121°C (250°F) for two hours |
| 50% or 95% Ethanol(a),(b) |
121°C (250°F) for two hours |
(a)Requires a pressure cell
or autoclave, see Appendix V. Appropriate
safety precautions should be exercised when using equipment generating pressures
above 1 atmosphere.
(b)Depends on food-contact layer, see Appendix I. |
After two hours at elevated temperatures, continue the tests at 40°C (104°F) for
238 hours to a total of 240 hours (10 days). Analyze the test
solutions at the end of the initial two hour period, and after 24, 96 and 240 hours.
Boiling water sterilized.
The protocol remains the same as for Condition of Use A. except that the highest test
temperature is 100°C (212°F).
Hot filled or pasteurized above 66°C (150°F).
Add solvents to the test samples at 100°C (212°F), hold for 30 minutes, and then
allow to cool to 40°C (104°F). Maintain the test cells
at 40°C (104°F) for ten days with samples taken for analysis after the intervals
indicated for the previous protocols. If the maximum hot fill
temperature will be lower than 100°C (212°F), test solvents may be added at this lower
temperature.
Alternatively, perform migration studies for 2 hours at 66°C (150°F) followed by 238
hours at 40°C (104°F). For the alternative method,
the longer time at the lower
temperature (2 hours at 66°C vs 30 minutes at 100°C) compensates for the shorter time
at 100°C.
Hot filled or pasteurized below 66°C (150°F).
The recommended
protocol is analogous to that for C. except that all test solvents are added to the test samples at
66°C (150°F) and held for 30 minutes
before cooling to 40°C (104°F).
Room temperature filled and stored (no thermal treatment in the
container).
The notifier should
conduct migration studies for 240 hours at 40°C (104°F). Analyze the test solutions
after 24, 48, 120 and 240 hours.
Refrigerated storage (no thermal treatment in the container)
The recommended
protocol is identical to that for E. except that the test temperature is 20°C (68°F).
Frozen storage (no thermal treatment in the container)
The recommended
protocol is identical to F. except that the test time is five (5) days.
Frozen or refrigerated storage;
ready-prepared foods intended to be reheated in container at time of
use:
| 10% Ethanol(a) |
100°C (212°F) for two hours |
| Food Oil (e.g., corn oil) or HB307 or Miglyol
812TM |
100°C (212°F) for two hours |
| 50% or 95% Ethanol(a),(b) |
100°C (212°F) for two hours |
(a)Requires a pressure cell
or autoclave, see Appendix V.
(b)Depends on food-contact layer, see Appendix I. |
Applications involving the heating and cooking of food at temperatures exceeding
121°C (250°F) are not included under conditions of use A.-H.
Migration testing protocols for these applications are discussed in Section 11. of this Appendix.
Adjuvants for Polyolefins
In general, under identical testing conditions, levels of migrants from low-density
polyethylene (LDPE) are higher than from high-density polyethylene
(HDPE) or polypropylene (PP). Migration studies done solely on LDPE (complying with
§177.1520(a)(2)) at 100°C (approximately the highest
temperature at which LDPE remains functional) are, therefore, generally sufficient to provide
coverage for all polyolefins including PP, which may be used
for retort applications. In such a case, the CF for all polyolefins (CF = 0.33) generally will be
used instead of the individual CF for LDPE (see
Appendix IV. Table I). Levels of migrants from PP
are generally higher than from HDPE.
Thus, migration studies conducted
with PP are generally adequate to permit coverage in HDPE under the same conditions of use. In
such a case, the combined CF of PP and HDPE will be used in
deriving exposure estimates. However, when seeking coverage for all polyolefins it is usually
advantageous to perform migration testing on HDPE, PP and
LLDPE, complying with §177.1520, as well as LDPE. By doing this, actual migration
values for these polyolefins, which will likely be lower that those
obtained from LDPE, may be used to calculate the EDI.
Adjuvants for Polymers (other than Polyolefins)
Adjuvants for More than One Polymer
The migration testing protocols for polymers other than polyolefins are the same as those in
Section 1. of this
Appendix. Consult Appendix I. for the recommended
fatty-food simulant.
If use of an FCS is sought without limitation to specific polymers, the notifier may obtain
this broad coverage by testing with an unoriented
LDPE sample complying with §177.1520(a)(2). The test protocol depends on the
anticipated conditions of use (refer to
Section 1. of this Appendix). If the most rigorous
applications correspond to Condition of Use A.
(Section 1.A.), the test temperature should be the
highest temperature at which the polymer remains functional
(ca.
100°C for LDPE). The CF for all polymers (Appendix
IV. Table 1, CF = 0.8) should be used with the migration data to
calculate the concentration of the FCS in the daily diet. In general, a lower calculated
concentration in the daily diet will result if a series of
representative polymers are separately tested and individual consumption factors are applied
(refer to the examples in
Appendix IV.). A notifier should consult with FDA to
determine which representative polymers should be tested.
Articles Intended for Repeated Use
A notifier should test the article with 10% and 50% ethanol and a food oil (e.g., corn oil) or
other fatty-food simulant (e.g., HB307 or
Miglyol 812) for 240 hours at the highest intended temperature of use. The test solutions should
be analyzed for migration of the FCS after 8, 72, and 240
hours. Notifiers should provide estimates of the weight of food contacting a known area of
repeat-use article in a given time period as well as an estimate
of the average lifetime of the article. Together with the migration data, this will allow calculation
of migration to all the food processed over the
service life of the article.
In the case of an adjuvant in a repeat-use article, FDA strongly
recommends an initial calculation of a "worst case" level in food by assuming 100% migration
of the adjuvant over the service life of the article and dividing that value by the quantity of food
processed. If this calculated concentration is
sufficiently low, migration studies will be unnecessary.
Coatings for Cans
The migration testing protocol is usually that outlined in Section 1.A. of this Appendix for high temperature, heat
sterilized or retorted products. If broad coverage is sought for all types of coatings, the notifier
should consult with FDA to determine which coatings
should be tested. For use conditions less severe than retort sterilization at 121°C, notifiers
should follow the migration test protocols outlined in
Sections 1.B.-G. of this Appendix which most closely
approximate the most severe expected use conditions.
Uncoated & Clay-Coated Papers with Latex Binders
These papers are intended for contact with food at temperatures less than 40°C for short
periods of time. The FDA recommended protocol is the
following:
| 10% Ethanol |
40°C (104°F) for 24 hours |
| 50% Ethanol |
40°C (104°F) for 24 hours |
| Food Oil (e.g., corn oil) or HB307 or Miglyol 812 |
40°C (104°F) for 24 hours |
Migration studies conducted on uncoated or clay-coated papers typically result in a high level
of extractives due to the large number of low-molecular
weight, soluble components in both paper and paper coatings. Therefore, when total nonvolatile
or chloroform-soluble total nonvolatile extractives are
determined for a paper coating, FDA recommends that the corresponding extractives should not
be subtracted from uncoated paper as a blank correction.
Rather than using paper as a support for the coating, it is often useful to apply the coating to a
suitable inert substrate, such as glass or metal, for
use in migration testing. For a new adjuvant in paper coatings, FDA recommends the
analysis of the test solutions for the unregulated adjuvant. For
a new polymer used in paper coatings, FDA recommends the analysis of the test
solutions for constituent oligomers and monomers.
Specially Treated Papers
This class includes such types as fluoropolymer- and silicone-treated papers that have
oil-resisting and heat-resisting properties. The specific
protocol depends on the particular uses anticipated. It is recommended that the notifier either
devise a protocol and submit it to FDA
for comment or request
comment from FDA about appropriate test conditions.
Adhesives (Room temperature or below)
In previous chemistry documents for indirect additives, migration tests were not
recommended for adhesives intended for use at room temperature or
below and in accordance with §175.105. (High temperature applications are discussed in
Section 9.). This
recommendation was based on consideration of subparagraph (a)(2) of §175.105 which
specifies that the adhesive is either separated from food by a
functional barrier, or the quantity of adhesive that contacts aqueous and fatty food is limited to
the trace amount at seams and edges.
If a notifier proposes to use the adhesive or adhesive component in concordance with the
limitations of §175.105, migration levels for the
notified substances will generally be assumed to be no greater than 50 ppb, as is the case for
petitioned adhesives components. Applying a CF of 0.14 for
adhesives gives a dietary concentration of 7 ppb. If the assumptions of §175.105 cannot be
supported, notifiers should submit data or calculations to
model the intended use of any adhesive component. If a notifier wishes to perform migration
testing, multilaminate samples should be fabricated with the
maximum anticipated amount of the adhesive component and with the minimum thickness of the
food contact layer. The migration protocol corresponds to
condition of use E. Alternatively, migration levels in food can be estimated based on migration
modeling (see
Section II.D.5.).
Laminates & Coextrusions
Components of multilayer structures used above room temperature are the subject of two
regulations. One covers laminates used in the temperature range
120°F (49°C)-250°F (121°C) (21 CFR 177.1395) and the other covers
laminate structures used at temperatures of 250°F (121°C) and
above (21 CFR 177.1390). Layers not separated from food by barriers preventing migration
during expected use must be listed in these regulations, or be
the subject of an effective PMN, unless they are authorized elsewhere for the intended use
conditions as specified in 21 CFR 177.1395(b)(2) and 21 CFR
177.1390(c)(1). Test protocols presented in Sections
1.A.-H. may be appropriate for evaluating the level of migration
from non-food-contact layers of some laminate structures. End uses that differ considerably from
those considered in these Guidelines, however, should be
the subject of special protocol development in consultation with FDA.
Boil-In-Bags
FDA recommends the protocol employed in Condition of Use C.
Special High-Temperature Applications
Advances in packaging technology have led to the development of food packaging materials
that can withstand temperatures substantially exceeding
121°C (250°F) for short periods of time for the purposes of heating and cooking of
ready-prepared food. FDA recommends use of the following
protocols for migration testing of dual-ovenable containers and microwave heat susceptor
materials.
-
DUAL-OVENABLE TRAYS
For high temperature oven use (conventional and microwave), FDA recommends migration
testing at the maximum intended conventional oven cooking
temperature for the longest intended cooking time, using a food oil, or a fatty-food simulant such
as Miglyol 812.
MICROWAVABLE CONTAINERS
The temperature ultimately experienced by a food-contact material when cooking foods in a
microwave oven is dependent on many factors. Some of these
are food composition, heating time, mass and shape of the food, and shape of the container. For
example, food with mass in excess of 5 g/in2
container surface area and having a thick shape will require longer cooking times to achieve the
desired degree of interior cooking than if it had a lower
mass-to-surface area ratio and were thinner. Because the ultimate temperature of the container
will depend on many factors and is, therefore, not readily
predicted, it is recommended that notifiers consult with FDA on any planned testing protocol
prior to initiating migration testing.
MICROWAVE HEAT-SUSCEPTOR PACKAGING
The high temperatures attained by packaging using susceptor technology may result in (a) the
formation of significant numbers of volatile chemicals
from the susceptor components and (b) loss of barrier properties of food-contact materials
leading to rapid transfer of nonvolatile adjuvants to foods.
Studies by FDA, with hot vegetable oil in contact with a susceptor, have shown that the susceptor
materials liberate volatile chemicals that may be
retained in the oil at parts-per-billion (ppb) levels. FDA recommends the use of the protocol
outlined in an article by McNeal and Hollifield
(McNeal and Hollifield, 1993) for the identification and quantification of
volatiles from susceptors.
To isolate and identify the total available nonvolatile extractives, notifiers should
perform Soxhlet extractions on finely shredded portions of
laminated susceptor materials using polar and nonpolar solvents as outlined in Appendix X1 of
ASTM method F1349-91. Migration protocols for
UV-absorbing nonvolatiles are also outlined in ASTM method F1349-91 and in an article
by Begley and Hollifield (Begley and Hollifield, 1991). The ASTM method relies on
the determination of a time-temperature profile based
on cooking a food product according to label directions, for the maximum cooking time. The
temperature reached by a microwave heat susceptor, however, is
dependent on the amount and characteristics of the food product. Testing methods should involve
a standard set of conditions that represent the maximum
anticipated use conditions. Therefore, FDA recommends that migration studies be conducted in a
manner similar to that outlined in the article by Begley
and Hollifield. The recommended standard test conditions are as follows:
- 1) use laminated susceptor stock representative of the
proposed application(s);
- 2)
use a microwave oven with an output wattage on the order of 700 watts;
- 3)
use a maximum microwave time of 5 minutes;
- 4)
use an oil mass-to-susceptor surface area on the order of 5 g/in2; and
- 5)
use a water load on the order of 5 g/in2.
Exposure estimates may be based, in the absence of validated migration studies, on the
assumption of 100% migration of the total nonvolatile
extractives to food, as determined by Soxhlet extractions.
Validated migration protocols for the direct determination of aliphatic migrants are not
available at this time. However, the amount of aliphatic
migrants may be estimated by subtracting the UV-absorbing nonvolatiles and inert materials
from the total nonvolatiles obtained by Soxhlet extraction (see
Appendix X1 in ASTM method F1349-91). Exposure estimates for aliphatic migrants should be
based on the assumption of 100% migration to food.
Colorants for Plastics
Some colorants, pigments in particular, may be quite insoluble in the food simulants 10%-
and 95%-ethanol. In such cases, solubility information may
provide a basis for an alternative to migration testing for evaluating worst-case exposure since
migration levels would not be expected to exceed the
limits of solubility of the colorant at the proposed use temperature. If the colorant is to be used in
all plastic packaging, for which a CF = 0.05 would
be used, a solubility below ca. 100 µg/kg
at 40°C would lead to a dietary concentration no greater than 5 ppb under conditions as
severe as
condition of use E. A solubility less than 10 µg/kg
would lead to an exposure below the threshold level of 0.5 ppb dietary concentration (see 21
CFR 170.39).
Dry Foods with Surface Containing No Free Fat or Oil
(21 CFR 176.170(c), Table 1, Food Type
VIII)
Although studies have shown migration of certain adjuvants into
dry foods (e.g., low molecular weight adjuvants in contact with porous or
powdered foods), at the present time no migration testing is recommended.
Wet-End Additives used in the Manufacture of Paper and Paperboard
Paper additives used in the wet-end of papermaking include those designed to improve the
papermaking process, such as processing aids, and those
designed to modify the properties of the paper, such as functional aids. Functional aids, mostly
organic resins or inorganic fillers, are designed to bond
to the paper fibers and, thus, are substantive to paper. For those FCSs that are substantive to
paper, migration studies should be conducted and the test
solutions analyzed for constituents of the substance. For example, in the case of a polymeric
retention aid, the test solutions should be analyzed for
constituent oligomers and monomers. On the other hand, processing aids are intended to remain
with the process water slurry and, thus, are generally not
substantive to paper. Exposure estimates for non-substantive additives may be based on
migration studies, or alternatively, on scenarios involving
partitioning of the additive between paper fibers and slurry water. The following example
illustrates this approach:
Consider an adjuvant added prior to the sheet-forming operation in the manufacture of
paper. The intended use level is reported to be 10 mg/kg
in
the slurry. Since the additive is not substantive to paper, the mass of water (containing the
additive) in contact with the pulp at the point in the
papermaking process where the slurry enters the drier determines the level of the adjuvant
retained in paper. Prior to entering the driers, the slurry is
concentrated to contain approximately 33% pulp and 67% water. This corresponds to an adjuvant
level of 20 mg/kg
relative to the pulp. Assuming that finished
paper contains 92% pulp, a paper basis weight of 50 mg/in2, 100% migration of
the adjuvant to food, and that 10 g of food contacts 1
in2 paper, the
results in an
adjuvant concentration in food of 0.09 mg/kg,
or 90 µg/kg. Applying a CF of 0.1 for uncoated and clay-coated paper gives a
dietary concentration of 9 ppb.
APPENDIX III.
ILLUSTRATIVE EXAMPLE OF VALIDATION OF ANALYSES
Polyethylene film containing a new antioxidant was subjected to migration testing with 10%
ethanol. The test solutions were analyzed for antioxidant
migration. Tests were carried out in separate cells each containing 100 in2 of
film. Four sets of test solutions (in triplicate) were analyzed
at 2, 24, 96 and 240 hours for a total of 12 test solutions. After each time interval, each solution
from one set was evaporated to dryness, the residue
dissolved in an appropriate organic solvent, and a known aliquot injected into a gas
chromatograph.
Validation experiments are carried out with the set of test simulants exhibiting the highest
level of antioxidant migration. To validate the analytical
method, an additional three sets (in triplicate) using 10% ethanol can be run for 240 hours. Each
set of these test solutions can then be fortified with
the antioxidant at levels corresponding to one-half (1/2), one (1) and two (2) times, respectively,
the average migration value determined for the regular
(unfortified) 240 hour test solutions.
Instead, the notifier decided to carry out one large test using enough film and solvent for
twelve analyses (three at each of the four time intervals).
After 240 hours, the test solution was divided into twelve (12) equal solutions (i.e., four sets of
triplicate samples). One set (three solutions)
was found to contain antioxidant at an average level of 0.00080 mg/in2. This
value corresponds to 0.080 mg/kg
in food if it is assumed that 10
grams of food contacts 1 in2 of film. Of the remaining nine solutions (three sets),
three solutions were fortified at concentrations
corresponding to 0.00040 mg/in2, three were fortified at 0.00080
mg/in2 and three were fortified at 0.00160 mg/in2. Each
solution was worked up and analyzed as described above. To illustrate the recovery calculations,
the results for the set of three solutions fortified at
one-half times the average migration (0.00040 mg/in2) are summarized in the
following table:
|
Measured Level in each Sample
(mg/in2)(a) |
Recovery
(mg/in2)(b) |
Percent Recovery (%)(c) |
| 0.00110 |
0.00030 |
75.0 |
| 0.00105 |
0.00025 |
62.5 |
| 0.00112 |
0.00032 |
85.0 |
(a)includes 0.00040
mg/in2 fortification.
(b)calculated by subtracting the average level (0.00080
mg/in2) from the measured levels in each sample.
(c)calculated by dividing the recovery by the fortification
level (0.00040 mg/in2), and multiplying by 100
(see Section
II.D.3.e.). |
The average percent recovery is 74.2%, and the relative standard deviation is 15.2%. These
are within the limits specified (see
Section II.D.3.e.) for a concentration in food of 0.080
mg/kg (percent recovery 60-110%, relative standard deviation not
exceeding 20%). If the corresponding percentages for the other two fortification levels are also
within these limits, the validation for the 10% ethanol
migration studies would be acceptable. The actual validation procedure used will, of course,
depend on the particular type of analysis.
APPENDIX IV.
CONSUMPTION FACTORS, FOOD-TYPE DISTRIBUTION FACTORS, AND EXAMPLE
OF EXPOSURE ESTIMATE
CALCULATIONS
This appendix summarizes packaging data recommended by FDA for evaluating exposure to
FCS. An example of how these data are combined with levels of an FCS in food is also
presented. A more complete discussion of the source of these data and their use in exposure
calculations is presented in
Section II.E.
|
TABLE I -
CONSUMPTION FACTORS
(CF) |
| Package Category |
CF |
Package Category |
CF |
| A. General |
| Glass |
0.1 |
Adhesives |
0.14 |
| Metal- Polymer coated |
0.17 |
Retort pouch |
0.05 |
| Metal- Uncoated |
0.03 |
Microwave susceptor |
0.01 |
| Paper- Polymer coated |
0.2 |
|
|
| Paper- Uncoated and clay-coated |
0.1 |
|
|
| Polymer |
0.4 |
|
|
| B. Polymer |
| Polyolefins |
0.35 |
PVC |
0.1 |
| LDPE |
0.12 |
rigid |
0.05 |
| LLDPE |
0.06 |
semirigid |
0.05 |
| HDPE |
0.13 |
PET |
0.21 |
| PP |
0.04 |
Other Polyesters |
0.05 |
| Polystyrene |
0.1 |
Cellophane |
0.01 |
| impact |
0.04 |
Nylon |
0.02 |
| non-impact |
0.06 |
Acrylics, phenolics, etc. |
0.15 |
| EVA |
0.02 |
All Others(a) |
0.05 |
| (a)As discussed in the text,
a minimum CF of 0.05 will be used initially for all exposure
estimates. |
|
TABLE II - FOOD-TYPE
DISTRIBUTION FACTORS (fT) |
| Package Category |
Food-Type Distribution
(fT) |
| |
Aqueous(a) |
Acidic(a) |
Alcoholic |
Fatty |
| A. General |
| Glass |
0.08 |
0.36 |
0.47 |
0.09 |
| Metal- Polymer coated |
0.16 |
0.35 |
0.40 |
0.09 |
| Metal- Uncoated |
0.54 |
0.25 |
0.01(b) |
0.20 |
| Paper- Polymer coated |
0.55 |
0.04 |
0.01(b) |
0.40 |
| Paper- Uncoated and clay-coated |
0.57 |
0.01(b) |
0.01(b) |
0.41 |
| Polymer |
0.49 |
0.16 |
0.01(b) |
0.34 |
| B. Polymer |
| Polyolefins |
0.67 |
0.01(b) |
0.01b |
0.31 |
| Polystyrene |
0.67 |
0.01(b) |
0.01(b) |
0.31 |
| -impact |
0.85 |
0.01(b) |
0.04 |
0.10 |
| -nonimpact |
0.51 |
0.01 |
0.01 |
0.47 |
| Acrylics, phenolics, etc. |
0.17 |
0.40 |
0.31 |
0.12 |
| PVC |
0.01(b) |
0.23 |
0.27 |
0.49 |
| Acrylonitrile, ionomers, PVDC |
0.01(b) |
0.01(b) |
0.01(b) |
0.97 |
| Polycarbonates |
0.97 |
0.01(b) |
0.01(b) |
0.01(b) |
| Polyesters |
0.01(b) |
0.97 |
0.01(b) |
0.01(b) |
| Polyamides (nylons) |
0.10 |
0.10 |
0.05 |
0.75 |
| EVA |
0.30 |
0.28 |
0.28 |
0.14 |
| Wax |
0.47 |
0.01(b) |
0.01(b) |
0.51 |
| Cellophane |
0.05 |
0.01(b) |
0.01(b) |
0.93 |
(a)For 10% ethanol as the
food simulant for aqueous and acidic foods, the food-type distribution factors
should be summed.
(b)1% or less |
Examples of Exposure Estimate
Calculations
The following hypothetical examples are intended to illustrate the calculation of the
concentration of an FCS in the daily diet (CF x <M>,
i.e., the fraction of food in the diet contacting the packaging material times the average
concentration of the FCS in food) and its EDI.
Example 1
A PMN is received that describes the use of a new antioxidant at a maximum level of 0.25%
w/w in polyolefins contacting food at or below room
temperature (see Appendix II. Sections 1.E. through
1.G.). Migration values from LDPE reported to FDA for the three food
simulants are given below:
|
Solvent (i) |
Mi (mg/kg) |
| 10% aqueous ethanol |
0.060 |
| 50% aqueous ethanol |
0.092 |
| Miglyol 812 |
7.7 |
The notifier used a solvent volume-to-exposed surface area ratio of 10
mL/in2. Therefore, solution concentrations are essentially equivalent
to food concentrations (under the assumption that 10 g food contacts 1 in2 of
surface area). The CF and fTs for polyolefins are
given in Tables I and II, respectively. The <M> for the antioxidant would
be
calculated as follows:
| <M> |
= |
(faqueous+facidic)(M10% Ethanol) +
falcohol(M50% Ethanol) +
ffatty(MMiglyol 812) |
| |
= |
0.68(0.060 mg/kg
)+0.01(0.092 mg/kg)+0.31(7.7 mg/kg) |
| |
= |
2.4 mg/kg |
The concentration of the antioxidant in the daily diet resulting from the proposed use would
be:
| CF x <M> |
= |
0.35 x 2.4 mg/kg |
| |
= |
0.84 mg/kg |
If there were no other permitted uses, then the EDI would be calculated using the above
value:
| EDI |
= |
3 kg food/person/day x 0.84 mg antioxidant/kg
food |
| |
= |
2.5 mg/person/day |
Example 2
In a subsequent notification, expanded use of the same antioxidant in polycarbonate and
polystyrene food contact articles is described. Each polymer
would contact food at or below room temperature. Migration levels are given below:
| Solvent |
Migration to Food (mg/kg) |
| |
Polycarbonate |
Polystyrene |
Impact Polystyrene |
| 10% aq. ethanol |
0.020 |
0.020 |
0.020 |
| 50% aq. ethanol |
0.025 |
0.035 |
0.22 |
| Miglyol 812 |
0.033 |
0.15 |
6.2 |
The concentration of the antioxidant in the daily diet resulting from each of the proposed
uses is calculated below. A CF of 0.04 for impact
polystyrene and a CF of 0.06 for all other polystyrenes was used in the calculation.
Polycarbonates
| CF x <M> |
= |
0.05(0.98(0.020 mg/kg)
+0.01(0.025 mg/kg)+0.01(0.033 mg/kg)) |
| |
= |
0.001 mg/kg |
Polystyrene
| CF x <M> |
= |
0.06(0.52(0.020 mg/kg)
+0.01(0.035 mg/kg)+0.47(0.15 mg/kg)) |
| |
= |
0.0049 mg/kg |
Impact Polystyrene
| CF x <M> |
= |
0.04(0.86(0.020 mg/kg)
+0.04(0.22 mg/kg)+0.10(6.2 mg/kg)) |
| |
= |
0.026 mg/kg |
The total concentration of the antioxidant in the daily diet resulting from the additional uses
in polycarbonate and polystyrene is approximately
0.032 mg/kg or 0.032 parts per million (ppm).
The contribution to the EDI is:
| EDI |
= |
3 kg food/person/day x 0.032 mg antioxidant/kg
food |
| |
= |
0.096 mg/person/day |
The cumulative exposure from the previously regulated use (Example 1, 2.5 mg/person/day)
and the additional proposed uses would be 2.6 mg/person/day.
APPENDIX V.
REFERENCES
General References
American Society for Testing and Materials (ASTM), E 1303-95, Standard Practices for
Refractive Index Detectors used in Liquid Chromatography. ASTM, West
Conshohocken, PA 19428-2959.
Arthur D. Little, Inc., July 1983: A Study of Indirect Food
Additive Migration. Final Summary
Report. 223-77-2360.
Arthur D. Little, Inc., September 30, 1988: High Temperature
Migration Testing of Indirect Food
Additives. Final Report. FDA Contact No. 223-87-2162.
Arthur D. Little, Inc., August 1990: High Temperature Migration
Testing of Indirect Food
Additives to Food. Final Report. FDA Contract No. 223-89-2202.
ASTM E 1511-95, Standard Practice for Testing Conductivity Detectors Used in Liquid or
Ion
Chromatography. ASTM, West Conshohocken, PA 19428-2959.
Baner, A., Brandsch, J., Franz, R. and Piringer, O. , 1996, The
Application of a predictive
migration model for evaluating the compliance of plastic materials with European food
regulations. Food Additives and Contaminants, 13 (5),
587-601.
Begley, T. H. and Hollifield, H. C., 1991, Application of a
polytetrafluoroethylene single-sided
migration cell for measuring migration through microwave susceptor films. American Chemical
Society Symposium Series 473: Food and Packaging Interactions II, Chapter 5, 53-66.
Chang, S., 1984, Migration
of low molecular weight components from polymers: 1.
Methodology and diffusion of straight-chain octadecane in polyolefins. Polymer,
25, 209-217.
Currie, L. A., 1968, Limit
of qualitative detection and quantitative determination, application to
radiochemistry. Analytical Chemistry, 40(3),
586-593.
Goydan, R., Schwope, A., Reid, R., and Cramer, G.,
1990, High temperature migration of
antioxidants from polyolefins. Food Additives and Contaminants,
7(3), 323-337.
Keith, L. H., Crummett, W., Deegan, Jr., J., Libby,
R. A., Taylor, J. K., and Wentler, G., 1980,
Principles of environmental analysis. Analytical Chemistry,
55, 2210-2218.
Limm, W. and Hollifield, H. C.,
1995, Effects of temperature and mixing on polymer adjuvant
migration to corn oil and water. Food Additives and Contaminants,
12 (4), 609-624.
Limm, W. and Hollifield, H.,
1996, Modelling additive diffusion in polyolefins. Food
Additives
and Contaminants, 13(8), 949-967.
McNeal, T. P. and Hollifield, H. C.,
1993, Determination of volatile chemicals released from
microwave-heat-susceptor food packaging. J. AOAC International,
76(6), 1268-1275.
National Bureau of Standards, March
1982: Migration of Low Molecular Weight Additives in
Polyolefins and Copolymers. Final Project Report, NBSIR 82-2472. NTIS PB 82-196403,
National Technical Information Services, Springfield, VA.
Schwope, A. D. and Reid, R. C.,
1988, Migration to dry foods. Food Additives and
Contaminants, 5 (Suppl. 1), 445-454.
Schwope, A. D., Till, D. E., Ehntholt, D. J., Sidman,
K. R., Whelan, R. H., Schwartz, P. S., and
Reid, R. C., 1986, Migration of an organo-tin stabilizer from
polyvinyl chloride film to food
and food simulating liquids. Deutsche Lebensmittel Rundschau,
82(9), 277-282.
Schwope, A. D., Till, D. E., Ehntholt, D. J., Sidman,
K. R., Whelan, R. H., Schwartz, P. S., and
Reid, R. C., 1987, Migration of Irganox 1010 from ethylene-vinyl
acetate films to foods and
food-simulating liquids. Food and Chemical Toxicology,
25(4), 327-330.
Schwope, A. D., Till, D. E., Ehntholt, D. J., Sidman,
K. R., Whelan, R. H., Schwartz, P. S.,
and Reid, R. C., 1987, Migration of BHT and Irganox 1010 from
low-density polyethylene
(LDPE) to foods and food-simulating liquids. Food and Chemical Toxicology,
25(4), 317-326.
Snyder, R.C. and Breder, C.V.,
1985, New FDA migration cell used to study migration of styrene
from polystyrene into various solvents. Journal of Association Official
Analytical Chemist,
68(4), 770-775.
Till, D., Schwope A. D., Ehntholt, D. J., Sidman, K.
R., Whelan, R. H., Schwartz, P. S., and
Reid R. C., 1987, Indirect food additive migration from polymeric
food packaging materials.
CRC Critical Reviews in Toxicology, 18(3),
215-243.
Till, D. E., Ehntholt, D. J., Reid, R. C., Schwartz, P.
S., Sidman, K. R., Schwope, A. D., and
Whelan, R. H., 1982, Migration of BHT antioxidant from high density
polyethylene to foods and
food simulants. Industrial & Engineering Chemistry, Product Research and
Development, 21(1),
106-113.
Till, D. E., Ehntholt, D. J., Reid, R. C., Schwartz, P.
S., Schwope, A. D.; Sidman, K. R., and
Whelan, R. H., 1982, Migration of styrene monomer from crystal
polystyrene to foods and food
simulating liquids. Industrial & Engineering Chemistry, Fundamentals,
21(2), 161-168.
Till, D. E., Reid, R. C., Schwartz, P. S., Sidman, K.
R., Valentine, J. R., and Whelan, R. H.,
1982, Plasticizer migration from polyvinyl chloride film to solvents
and foods. Food and
Chemical Toxicology, 20(1), 95-104.
The following are lists of references that contain
descriptions, photos, or drawings of migration
cells for conducting migration testing for different packaging applications.
Cells for Migration Testing
Conventional
Applications
ASTM F34-98, Standard Practice for Construction of
Test Cell for Liquid Extraction of Barrier
Materials. ASTM, West Conshohocken, PA
19428-2959.
Dow Chemical, Inc., A single-sided migration cell,
known as the Dow cell, has been used with
food oil at 175°C. The cell is available from: Kayeness, Inc., 115 Thousand Oaks Blvd., Suite
101, P.O. Box 709, Morgantown, PA 19543 (610-286-7555). Model no. D9030.
Figge, K. and Koch, J.,
1973, Effect of some variables on the migration of additives from
plastics
into edible fats. Food Cosmetics Toxicology, 11,
975-988. The cell used was a single-sided cell
in contact with food oil at 80°C.
Goydan, R., Schwope, A. D., Reid, R. C., and
Cramer, G., 1990. The cell used was a double-sided (immersion),
stainless steel cell, with water, 95% ethanol, and oil at 130°C.
Limm, W. and Hollifield, H.,
1995. The cell used was a single-sided glass cell with water, food
oil, and food at 135°C.
Snyder, R.C. and Breder, C.V.,
1985. The cell used was a double-sided (immersion) glass cell
with water, 3% acetic acid, 95% ethanol, and oil at 40°C and 50% aqueous ethanol at 70°C.
This cell is also specified in ASTM D4754-87 "Standard Test Method for the Two-Sided Liquid
Extraction of Plastic Materials Using FDA Migration Cell." ASTM,
West Conshohocken, PA 19428-2959.
Till, D.E., Ehntholt, D. J., Reid, R. C., Schwartz, P.
S., Sidman, K. R., Schwope, A. D., and
Whelan, R. H., 1982. The cells used were glass, single-sided and
double-sided (immersion)
cells, with water, 3% acetic acid, 95% ethanol, and oil at 40°C.
Microwave Applications
ASTM F1349-91, Standard Test Method for
Nonvolatile Ultraviolet (UV) Absorbing
Extractables from Microwave Susceptors. ASTM, West
Conshohocken, PA 19428-2959.
Begley, T. and Hollifield, H.,
1991. The cell was used with food oil at temperatures up to
240°C.
Rijk, R. and De Kruijf, N.,
1993, Migration testing with olive oil in a microwave oven. Food
Additives and Contaminants, 10(6), 631-645.

1. CAS Registry Numbers for new compounds and assistance with
nomenclature can be obtained by writing to Chemical Abstracts Service
(CAS) Client Services, 2540 Olentangy River Road, P.O. Box 3343, Columbus, OH 43210, or by
visiting their website at
http://www.cas.org.
2. Migration into food depends on the chemical structure of the FCS,
the nature of the food matrix contacting the FCS, the type of
food with which it is in contact, and the temperature and duration of food contact. Prior to the
submission of a PMN, a potential notifier may wish to
meet or correspond with FDA to discuss appropriate migration testing protocols (see Section III.).
3. In the past, FDA recommended 8% ethanol as an aqueous food
simulant. Increasing the ethanol concentration from 8% to 10% will have a minimal
impact on migration studies conducted on adjuvant/polymer systems. This change also
harmonizes more closely FDA's migration protocols with those of other
nations. See the reference list at the end of Appendix II.
relating to FDA's development of the use of food simulants.
4. Miglyol 812, a product of Dynamit Nobel Chemicals, is available
from HULS America, Inc., 80 Centennial Ave., P. O. Box 456,
Piscataway, NJ 08855-0456.
5. HB307 is available from NATEC, Behringstrasse 154, Postfach
501568, 2000 Hamburg 50, Germany.
6. Previous test protocols (prior to 1995) recommended a test
temperature of 49°F for 10 days. Recent studies by FDA, however,
have shown little difference in migration levels at 49°C and 40°C (104°F).
Furthermore, the differences in migration levels between 49°C
and 40°C are of even less significance for migration studies requiring elevated temperatures
(e.g., 100°C or 121°C) for the first two
hours. Up to 80% of the total migration observed over the 10 day period is usually completed
within this two hour period at the higher temperature.
Therefore, 40°C is regarded as acceptable for migration studies for room-temperature
applications and for the portion of the migration test for
elevated-temperature applications intended to reflect long term ambient storage.
7. The LOD is the lowest concentration of analyte that the analytical
method can reliably detect above a blank (or control). It is
preferable that the LOD be determined from analyses of five blank samples. The blank signal
(i.e., the analyte response for the blank sample or the
width of the baseline close to the actual or expected analyte peak) is measured, and the average
signal and standard deviation for the blank are
calculated. The signal corresponding to the LOD is located three standard deviations above the
average blank signal. The blank signal for the LOD is
usually determined from the peak-to-peak noise measured on the baseline close to the actual or
expected analyte signal. See American Society for Testing
and Materials (ASTM), E 1303-95 or ASTM E 1511-95.
The region for quantitation of the analyte should clearly be above the LOD. The signal
corresponding to the LOQ is located ten standard deviations
above the average blank signal. See (Currie, 1968) and (Keith., et al,
1980).
This document was issued in May 2000.
For more recent information on Guidance and Reference Documents for
Petitions and Notifications
see
http://www.cfsan.fda.gov/~dms/opa-guid.html
The latest version of
this guidance issued on April 10, 2002.
Food Ingredients and Packaging
|
Guidance for Submitting Petitions
Foods Home
|
FDA Home
|
Search/Subject Index
|
Disclaimers & Privacy Policy
|
Accessibility/Help
Content last updated by abb/emw/hrw on 2004-JAN-06
Hypertext updated by cjm/ear/emw/kwg/dms/cjm/hrw/msl 2004-DEC-06
![]() g/kg. It may be
expected that this low concentration in food would lead to a
commensurately low EDI
for the FCS. Therefore, although
migration studies which could result in further lowering of the estimate of daily intake, such
studies might be unnecessary.
g/kg. It may be
expected that this low concentration in food would lead to a
commensurately low EDI
for the FCS. Therefore, although
migration studies which could result in further lowering of the estimate of daily intake, such
studies might be unnecessary.