
Volume 6, No. 10, October 2008
Features
American College of Obstetricians and Gynecologists
ACOG Practice Bulletin No. 97: Fetal Lung Maturity
Summary of Recommendations and Conclusions
The following recommendations and conclusions are based on limited
or inconsistent scientific evidence (Level B :)
- Testing for fetal lung maturity should not be performed, and is contraindicated, when delivery is mandated for fetal or maternal indications.
- Fetal pulmonary maturity should be confirmed before scheduled delivery at less than 39 weeks of gestation unless fetal maturity can be inferred from historic criteria.
- The probability of neonatal RDS is dependent on both the fetal lung maturity test result and the gestational age at which the fetal lung maturity test was performed.
- Fluorescence polarization assays (TDx FLM II) using a defined mature profile of 55 mg/g or greater is appropriate for the determination of risk of neonatal RDS in pregnancies of women with diabetes mellitus.
- Fetal lung maturity test results from amniotic fluid collected vaginally compared with those from fluid collected by transabdominal amniocentesis demonstrate that when results from fluid collected vaginally are mature, the results are reliable.
- Complications from third-trimester amniocentesis for fetal lung maturity are uncommon when performed with ultrasound guidance.
The following conclusions are based primarily on consensus and expert opinion (Level C):
- In general, the same threshold values for fetal lung maturity tests that predict low risk of neonatal RDS in pregnancies of women who do not have diabetes mellitus apply to pregnancies of women who have diabetes mellitus, whether it is gestational diabetes mellitus or pregestational diabetes mellitus.
- Data suggest that amniocentesis of both twins be performed when the gestation is between 30 0⁄7 weeks and 32 6⁄7 weeks of gestation. Amniocentesis of one twin appears to be sufficient when gestation is greater than 32 6⁄7 weeks.
- Prior to elective delivery, fetal lung maturity testing in twins with well defined gestational ages at 38 0⁄7 weeks or greater may not be necessary.
Proposed Performance Measure
Documentation of discussion or performance of fetal lung maturity testing in elective cesarean deliveries at a gestation less than 39 0⁄7 weeks
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 97: fetal lung maturity. Obstet Gynecol. 2008 Sep;112(3):717-26. http://www.ncbi.nlm.nih.gov/pubmed/18757686
ACOG Committee Opinion No. 415: Depot Medroxyprogesterone Acetate and Bone Effects
ABSTRACT: Although depot medroxyprogesterone acetate (DMPA) is associated with bone mineral density (BMD) loss during use, current evidence suggests that partial or full recovery of BMD occurs at the spine and at least partial recovery occurs at the hip after discontinuation of DMPA. Given the efficacy of DMPA, particularly for populations such as adolescents for whom contraceptive adherence can be challenging or for those who feel they could not comply with a daily contraceptive method or a method that must be used with each act of intercourse, the possible adverse effects of DMPA must be balanced against the significant personal and public health impact of unintended pregnancy. Concerns regarding the effect of DMPA on BMD should neither prevent practitioners from prescribing DMPA nor limit its use to 2 consecutive years. Practitioners should not perform BMD monitoring solely in response to DMPA use because any observed short-term loss in BMD associated with DMPA use may be recovered and is unlikely to place a woman at risk of fracture during use or in later years.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion
No. 415: depot medroxyprogesterone acetate and bone effects. Obstet Gynecol.
2008 Sep;112(3):727-30.
http://www.ncbi.nlm.nih.gov/pubmed/18757687
ACOG Committee Opinion No. 416: The Uninsured
ABSTRACT: The
United States is one of the few industrialized nations in the world that do not
guarantee health care for their populations. Access to health care for all women
is of paramount concern to obstetrician–gynecologists and
the American College of Obstetricians and Gynecologists. Pregnant women and infants
are among the most vulnerable populations in the United States and the American
College of Obstetricians and Gynecologists believes that providing them with
full insurance coverage and access to health care must be a primary step in the
process of providing coverage for all individuals within the U.S. borders. Health
care professionals can play a pivotal role in improving access to needed health
care by helping society and our political representatives understand the importance
of broadening health insurance coverage.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No.
416: the uninsured. Obstet Gynecol. 2008 Sep;112(3):731-4. http://www.ncbi.nlm.nih.gov/pubmed/18757688
ACOG Committee Opinion No. 417: Addressing Health Risks of Noncoital Sexual Activity
ABSTRACT: Noncoital sexual behaviors, which include mutual masturbation, oral sex, and anal sex, are common expressions of human sexuality. Couples may engage in noncoital sexual activity instead of penile–vaginal intercourse hoping to reduce the risk of sexually transmitted diseases and unintended pregnancy. Although these behaviors carry little or no risk of pregnancy, women engaging in noncoital behaviors may be at risk of acquiring sexually transmitted diseases. Practitioners can assist by assessing patient risk and providing risk reduction counseling for those participating in noncoital sexual activities.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 417: addressing health risks of noncoital sexual activity. Obstet Gynecol. 2008 Sep;112(3):735-7. http://www.ncbi.nlm.nih.gov/pubmed/18757689
ACOG Committee Opinion No. 418: Prenatal and Perinatal Human Immunodeficiency Virus Testing: Expanded Recommendations
ABSTRACT: Early identification and treatment of all pregnant women with human immunodeficiency virus (HIV) is the best way to prevent neonatal disease and improve the woman’s health. Human immunodeficiency virus screening is recommended for all pregnant women after they are notified that they will be tested for HIV infection as part of the routine panel of prenatal blood tests unless they decline the test (i.e., opt-out screening). Repeat testing in the third trimester, or rapid HIV testing at labor and delivery as indicated or both also are recommended as additional strategies to further reduce the rate of perinatal HIV transmission. The American College of Obstetricians and Gynecologists makes the following recommendations: obstetrician–gynecologists should follow opt-out prenatal HIV screening where legally possible; repeat conventional or rapid HIV testing in the third trimester is recommended for women in areas with high HIV prevalence, women known to be at high risk for acquiring HIV infection, and women who declined testing earlier in pregnancy; rapid HIV testing should be used in labor for women with undocumented HIV status following opt-out screening; and if a rapid HIV test result in labor is positive, immediate initiation of antiretroviral prophylaxis should be recommended without waiting for the results of the confirmatory test.
ACOG Committee Opinion No. 418: prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Obstet Gynecol. 2008 Sep;112(3):739-42. http://www.ncbi.nlm.nih.gov/pubmed/18757690
American Family Physician
Health maintenance for postmenopausal women
Menopause is the permanent cessation of menstruation resulting from the loss of ovarian and follicular activity. It usually occurs when women reach their early 50s. Vasomotor symptoms and vaginal dryness are frequently reported during menopause. Estrogen is the most effective treatment for management of hot flashes and night sweats. Local estrogen is preferred for vulvovaginal symptoms because of its excellent therapeutic response. Bone mineral density screening should be performed in all women older than 65 years, and should begin sooner in women with additional risk factors for osteoporotic fractures. Adequate intake of calcium and vitamin D should be encouraged for all postmenopausal women to reduce bone loss. Coronary artery disease is the leading cause of death in women. Postmenopausal women should be counseled regarding lifestyle modification, including smoking cessation and regular physical activity. All women should receive periodic measurement of blood pressure and lipids. Appropriate pharmacotherapy should be initiated when indicated. Women should receive breast cancer screening every one to two years beginning at age 40, as well as colorectal cancer screening beginning at age 50. Women younger than 65 years who are sexually active and have a cervix should receive routine cervical cancer screening with Papanicolaou smear. Recommended immunizations for menopausal women include an annual influenza vaccine, a tetanus and diphtheria toxoid booster every 10 years, and a one-time pneumococcal vaccine after age 65 years.
Rao SS, Singh M, Parkar M, Sugumaran R. Health maintenance for postmenopausal women. Am Fam Physician. 2008 Sep 1;78(5):583-91. http://www.ncbi.nlm.nih.gov/pubmed/18788234
Patient Education:
Information from your family doctor. Menopause: what you should know
Rao SS, Singh M, Parkar M, Sugumaran R. Information from your family doctor.
Menopause: what you should know. Am Fam Physician. 2008 Sep 1;78(5):593-4. http://www.ncbi.nlm.nih.gov/pubmed/18788235
AHRQ
US Surgeon General Issues "Call to Action" on DVT and PE
The Office of the Surgeon General has called for a coordinated, multifaceted plan to reduce the incidence of deep vein thrombosis (DVT) and pulmonary embolism (PE) in the US [1]. The "call to action" is intended to increase awareness about these silent conditions and emphasizes the importance of evidence-based practices in the management of DVT.
Acting Surgeon General Dr Steve Galson announced the call to action at a press conference in Washington, DC, and noted that DVT and PE contribute to approximately 100 000 deaths each year. "To put this number into context, the population of Cambridge, MA is roughly 100 000," said Galson. "The number of deaths attributable to blood clots is like having an entire city, like Cambridge, wiped out each year." "The good news," added Galson, "is that DVT and PE are preventable and treatable."
Published by the US Department of Health and Human Services, the Surgeon
General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism documents
the major public-health problems DVT and PE pose, as well as strategies to reduce
the risk of developing the conditions. The report highlights gaps in the awareness
and application of evidence-based interventions and discusses the public-health
response to reducing the incidence of DVT and PE.
In a concluding section on a vision for the future, the surgeon general asks
stakeholders to take action to create a future where the public is knowledgeable
about risk factors, triggering events, and symptoms of the disease; and where
evidence-based practices for screening, prevention, diagnosis, and treatment
of DVT/PE are routinely applied, and new scientific evidence is being discovered
to fill in gaps in knowledge.
Drs Samuel Goldhaber (Brigham and Women's Hospital, Boston, MA) and Thomas Ortel
(Duke University Medical Center, Durham, NC) are the scientific editors of the
surgeon general's report.
NHLBI Involved in Collaboration. The National Heart, Lung, and Blood Institute
(NHLBI) collaborated with the office of the surgeon general on the call to action,
and speaking to the media, Dr Elizabeth Nabel, director of the NHLBI, said the
institute is committed to supporting research that focuses on understanding the
causes of DVT and PE, as well as safe and effective detection methods, treatments,
and preventive measures. Nabel said the NHLBI is set to launch the first multicenter,
randomized, clinical trial of genotype-guided dosing of warfarin therapy.
"The study will examine whether the use of clinical plus genetic information
during the initiation of warfarin can lead to better and safer treatment in patients,
especially those with DVT, atrial fibrillation, who are at risk for stroke, or
who require warfarin therapy following orthopedic surgery," said Nabel.
Another NHLBI-sponsored trial is the Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial, a study evaluating whether the use of pharmacotherapy in combination with a catheter-mounted thrombus-removal device can prevent postthrombotic syndrome (PTS) in patients with DVT. PTS develops when the thrombus continues to block the vein, damaging the one-way valves, and resulting in venous pooling, chronic leg pain, swelling, fatigue, and skin ulcers. The drug-and-device therapy is intended to break up the venous clot and then remove it from the vein to restore blood flow.
ATTRACT is led by Dr Suresh Vedantham (Washington University School of Medicine, St Louis, MO) and will enroll approximately 700 patients at 28 centers. The end points include the severity of PTS, quality of life, relief of pain and swelling, safety, and cost. Vedantham, along with Goldhaber, also leads the Venous Disease Coalition, which was formed in 2006 after a surgeon general's workshop on DVT.
In addition to the surgeon general's report, the Agency for Healthcare Research and Quality (AHRQ) has also developed two evidence-based DVT/PE resources, one for physicians and the other for patients [2,3]. The guide for clinicians is entitled Preventing Hospital-Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement and is designed as a tool to "help hospitals and clinicians implement processes to prevent dangerous blood clots." The consumer resource, known as Your Guide to Preventing and Treating Blood Clots, is intended to help patients and their families learn about the causes, symptoms, and prevention of DVT.
- US Department of Health and Human Services. The surgeon general's call to action to prevent deep vein thrombosis and pulmonary embolism. Available at: www.surgeongeneral.gov/topics/deepvein/calltoaction/call-to-action-on-dvt-2008.pdf.
- Agency for Healthcare Research and Quality. Preventing hospital-acquired venous thromboembolism: A guide for effective quality improvement. Available at: www.ahrq.gov/qual/vtguide.
- Agency for Healthcare Research and Quality. Your guide to preventing and treating blood clots. Available at: www.ahrq.gov/consumer/bloodclots.htm.
The complete contents of Heartwire, a professional news service of WebMD, can be found at www.theheart.org, a Web site for cardiovascular healthcare professionals.
Ask A Librarian - Diane Cooper, IHS National Library Informationist
BMJ Clinical Evidence Adds Medical Conditions
Clinical Evidence added 50 more conditions for a total of 250. The resource offers summaries of conditions with treatment options. It’s quick, brief, and easy to use.
- Includes evidence-based research sourced from over 10,000 peer-reviewed references and covers over 570 clinical questions and 3000+ interventions.
- Provides background information with references.
- Sends alerts from monthly updates and new reviews.
- Offers drug safety alerts.
- Contains emerging research information.
- Links to practice guidelines, site tools and EBM resources.
To access Clinical Evidence from the HSRL website go to:
Research Tools > Databases > Clinical Evidence.
If you have questions about accessing or using Clinical Evidence, contact me at cooperd@mail.nih.gov or 301.594.2449.
Behavioral Health Insights - Peter Stuart, IHS Psychiatry Consultant
Antipsychotics – The Old versus the New - What Should I Use?
A number of recent reports suggest that the differences in efficacy and side effect burden between the old “typical” antipsychotics and the new “atypical” antipsychotics are not as significant as once proposed. The CATIE (Clinical Antipsychotic Trials in Intervention Effectiveness) trial compared four of the new agents to an old agent, perphenazine, and found that there was no substantial advantage of the newer agents over perphenazine in the treatment of schizophrenia. This came as quite a surprise to many who assumed the newer agents would outperform perphenazine. A large VA funded study came out shortly thereafter comparing olanzapine and haloperidol – again there was little overall advantage to the use of either medication. More recently the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) also found little clinical difference even for clinical concerns such as extrapyramidal symptoms between the older agent(s) and the atypicals used. Finally, just out in September in the American Journal of Psychiatry, findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study suggest similar outcomes in youth when newer agents were compared to molindone; an older though infrequently used antipsychotic.
Of particular interest and import for the aforementioned studies – all of them received no industry funding.
Recent reports of increased death rates in elderly patients taking risperidone and other atypicals as well as older agents, and the now well documented metabolic issues and clinically detrimental weight gain with many of the atypicals only add to concerns.
On the other hand, despite the black box warnings for increased rates of death in elderly populations using the newer atypicals – first generation antipsychotics may actually have associated rates of death that are even higher than the atypicals.
As Dr. Jeffrey Lieberman, the lead investigator of the CATIE trials was quoted as saying, the second generation antipsychotics or atypicals represent an “incremental” improvement over first generation drugs – not game-changing medications. Clozapine remains the gold standard for the treatment of schizophrenia of all the antipsychotics. It was, however, excluded from the first arms of these studies as it is replete with side effects and serious, unpredictable toxicity and as such is not as commonly used except for treatment of refractory psychosis.
So where does this leave primary care practitioners in busy practices – particularly when using these medications for off-label uses such as managing aggressive behavior in patients with dementia? Judged solely by prescriptive volume the atypicals remain the first choice for most providers. While the reduction in the occurrence of tardive dyskinesia remains only partially proven, it is a particularly troubling and disfiguring consequence and a strong reason to continue first line use of atypicals – though the bulk of the evidence now suggests that otherwise efficacy and total side effect burdens are similar compared to many first generation antipsychotics.
Some suggested rules
Rule #1 – consider your patient’s particular risks – is the main risk obesity and diabetes (try aripiprazole or ziprasidone), or sedation (try aripiprazole, ziprasidone or risperidone), or sensitivity/risk of tardive dyskinesia and EPS (elderly, those with histories of brain injury or dysfunction) (try quetiapine)?
Rule #2 – choose your antipsychotic based on its side effect profile. In our populations that often means starting with one that has a benign metabolic profile and limited EPS symptoms – aripiprazole remains a good starting point. Quetiapine in small amounts particularly for the elderly (25-75 mg daily) is helpful for situations in which some sedation is needed.
Rule #3 – if using any of the atypicals get a good metabolic baseline including lipid status and watch your patient’s weight. These effects are not specifically dose dependent and the weight gain can be quite rapid. Nutritional counseling at the outset is suggested.
Rule #4 – the old standby, haloperidol, remains a good option in the ICU setting for the management of acute delerium and agitation. Its distribution, metabolism and pharmacological effects are well understood and used thoughtfully remains the gold standard. IV use, while not included in its FDA indication, appears to reduce its EPS profile. Caution is indicated for IV use – EKG monitoring for torsades is recommended along with repletion of potassium and magnesium levels if indicated. See the article “Postoperative Delerium” by Fricchione et al in a recent issue of AJP for an excellent review. Psychotic agitation in the ER is better managed with one of the newer atypical agents available in IM formulation (olanzapine, aripiprazole) due to their lower propensity to induce EPS symptoms particularly in young males. Liquid formulations (risperidone) and disintegrating tablets (risperidone and olanzapine) are also available.
Rule #5 – always document counseling your patient and/or their guardian/family members for any off-label uses. The risks of use are significant – but often there are few alternate options. Chronic use should be regularly reviewed and attempts made to discontinue use where appropriate.
Rule #6 – consult your local psychiatrist.
References
Lieberman JA, Stroup TS, McEvoy JP, Swartz, MS, Rosenheck, RA, Perkins, DO, et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. N Engl J Med. 2005 Sep 22;353(12):1209-23. Epub 2005 Sep 19. http://www.ncbi.nlm.nih.gov/pubmed/16172203
Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006 Oct;63(10):1079-87. http://www.ncbi.nlm.nih.gov/pubmed/17015810
Rosenheck R, Perlick D, Bingham S, Liu-Mares W, Collins J, Warren S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. JAMA. 2003 Nov 26;290(20):2693-702. http://www.ncbi.nlm.nih.gov/pubmed/14645311
Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, et al. Double-Blind Comparison of First- and Second-Generation Antipsychotics in Early-Onset Schizophrenia and Schizo-affective Disorder: Findings From the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study. Am J Psychiatry. 2008 Sep 15. [Epub ahead of print]. http://www.ncbi.nlm.nih.gov/pubmed/18794207
Lieberman JA. Comparative effectiveness of antipsychotic drugs. A commentary on: Cost Utility Of The Latest Antipsychotic Drugs In Schizophrenia Study (CUtLASS 1) and Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE). Arch Gen Psychiatry. 2006 Oct;63(10):1069-72. http://www.ncbi.nlm.nih.gov/pubmed/17015808
Setoguchi S, Wang PS, Alan Brookhart M, Canning CF, Kaci L, Schneeweiss S. Potential Causes of Higher Mortality in Elderly Users of Conventional and Atypical Antipsychotic Medications. J Am Geriatr Soc. 2008 Aug 4. [Epub ahead of print] http://www.ncbi.nlm.nih.gov/pubmed/18691283
Fricchione GL, Nejad SH, Esses JA, Cummings TJ Jr, Querques J, Cassem NH, Murray GB. Postoperative delirium. Am J Psychiatry. 2008 Jul;165(7):803-12. http://www.ncbi.nlm.nih.gov/pubmed/18593786
SAMHSA Awards More Than $16 Million to Tribes/Tribal Organizations for Youth Suicide Prevention and Early Intervention Programs
The Substance Abuse and Mental Health Services Administration (SAMHSA) has announced the award of 12 grants totaling more than $16 million over three years to support suicide prevention efforts undertaken by tribes/tribal organizations. This grant program is authorized under the Garrett Lee Smith Memorial Act, which provides funding for programs to combat suicide.
SAMHSA Deputy Administrator Eric Broderick, D.D.S., M.P.H., made the announcement at the Interdepartmental Tribal Justice, Safety and Wellness Government-to-Government Consultation, Training and Technical Assistance Session in Billings, Mont. “As a result of the Garrett Lee Smith Memorial Act, SAMHSA works with state and local governments, communities and tribes/tribal organizations to stem the number of youth suicides in our country,” said Dr. Broderick. “These new grantees will help fill a significant need in their communities.”
Nationally, an estimated 900,000 youth had made a plan to commit suicide during their worst or most recent episode of major depression, and 712,000 attempted suicide during such an episode. The data are from SAMHSA’s National Survey on Drug Use and Health, which asked youth ages 12-17 about symptoms of depression, including thoughts about death or suicide.
The total approximate amount for the grant award period, which ranges from one to three years, is $16 million. First-year funding totals nearly $6 million. Continuation of these awards is subject to both availability of funds and progress achieved by awardees. The grants announced today will be administered by SAMHSA’s Center for Mental Health Services and will be awarded to the following organizations:
- Gila River Behavioral Health Authority Youth Suicide Prevention Project, The Gila River Indian Community, Sacaton, Ariz. -- $496,889 for the first year to provide suicide prevention and intervention services through Saving Lives for Tomorrow.
- Omaha Nation Community Response Team - Project Hope, Walthill, Neb. -- $500,000 for the first year to build on prior suicide prevention efforts in order to develop and implement a tribal youth suicide prevention initiative, grounded in strong partnerships and collaborations.
- Mescalero Apache School Youth
Suicide Prevention and Early Intervention Initiative,
Mescalero, N.M. -- $432,282 for the first year to implement and evaluate a comprehensive early intervention and suicide prevention model. - Wiconi Wakan Health & Healing Center, Rosebud Sioux Tribe, Rosebud, S.D. -- $477,570 for the first year to establish the Wiconi Wakan Health & Healing Center, a place to implement the Tribal Youth Suicide Prevention and Early Intervention Project Plan.
- Circle of Trust Youth Suicide Prevention Program, The Confederated Salish Kootenai Tribes of the Flathead Indian Nation, Pablo, Mont. -- $166,667 for the first year to implement a prevention project that will include both CSKT members and nonmembers.
- Preserving Life: Nevada Tribal Youth Suicide Prevention Initiative, Inter-Tribal Council of Nevada, Sparks, Nev. -- $500,000 for the first year to support, expand, and enhance suicide prevention efforts within the communities of the Nevada Tribes by implementing goals in the three areas of interest of the Indian Health Service Suicide Prevention Plan--Awareness, Interventions, and Methodology.
- Youth Suicide Prevention, The Crow Creek Sioux Tribe, Ft. Thompson, S.D. -- $450,390 for the first year to enhance the Tribe’s suicide prevention strategies and meet the objectives of its suicide prevention plan.
- Tribal Youth Suicide Prevention Program, Oglala Sioux Tribe, Pine Ridge, S.D. -- $500,000 for the first year to develop and implement a comprehensive and sustainable program to prevent suicide.
- Wiconi Ohitika Project, Cankdeska Cikana Community College, Fort Totten, N.D. -- $485,857 for the first year to provide suicide prevention for the Spirit Lake Nation.
- Sault Tribe Alive Youth (STAY) Project, Sault Ste Marie Tribe Chippewa Indians, Sault Ste Marie, Mich. -- $500,000 for the first year to work with tribal and non-tribal stakeholders to develop and implement a broad-based, culturally competent suicide prevention and early intervention program.
- Bering Strait Suicide Prevention Program, Kawerak, Inc., Nome, Alaska -- $500,000 for the first year to assist villages in developing prevention strategies through capacity building, education, training, and strong interdisciplinary collaboration and elder guidance.
- Native Youth Suicide Prevention Project, Native American Rehabilitation Association, Portland, Ore. -- $500,000 for the first year to expand and strengthen youth suicide prevention networks.
Additional grants will be awarded this year for suicide prevention efforts under the Garrett Lee Smith program. http://www.samhsa.gov/newsroom/advisories/0808203705.aspx
Breastfeeding - Suzan Murphy
Mastitis and Plugged Ducts
Author: Tony Nazario, Senior Nursing Student, Arizona State University, Summer Student Nurse Extern – PIMC (With a special thank you to LT Jing Li, PharmD for generously providing technical expertise for this article.)
What is mastitis?
Mastitis is an acute inflammation of the breast tissue that nearly one quarter of women report experiencing. It often occurs within the first six weeks after delivering but women are at risk for getting mastitis throughout breastfeeding. It is an uncomfortable condition and some women may prematurely wean their children as a result, however, this is discouraged, as continued breast-feeding is part of the treatment process.
Signs and Symptoms
Signs and symptoms are the first indicators of a problem and if they are present, referring to breastfeeding support and/or scheduling an appointment with a physician are recommended.
Signs and Symptoms of an infection:
-sudden onset
-localized intense pain
-redness or swelling of the breast
-the breast may feel warm to touch
- Flu-like symptoms
-a fever of 101º F or higher
Signs and Symptoms of a plugged duct:
-gradual onset
-mild localized pain
-location may shift
-breast may feel slightly warm to touch
-a low-grade fever 101º F or lower
-generally feels well
Causes
An infection can occur anytime a pathogen (disease causing micro-organism such as bacteria) enters the breast and infect the tissue. This can occur through vulnerable areas of the breast/nipple where the skin is cracked/broken or sore.
Inflammatory mastitis occurs due to “milk stasis.” Milk stasis is when milk does not completely drain from the breast. When the breast does not fully drain after feeding, the milk that is remaining in the breast can clog the mammary duct(s).
Risk Factors for mastitis include:
-Cracked or sore nipples
-A previous history of mastitis
-Only feeding the child in one position (This increases the risk for mastitis
because the breast may not be able to completely drain.)
-Feeding only one side at each feeding
-Wearing a tight fitting bra (This can restrict the flow of milk).
-Extreme fatigue and stress can cause the normal body defenses to be weakened
and that can place the mother at an increased risk for bacterial infection.
Treatment
If mastitis is suspected a physician should be contacted to make a proper diagnosis. If the woman is experiencing mastitis related to an infection, antibiotic therapy will be required. Antibiotics therapy often provides rapid results and relief within days of beginning treatment. In order to fully combat bacterial mastitis the client will need to complete the full round of antibiotics* even when signs and symptoms have gone away. Continued breastfeeding is highly encouraged to prevent additional blockages and maintain milk flow.
In cases of plugged ducts (inflammatory mastitis) self-care remedies are suggested. Applying wet or dry heat to the affected area followed by gentle massages. Women are also encouraged to shower or apply hot wet packs between feedings. As with mastitis, continued breastfeeding is highly encouraged to prevent additional blockages and maintain milk flow.
Prevention
Frequent feedings that fully allow the breast to drain are recommended to prevent blockages.
*Information provided by Jing Li, PharmD, LT USPHS. According to UpToDate, current antibiotics used for mastitis are dicloxacillin or cloxacillin 500 mg four times a day for ten to fourteen days. If there is no response in 24-48 hours, a regimen of cephalexin or augmentin is advised. If mastitis recurs repeatedly in the same area it is advisable to rule out breast cancer.
According to NIH Lactnet (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT) dicloxacillin, cloxacillin, cephalexin and augmentin are compatible with breastfeeding.
For more information, please see:
Biancuzzo, M, Breastfeeding the Newborn: Clinical Strategies for Nurses, Mosby
Inc. 2003.
Lawrence RA, Breastfeeding: A Guide for the Medical Profession, 6th Edition,
Mosby, Inc. 2005.
The IHS/MCH Website includes many Breastfeeding Resources:
http://www.ihs.gov/MedicalPrograms/MCH/M/bf.cfm
CCC Corner Digest
Nicely laid out hard copy - A compact digest of last month’s CCC Corner
If you want a copy of the CCC Digest mailed to you each month, please contact Jean Howe
Domestic Violence - Denise Grenier, Tucson / Rachel Locker, Warm Springs
Break the Silence: Stop the Violence
It may shock you to know that one out of every eleven teens reports being hit or physically hurt by a boyfriend or girlfriend in the past twelve months. But why is that, and how can we change it? In "Break The Silence: Stop the Violence," parents talk with teens about developing healthy, respectful relationships before they start dating.
Source: National Center for Injury Prevention
and Control (NCIPC)
Running Time: (4:12) Release Date: 8/4/2008
http://www.cdc.gov/CDCTV/BreakTheSilence/index.html
Elder Care News - Bruce Finke, Elder Care Initiative
Less Sleep May Equal Greater Risk of Falling in Elderly Women
BACKGROUND:
Prior studies have suggested that insomnia and self-reported poor sleep are associated
with increased risk of falls. However, no previous study, to our knowledge, has
tested the independent associations of objectively estimated characteristics
of sleep and risk of falls, accounting for the use of commonly prescribed treatments
for insomnia.
METHODS: Study subjects were participants in the Study of Osteoporotic Fractures.
In 2978 primarily community-dwelling women 70 years and older (mean age, 84 years),
sleep and daytime inactivity were estimated using wrist actigraphy data collected
for a minimum of 3 consecutive 24-hour periods (mean duration, 86.3 hours). Fall
frequency during the subsequent year was ascertained by a triannual questionnaire.
Use of medications was obtained by examiner interview.
RESULTS: In multivariate-adjusted models, relative to those with "normal" nighttime
sleep duration (>7 to 8 hours per night), the odds of having 2 or more falls
in the subsequent year was elevated for women who slept 5 hours or less per night
(odds ratio, 1.52; 95% confidence interval, 1.03-2.24). This association was
not explained by the use of benzodiazepines. Indexes of sleep fragmentation were
also associated with an increased risk of falls. For example, women with poor
sleep efficiency (<70% of time in bed spent sleeping) had 1.36-fold increased
odds of falling compared with others (odds ratio, 1.36; 95% confidence interval,
1.07-1.74).
CONCLUSION: Short nighttime sleep duration and increased sleep fragmentation
are associated with increased risk of falls in older women, independent of benzodiazepine
use and other risk factors for falls.
Stone KL, Ancoli-Israel S, Blackwell T, Ensrud KE, Cauley JA, Redline S, Hillier TA, Schneider J, Claman D, Cummings SR. Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008 Sep 8;168(16):1768-75.http://www.ncbi.nlm.nih.gov/pubmed/18779464
Family Planning
ACOG Press Release: Bone Mineral Density Concerns Should Not Discourage Use of Contraceptive Shot
Concerns about the effects of depot medroxyprogesterone acetate (DMPA)—the contraceptive shot—on bone mineral density (BMD) should not prevent clinicians from prescribing this method of contraception nor should its use be limited to two years, according to a new Committee Opinion released today by The American College of Obstetricians and Gynecologists (ACOG). Though DMPA has a known link to BMD loss, studies show that most of the loss is temporary and is similar to the BMD loss caused by pregnancy and breastfeeding.
"Women should be thoroughly counseled about the risks and benefits of DMPA so they can make an informed decision about whether it's right for them," said Denise Jamieson, MD, chair of ACOG's Committee on Gynecologic Practice. "Many women would choose the theoretical risk of future fracture over the very real risk of an unintended pregnancy. For example, a teen at high risk of pregnancy—who faces a similar rate of bone loss from either pregnancy or DMPA use—may find the risk worthwhile."
In 2004, the US Food and Drug Administration issued a black box warning discouraging the use of DMPA for more than two consecutive years. The warning states that prolonged use of DMPA may result in significant loss of BMD, that bone loss is greater the longer the drug is used, and that the loss may not be completely reversible after discontinuation. It also advises that DMPA use beyond two years should only be considered if other contraceptive methods are inadequate. Additionally, one DMPA manufacturer has recommended bone scans for women after two years of use.
Bone loss does occur, especially within the first two years of DMPA use. But evidence suggests that within one to two years after discontinuation, BMD in most adolescents and women rebounds to near baseline levels. The FDA's warning is based on intermediate effects on BMD, which may or may not be relevant to increased risk of fracture. Studies show that former adult DMPA users have BMD rates similar to women who have never used the drug.
Bone loss in reproductive-aged women is not exclusive to DMPA users. Adult
women show similar rates of temporary bone loss during pregnancy and breastfeeding
(2%-8% and 3%-5%, respectively) when compared with BMD loss sustained by DMPA
users (approximately 3%-5%).
Adolescents using DMPA lose BMD at a time when their BMD would typically increase,
which can be a cause of concern. However, while low BMD is linked to an increased
risk of fracture in older women, no studies have linked DMPA-related BMD loss
with increased rates of fracture in younger women with a low-fracture risk.
More than 2 million American women use DMPA, including approximately 400,000 teens. Injected once every three months, it is a safe method of birth control that is between 97% and 98% effective at preventing pregnancy. "DMPA has a number of characteristics that make it particularly appealing to certain populations such as adolescents or women who may have a hard time successfully using a daily or partner-dependent method of contraception," said Dr. Jamieson. "Some women also prefer it over other methods of contraception because of the privacy that it provides. Increased use of DMPA has likely been a contributing factor in the drop of adolescent pregnancy rates in the past decade."
Daily exercise
and age-appropriate calcium and Vitamin-D intake should be encouraged in DMPA
users, especially in teens, who often do not get enough calcium. Although studies
have shown that low-dose estrogen supplementation slows bone DMPA bone loss,
ACOG does not currently recommend it. Clinicians should counsel women on the
side effects of DMPA, such as breakthrough bleeding, to curb the high discontinuation
rate for this contraceptive method.
ACOG recommends that other effective, long-term methods of contraception that
have no effect on bone density—such as contraceptive implants and intrauterine
devices—should also be considered as first-line methods for adolescents.
See ACOG section of Features for the Abstract of Committee Opinion #415
Management of Breakthrough Bleeding with Continuous Use of the Transvaginal Contraceptive Ring
OBJECTIVE: To assess bleeding patterns with continuous use of the transvaginal
contraceptive ring.
METHODS: We did a prospective analysis of daily menstrual flow during a 21/7
cycle followed by 6 months of continuous use and institution of a randomized
protocol to manage breakthrough bleeding/spotting. Seventy-four women completed
the baseline 21/7 phase and were randomized equally into two groups during the
continuous phase. Group 1 was instructed to replace the ring monthly on the same
calendar day with no ring-free days. Group 2 was instructed to use the same process,
but if breakthrough bleeding/spotting occurred for 5 days or more, they were
to remove the ring for 4 days, store it, and then reinsert that ring.
RESULTS: Sixty-five women completed the continuous phase with reduced average
flow scores in the continuous phase compared with the 21/7 phase (P<.02).
Most patients had no to minimal bleeding during continuous use, with group 2
experiencing a statistically greater percentage of days without breakthrough
bleeding or spotting (95%) compared with group 1 (89%) (P=.016). Instituting
a 4-day hormone-free interval was more (P<.001) effective in resolving breakthrough
bleeding/spotting than continuing ring use.
CONCLUSION: A reduction in bleeding occurred during continuous use with replacement
of the transvaginal ring compared with baseline 21/7 use. Continuous vaginal
ring use resulted in an acceptable bleeding profile in most patients, reduction
in flow, reduction in pelvic pain, and a high continuation rate.
Sulak PJ, Smith V, Coffee A, Witt I, Kuehl AL, Kuehl TJ. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol. 2008 Sep;112(3):563-71. http://www.ncbi.nlm.nih.gov/pubmed/18757653
Featured Website
Welcome to WIN
The Weight-control Information Network provides the general public, health professionals, the media, and Congress with up-to-date, science-based information on weight control, obesity, physical activity, and related nutritional issues.
About WIN
The Weight-control Information Network (WIN) is an information service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH). WIN was established in 1994 to provide the general public, health professionals, the media, and Congress with up-to-date, science-based information on obesity, weight control, physical activity, and related nutritional issues. WIN also developed the “Sisters Together: Move More, Eat Better” national initiative to encourage Black women to maintain a healthy weight by becoming more physically active and eating healthier foods.
How can WIN help you?
WIN produces, collects, and disseminates materials on obesity, weight control, and nutrition.
WIN Provides:
- Publications, including fact sheets and brochures
- NIDDK’s Clinical Weight Loss and Control Lecture Series videos
- Information on Clinical Nutrition Research Centers (ONRCs) and Clinical Nutrition Research Units (CNRUs)
- WIN Notes, an electronic newsletter for health professionals featuring the latest information from NIDDK and other organizations on obesity, weight control, and related nutritional topics. WIN Notes also reports on activities of the NIDDK-sponsored Clinical Obesity Research Panel (CORP).
- Sisters Together: Move More, Eat Better, WIN's national initiative to encourage Black women to maintain a healthy weight.
http://www.win.niddk.nih.gov/index.htm
Frequently Asked Questions - Neil Murphy; SCF, ANMC
Q. Can a patient receive outpatient cervical ripening?
A. Yes, selected patients can receive outpatient cervical ripening with careful monitoring
Outpatient cervical ripening with low dose prostaglandins has been found to be a convenient, safe, cost- and time-saving procedure for women with a medical need for induction, but without an urgent need to be delivered. This process should be undertaken with care and forethought. It should not be undertaken unless there is an ongoing quality assurance system to maximize patient safety.
Outpatient ripening should only be scheduled through an antenatal testing clinic with appropriate monitoring equipment and staff. To maximize resources there could be two staggered sessions per day. One possible approach could be the first session beginning at 0700 and the second session will begin at 1100. This staggered approach would allow for more cervical ripening without disrupting flow within the clinic.
Proposed Guidelines: Outpatient Pre-induction Cervical Ripening with Low-Dose Misoprostol in OB Triage/Antenatal Testing Clinic
A. Background
Outpatient cervical ripening with low dose prostaglandins can be a convenient, safe, cost- and time-saving procedure for women with a medical need for induction, but without an urgent need to be delivered. Outpatient ripening will be scheduled through the Antenatal Testing Clinic, Monday-Friday, starting in two sessions. The first session will begin at 0700 and the second session will begin at 1100 for a total of four ripenings. No weekend cervical ripenings will be scheduled in OB Triage. Medically-indicated ripenings will be scheduled in Labor & Delivery on holidays and weekends.
B. Candidates for Outpatient Pre-induction Cervical Ripening:
Women at term with an unripe cervix (defined as Bishop Score < 5) with a medical indication for delivery, such as:
- 41 week ripening/induction option
- diabetes well-controlled
- chronic hypertensive disease, well controlled*
- mild pre-eclampsia not requiring urgent delivery, after 39 weeks*
- patients requiring non-emergent ripening/induction with complications of pregnancy warranting delivery
Women who MAY BE candidates depending on clinical judgment:
- underlying maternal disease—cardiac/respiratory/coagulopathy/autoimmune
- Bishop’s score >5
Women who are NOT candidates include:
- previous cesarean delivery or other uterine incision
- non-reassuring fetal heart tracing
- unexplained vaginal bleeding, placental abruption, placenta previa
- symptomatic unstable pre-eclampsia/hypertension
- diabetic, not well-controlled
- women with a history of 5 or more vaginal deliveries
- suspected fetal growth restriction, <10th percentile
- multiple gestations
- malpresentation or pelvic structural deformity
- women in labor
- intrauterine fetal demise
- history of asthma
- women pregnant with identified at-risk fetuses
- cholestasis at 36 weeks
All patients who undergo the pre-induction cervical ripening protocol will be reported in a log on the Antenatal Testing Unit and subjected to a QA review quarterly.
*need baseline labs: CBC, platelets, LFTs, urine Protein:Creatinine Ratio
C. Bishop Score:
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Dilation (cm) | closed | 1-2 | 3-4 | >4 |
| Effacement (%) | 0-25 | 25-50 | 50-75 | >75% |
| Station | -3 | -2 | 0/-1 | +1/+2 |
| Consistency | firm | med | soft | - |
| Position | post | mid | ant | - |
| TOTAL = ______ |
D. Procedure:
- The patient will be scheduled to present to the Antenatal Testing Clinic at 7 AM or 11 AM. She need not be fasting the day of the procedure. Please schedule with the antenatal testing nurse at XXX-XXXX. Up to four patients a day can be accommodated at the present time. Patients should be scheduled for formal induction on Labor and Delivery (L&D) 48 hours (Day #3) after beginning the ripening procedure.
- The patient will have a 20-minute pre-ripening non-stress test. If any baseline fetal heart rate abnormalities are noted, or if the patient is having regular painful contractions every 3-5 minutes, the procedure will be canceled and the on-call provider notified. An ultrasound to confirm presentation and AFI and a cervical check to determine Bishop Score will be done prior to the insertion of the misoprostol by the triage nurse or provider.
- The patient’s provider should provide a written order on the PCC+ form:
“Please insert misoprostol 25 micrograms (1/4 tablet moistened with normal saline) high in the posterior vaginal fornix x 1 and monitor x 4 hours. May repeat x1 within 24 hours, if no active labor.”
- The patient should remain recumbent for the first hour following insertion of the tablet. Beginning one half-hour post-insertion she should be monitored continuously for one hour. If the fetal tracing remains reassuring, she may then ambulate but should be monitored for at least 20 minutes every hour for the next 3 hours. She may take fluids by mouth ad lib.
- If the fetal tracing remains reassuring, the patient is not having painful contractions and the discharge cervical dilation does not indicate active labor, she may then be discharged home after 4 hours. Uterine tachysystole, without any worrisome fetal heart rate changes, will not be an indication to stop the procedure or to not send the patient home. If any fetal heart rate decelerations are noted, an attempt should be made to remove the tablet, and the patient transferred expeditiously to L&D for close observation. The on-call L&D provider will be notified and assume care.
- If active labor has not started by 7 AM the following day, the patient will return to the antenatal testing clinic for a repeat cervical examination. If the cervix is still unfavorable for induction, the above procedures will be repeated a second time.
- If no active labor by 6 AM the third day (48 hours after the first dose), the patient should call L&D for a time for formal induction of labor.
Literature Review
1.) To what degree can pre-clampsia be managed with outpatient cervical ripening?
2.) What are national trends in enrollment bishop score criteria?
Disclaimer:
Before we begin, please realize most of the studies are small and limited in
scope. It is even more limited if we confine our question to the use of low dose
misoprostol, so the size of these studies is even smaller. There are also a wide
variety of methods used: single dose, 3-4 day intervals, different doses of misoprostol,
different Bishop score criteria, multiple dose regimens, etc….
In virtually all cases, the ultimate clinical outcome will be based on the patient’s underlying clinical condition for which she qualified for cervical ripening, rather than a result of the cervical ripening process, per se.
1.) The first question was easy to answer. There is no body of data specific to cervical ripening in pre-eclampsia. Hypertension and pre-eclampsia were exclusion criteria in some studies. Most studies recruited among post-dates or diabetics patient populations.
McKenna 2004 did include one patient with pre-eclampsia and 12 patients with hypertension in their outpatient study arm with Foley bulb usage. They make no mention of the patient’s underlying condition‘s clinical outcome.
Sciscione et al 2001 presents a table of their patient’s clinical indications on page 753, which does not include any patients with pre-eclampsia in their Foley bulb trial.
In summary, it seemed clear that whatever the medical or obstetric condition, it should be considered ‘stable’ upon entry to the outpatient ripening process.
It is well documented in the pre-eclampsia literature that mild pre-eclampsia can be managed with outpatient clinical monitoring. That same management can be applied to a cervical ripening process if the patient is stable.
I would not plan to look to the existing literature to decide on the management
of specific clinical scenarios. Rather, as a team of providers and nurses we
may want to ask….
Would we manage this clinical condition differently whether the patient was an
inpatient or outpatient? e. g., regardless of the intercurrent use of a small
dose of misoprostol.
If so, then we should address those differences through the specific guidelines for that disease, e.g., not through the outpatient cervical ripening process.
2.) The bishop score is more reproducible than the topic above, but is limited by the wide variety of criteria and study methods. In addition, the studies varied in how they managed the ripening process, e. g., some studies had a 3-4 day interval between doses, single dose, multiple dose, etc…
Entry Bishop Score
Biem < 6
entry criteria
Chang 2005 “unfavorable
cervix”
Farmer 1996 < 4
entry criteria
Incerpi 2001 < 4
entry criteria
Kipikasa 2005 < 5
entry criteria
McKenna 2004 4.7
reported mean entry score
McKenna 2004 1.8
reported mean entry score
Meyer 2005 < 6
entry criteria
Oboro 2005 < 8
entry criteria
O’Brien 1995 < 6
entry criteria
Sawai 1991 < 9
entry criteria
Scisione 2001 < 5
entry criteria
Stitely 2000 < 4
entry criteria
In summary, use a Bishop’s score of < 5 for outpatient ripening is supported in the current literature, though there is wide range of entry criteria, e. g., up to < 9.
Resources:
Biem
SR, Turnell
RW, Olatunbosun
O, Tauh
M, Biem
HJ. A randomized controlled trial of outpatient versus inpatient labour induction
with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction.
J Obstet Gynaecol Can. 2003 Jan;25(1):23-31.
http://www.ncbi.nlm.nih.gov/pubmed/12548322
Chang DW; Velazquez MD; Colyer M; Klaus P; Mallipeddi SK; Rayburn WF Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs. inpatient administration. J Reprod Med. 2005 Oct;50(10):735-9. http://www.ncbi.nlm.nih.gov/pubmed/16320553
Cervical ripening. Clin Obstet Gynecol. 1998 Sep;41(3):606-10.
http://www.ncbi.nlm.nih.gov/pubmed/9742357
Farmer KC, Schwartz WJ 3rd, Rayburn WF, Turnbull G. A cost-minimization analysis of intracervical prostaglandin E2 for cervical ripening in an outpatient versus inpatient setting. Clin Ther. 1996 Jul http://www.ncbi.nlm.nih.gov/pubmed/8879901
Incerpi MH; Fassett MJ; Kjos SL; Tran SH; Wing DA Vaginally administered misoprostol for outpatient cervical ripening in pregnancies complicated by diabetes mellitus. Am J Obstet Gynecol. 2001 Oct;185(4):916-9. http://www.ncbi.nlm.nih.gov/pubmed/11641678
Kipikasa JH, Adair CD, Williamson J, Breen JM, Medford LK, Sanchez-Ramos L. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005 Feb;88(2):108-11. http://www.ncbi.nlm.nih.gov/pubmed/15694083
McKenna DS; Duke JM Effectiveness and infectious morbidity of outpatient cervical ripening with a Foley catheter. J Reprod Med. 2004 Jan;49(1):28-32. http://www.ncbi.nlm.nih.gov/pubmed/14976792
McKenna DS; Ester JB; Proffitt M; Waddell KR Misoprostol outpatient cervical
ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol.
2004 Sep;104(3):579-84.
http://www.ncbi.nlm.nih.gov/pubmed/15339772
Meyer M; Pflum J; Howard D Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005 Mar;105(3):466-72. http://www.ncbi.nlm.nih.gov/pubmed/15738009
Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent
induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand.
2005 Jul;84(7):628-31.
http://www.ncbi.nlm.nih.gov/pubmed/15954870
O'Brien JM; Mercer BM; Cleary NT; Sibai BM Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1995 Dec;173(6):1855-9. http://www.ncbi.nlm.nih.gov/pubmed/8610775
Sawai SK; Williams MC; O'Brien WF; Angel JL; Mastrogiannis DS; Johnson L Sequential outpatient application of intravaginal prostaglandin E2 gel in the management of postdates pregnancies. Obstet Gynecol. 1991 Jul;78(1):19-23. http://www.ncbi.nlm.nih.gov/pubmed/2047061
Sawai SK; O'Brien WF Outpatient cervical ripening. Clin Obstet Gynecol. 1995
Jun;38(2):301-9.
http://www.ncbi.nlm.nih.gov/pubmed/7554598
Sciscione AC; Muench M; Pollock M; Jenkins TM; Tildon-Burton J; Colmorgen GH Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001 Nov;98(5 Pt 1):751-6. http://www.ncbi.nlm.nih.gov/pubmed/11704164
Stitely ML, Browning J, Fowler M, Gendron RT, Gherman RB. Outpatient cervical
ripening with intravaginal misoprostol. Obstet Gynecol. 2000 Nov;96(5 Pt 1):684-8.
http://www.ncbi.nlm.nih.gov/pubmed/11042301
ACOG
New U.S. Food and Drug Administration labeling on Cytotec (misoprostol) use and pregnancy. ACOG Committee Opinion No. 283. American College of Obstetricians and Gynecologists. Obstet Gynecol 2003;101:1049–50. http://www.ncbi.nlm.nih.gov/pubmed/12834952
Induction of Labor Practice Bulletin NUMBER 10, NOVEMBER 1999. American College of Obstetricians and Gynecologists, Washington, DC
Cochrane Database
Abstract
BACKGROUND: Misoprostol (Cytotec, Searle) is a prostaglandin E1 analogue widely
used for off-label indications such as induction of abortion and of labour. This
is one of a series of reviews of methods of cervical ripening and labour induction
using standardised methodology.
OBJECTIVES: To determine the effects of vaginal misoprostol for third trimester
cervical ripening or induction of labour.
SEARCH STRATEGY: The Cochrane Pregnancy and Childbirth Group trials register
(February 2004), the Cochrane Central Register of Controlled Trials (The Cochrane
Library, Issue 4, 2003) and bibliographies of relevant papers.
SELECTION CRITERIA: Clinical trials comparing vaginal misoprostol used for third
trimester cervical ripening or labour induction with placebo/no treatment or
other methods listed above it on a predefined list of labour induction methods.
DATA COLLECTION AND ANALYSIS: A strategy was developed to deal with the large
volume and complexity of trial data relating to labour induction. This involved
a two-stage method of data extraction.
MAIN RESULTS: Seventy trials have been included. Compared to placebo, misoprostol
was associated with reduced failure to achieve vaginal delivery within 24 hours
(relative risk (RR) 0.36, 95% confidence interval (CI) 0.19 to 0.68). Uterine
hyperstimulation, without fetal heart rate changes, was increased (RR 11.66 95%
CI 2.78 to 49).
Compared with vaginal prostaglandin E2, intracervical prostaglandin E2 and oxytocin,
vaginal misoprostol was associated with less epidural analgesia use, fewer failures
to achieve vaginal delivery within 24 hours and more uterine hyperstimulation.
Compared with vaginal or intracervical prostaglandin E2, oxytocin augmentation
was less common with misoprostol and meconium-stained liquor more common. Lower
doses of misoprostol compared to higher doses were associated with more need
for oxytocin augmentation and less uterine hyperstimulation, with and without
fetal heart rate changes. Information on women's views is conspicuously lacking.
CONCLUSIONS: Vaginal misoprostol in doses above 25 mcg four-hourly was more effective
than conventional methods of labour induction, but with more uterine hyperstimulation.
Lower doses were similar to conventional methods in effectiveness and risks.
The studies reviewed were not large enough to exclude the possibility of rare
but serious adverse events, particularly uterine rupture, which has been reported
anecdotally following misoprostol induction. The authors request information
on cases of uterine rupture known to readers. Further research is needed to establish
the ideal route of administration and dosage, and safety. Professional and governmental
bodies should agree guidelines for the use of misoprostol, based on the best
available evidence and local circumstances.
Hofmeyr GJ, Gülmezoglu AM. Vaginal
misoprostol for cervical ripening and induction of labour. Cochrane Database
of Systematic Reviews 2003, Issue 1. Art. No.: CD000941. DOI: 10.1002/14651858.CD000941.
http://www.ncbi.nlm.nih.gov/pubmed/12535398
Indian Child Health Notes - Steve Holve, Pediatrics Chief Clinical Consultant
Quote of the month
“The function of the imagination is not to make strange things settled,
so much as to make settled things strange.”
G.K. Chesterton
Article of Interest
Universal screening for hearing loss in
newborns: US Preventive Services Task Force
Pediatrics. 2008 Jul;122(1):143-8. http://pediatrics.aappublications.org/cgi/content/full/122/1/143
The USPSTF recommends screening for hearing loss in all newborn infants stating that there is a high certainty that the net benefit is moderate to substantial. This follows on the recommendations of the Joint Committee on Infant Hearing that first recommended newborn hearing screening in 2000.
It is estimated that 1-3/1,000 live born infants have significant congenital hearing loss. Previous efforts with targeted screening of high-risk newborns missed over 50% of affected infants leading to the new recommendation for universal screening. Most programs involve a two-step process in which the first screen is an otoacoustic emission test and follow-up is done by auditory brainstem response. This procedure yields a screening sensitivity of 0.92 and a specificity of 0.98
There is also substantial evidence that early identification and intervention of hearing loss before 6 months of age will result in marked benefits. Children that receive timely intervention services perform 20-40 percentile points higher in vocabulary, social adjustment and behavior at 8 years of age. Intervention can include augmentation devices, cochlear implants or acquisition of sign language.
Editorial Comment
Since the first recommendation in 2000 that infants be screened for hearing loss the percentage of infants screened at birth has increased from 38% up to 95%. However, almost half of children who fail their first screen do not receive appropriate or timely follow-up care. Ensuring that all infants receive timely intervention is now our greatest challenge in newborn hearing screening. Reviewing the results of newborn hearing screening should be a part of the two week and six week well child care visit.
Recent literature on American Indian/Alaskan Native Health
Michael L. Bartholomew, MD
Wood D, Winterbauer N, Sloyer P, Jobli E, Hou T, McCaskill Q, Livingood WC.
A Longitudinal Study of a Pediatric Practice-based Versus an Agency-Based Model
of Care Coordination for Children and Youth with Special Health Care Needs. Matern
Child Health J. 2008.
http://www.ncbi.nlm.nih.gov/pubmed/18766431
Children with Special Health Care Needs (CSHCN) is defined as “those who have or are at increased risk for a chronic physical, developmental, behavioral, or emotional condition and who also require health and related services of a type or amounts beyond that required by children generally.”1 Recent estimates indicate that CSHCN account for approximately 14% of all U.S. children (10.2 million) and roughly 70% of all health care expenditures.1,2 The American Academy of Pediatrics defines the characteristics of the medical home as a primary care delivery model that is “accessible, continuous, comprehensive, family-centered, coordinated, compassionate, and culturally effective.”3 Care coordination, as a part of the medical home model, has been proven to be a vital piece of any integrated health services system for CSHCN. Positive outcomes such as patient satisfaction, reduced health care costs, reduced delay in care, and fewer hospitalizations have all been shown to be related to care coordination and the medical home model.1 In 2008, Robert McSwain, Director of the Indian Health Service, included the development of a “medical home” in his vision statement for improving the health care for patients.”4 Currently, tribal clinics and service units are advancing towards care coordination and the establishment of a medical home.
This prospective cohort study compares agency-based care coordination with practice-based models for CSHCN. Three pediatric practices that utilize an agency based model of care coordination were compared to three practices that utilize nurse care coordination and received medical home training. Families of CSHCN were monitored over 18 months through base-line and follow-up surveys. Families rated four care coordination measures: 1.) Help with needed services 2.) Support from the care coordinator 3.) Satisfaction with care coordination services 4.) Barriers to getting health services. Additionally, parents rated of pediatric services including treatment by the office staff, communication with the pediatrician, partnering in decision-making, and connecting to outside resources. Parents with higher scale scores at follow-up than at baseline were classified as “improved” whereas scores lower or equal at follow-up were labeled as “not improved.”
Although practiced based care coordination showed no significant difference in mean change scores between baseline and follow-up for the four care coordination measures, it had higher percentages of “improved” scores than did the agency based model. Of the ratings of pediatric services, practice based care coordination had a higher percentage of “improved” in one measure (treatment by office staff), while the percentages in the remaining measures were similar.
Despite the studies limitations (transient target population, lack of randomization, and combination of the medical home training and practice based care coordination in the participating practices possibly influencing favorable responses), the authors conclude that practice base care coordination leads to increased family satisfaction in the quality of care and the reduction of barriers to care for CSHCN.
References:
1American Academy of Pediatrics. (2005). Care Coordination in the Medical Home: Integrating Health and Related Systems of Care for Children With Special Health Care Needs. Policy Statement. Pediatrics, 116, 1238-1244.
2American Academy of Pediatrics. (2002). The Medical Home. Policy Statement. Pediatrics, 110, 184-186.
3American Academy of Pediatrics. The National Center of Medical Home Initiatives for Children With Special Health Care Needs. http://www.medicalhomeinfo.org/index.html
4Robert G. McSwain, Indian Health Service Director. Vision for the Indian Healthcare System.
Information Technology - Theresa Cullen, Chief Information Officer, IHS
RPMS iCare Population Management GUI v1.1 patch 2
Release Announcement Sept 8, 2008
Please notice the release of the iCare v1.1 Patch 2 and request that your Site
Manager load the patch as soon as possible on your RPMS server. An identified
problem in the Immunization Forecaster is causing server memory problems when
used by iCare. This patch will disable the iCare process to review and
display Immunization Forecaster Reminders in iCare panels. These Forecaster
reminders will, however, continue to display on individual Patients Records in
iCare.
We anticipate that the Immunization application will release a patch to fix the memory leak problem within the next few months. At that time, iCare will reinstate panel (population) identification and display of Immunization Forecaster reminders.
This patch does not affect the display of the other Immunization Health Maintenance Reminders in users' iCare panels.
The beta sites for this version of iCare were:
- Lame Deer Health Center
- Ft. Washakie Health Center
- Citizen Potawatomi Nation
For user support please contact the RPMS help desk at RPMSHelp@ihs.gov or call (888) 830-7280.
International Health Update - Claire Wendland, Madison, WI
Preventing and treating HIV/AIDS in Thai prisons
As antiretroviral therapy for HIV reaches poor and middle-income countries at last, health workers involved in the scale-up of treatment programs have increasingly tried to reach marginalized groups to offer testing and treatment. Treating such groups can be seen as both a moral and a public health imperative: in many countries, intravenous drug users, migrant laborers, men who have sex with men, and prisoners are sources of transmission in the community at large.
An article by David Wilson and colleagues from Doctors Without Borders (MSF) gives some sense of the difficulties and potential of prison-based programs. The health services at two large prisons in Bangkok asked MSF to provide clinical support for treatment of HIV in 2003. Once the MSF staff had established a good relationship with the prisons, they were also invited to expand into prevention. In Thailand, prisons are more than 50% over capacity. When out of their cells during the day, inmates stay in large “common rooms” packed with hundreds of others. Health services are poorly staffed and underfunded, but health needs, especially HIV-related needs, are high. Intravenous drug users, sex workers, and undocumented migrants (mostly from Burma/Myanmar, in which civil conflict rages) make up a huge proportion of the prison population – well over half of those imprisoned are there for drug-related offenses – and all three of these groups are at particularly high risk for HIV. Perhaps it is no surprise then that that HIV seroprevalence rates in prisoners are at least fifteen times as high as those in the general population (compared to roughly ten times as high in the United States, though in both cases the estimates are based on fairly limited data). In Thailand, this high rate is probably in large part a result of the considerable population of intravenous drug users in prison, but further spread of HIV in prison is related to unsafe sex – both consensual and non-consensual. To a much lesser extent, sharing of drug injection equipment and unsafe tattooing also contribute to in-prison HIV transmission.
Diagnosis is usually made when a prisoner presents with an opportunistic infection. Sometimes prisoners seek testing after other prisoners have died, though many are legitimately worried about maintaining confidentiality. Once diagnosed, taking one’s medicines in these overcrowded settings can be problematic because the disease is stigmatized, but Wilson and colleagues report that peer support systems (a central part of Thailand’s national HIV/AIDS strategy) have been very helpful in easing stigma and encouraging adherence to medication regimens. Treatment is more challenging once prisoners have left the facility. Frequent transfers of inmates to smaller, less crowded prisons can make follow-up tricky. It is more difficult yet to make sure prisoners can continue treatment once they are released; Thailand’s national health insurance program covers therapy, but undocumented migrants or those who have lost their ID cards face real obstacles in accessing care, and most prisoners have poor social support on release.
Effective prevention programs have also been a challenge. Inmates will listen to educational programs, but the disconnect between what is supposed to happen in prison and what actually does happen poses problems. For instance, prison guards, though they express strong support for HIV prevention, are generally not willing to participate in condom distribution, which they see as sanctioning sex. Nonetheless, Wilson and colleagues see some softening of these attitudes and have hope for the long-term success of both prevention and treatment programs. They encourage colleagues around the world by concluding that “barriers that prevent the provision of treatment in prisons when it is available outside are not technical or financial, but political.”
Wilson D, Ford N, Ngammee V, Chua A, Kyaw MK. HIV prevention, care, and treatment in two prisons in Thailand. PLoS Med. 2007 Jun;4(6):e204. http://www.ncbi.nlm.nih.gov/pubmed/17593894
MCH Alert
Report Explores Social, Emotional, and Physical Health and Workplace Challenges of Women in the Postpartum Period
New Mothers Speak Out: National Survey Results Highlight Women's Postpartum Experiences presents results from Listening to Mothers II and Listening to Mothers II Postpartum, two national surveys of women who gave birth in U.S. hospitals in 2005. The report, produced by Childbirth Connections, discusses women's experiences from before conception through the early postpartum months. The content is presented in four parts: maternal well-being; child well-being; family and relationships; and employment, maternity leave, and child care. A conclusion is provided. The appendices contain information about the survey methodology and a table comparing survey results to national birth records. The report and a fact sheet, titled Quick Facts from National New Mothers Speak Out Report: Challenges for Mothers with Young Children and Opportunities for Improvement, are available at http://www.childbirthconnection.org/article.asp?ck=10413.
Monograph Focuses on Opportunities to Improve Adolescent Health and Prevent Cardiovascular Disease
Prevention and Adult Cardiovascular Disease
Among Adolescents: Focusing on Risk Factor Reduction adopts a life-course health
development approach to cardiovascular disease (CVD); it examines the preventable
factors that lead to CVD by emphasizing how health problems in childhood and
adolescence directly affect cardiovascular health throughout the lifespan. The
monograph, produced by the National Institute for Health Care Management Research
and Educational Foundation with support from the Health Resources and Services
Administration's Maternal and Child Health Bureau, examines the prevalence of
CVD, its risk factors, and
promising strategies that health professionals and health plans can adopt to
reduce adolescent risk factors for developing CVD in the future. The discussion
is guided by recommendations for adolescent clinical preventive services and
the Healthy People 2010 Guidelines. Selected resources on reducing adolescent
risk factors for future chronic disease are included. The monograph is available
at http://www.nihcm.org/pdf/CVDPrevention_FINAL.pdf.
Special Notice: New Name Signals an Expanded Focus for The National Sudden Infant Death Syndrome and Other Infant Death (SIDS/ID) Cooperative Agreement Program
The National Sudden and Unexpected Infant/Child Death and Pregnancy
Loss Cooperative Agreement Program reflects both a new name and a broader focus
for the SIDS/ID Program. Supported by the federal Maternal and Child Health Bureau,
this cooperative agreement program
comprises a national consortium of four centers. Like many state and local initiatives,
the national consortium has expanded its program to include pregnancy loss (i.e.,
miscarriage) and stillbirth, as well as sudden and unexpected infant and child
death. All four centers serve this mission, yet each center has a unique purpose
and provides distinct resources and services. The centers maintain close collaborative
relationships as they address cross-cutting issues. They serve a broad constituency
in the public and private sectors, as well as individuals. Core services are
briefly listed below, along with contact information.
* The National Sudden and Unexpected Infant/Child Death and Pregnancy Loss Resource
Center at Georgetown University serves as a gateway to critical information on
risk reduction, prevention, and bereavement for pregnancy loss, stillbirth, and
sudden unexpected infant and child death.
Web: http://www.sidscenter.org
E-mail: info@sidscenter.org
Phone: (866) 866-7437
* The National Sudden and Unexpected Infant/Child Death and Pregnancy Loss Program
Support Center provides education, training, advocacy, and bereavement services,
including a 24-hour bilingual bereavement counseling helpline.
Web: http://www.firstcandle.org
E-mail: info@firstcandle.org
Phone: (800) 221-7437
* The National Sudden and Unexpected Infant/Child Death and Pregnancy Loss --
Project IMPACT serves as the communications hub for a national network of fetal,
infant, and child mortality programs -- convening, connecting, and providing
technical support to state and local efforts.
Web: http://www.sidsprojectimpact.com
E-mail: info@sidsprojectimpact.com
Phone: (800) 930-7437
* The National Sudden and Unexpected Infant/Child Death and Pregnancy Loss Project
at the National Center for Cultural Competence provides technical assistance
and develops resources on cultural and linguistic competence to help programs
effectively address racial and ethnic disparities in perinatal, infant, and child
mortality and pregnancy loss.
Web: http://www11.georgetown.edu/research/gucchd/nccc/projects/sids
E-mail: cultural@georgetown.edu
Phone: (800) 788-2066
Partners Engage in Focused Effort to Improve the Oral Health of Pregnant Women
The National Maternal and Child Oral Health Resource Center (OHRC)
has announced the availability of three new publications that provide ways to
improve access to oral health services for pregnant women as well as oral-health-promotion
and disease-prevention information for women and their families. The publications
were developed with support from the Health Resources and Services Administration's
Maternal and Child Health Bureau (MCHB) in follow-up to MCHB's Research to Policy
and Practice Forum: Periodontal Health and Birth Outcomes, held on December 11-12,
2006, in Washington, DC. Forum participants concluded that while scientific evidence
on the periodontal-preterm birth relationship remains inconclusive, sufficient
information and interest exists to warrant pursuing periodontal health strategies
that improve the oral health of pregnant women. Partners from the Altarum Institute,
the American Academy of Pediatric Dentistry, the American College of Obstetricians
and Gynecologists, the American Dental Association, the American Dental Education
Association, the Association of Maternal and Child Health Programs, the Children's
Dental Health Project, OHRC, and the National Oral Health Policy Center met regularly
over the past year and prepared the following documents:
* Access to Oral Health Care During the Perinatal Period: A Policy Brief provides an overview of the major barriers to addressing women's oral health needs during the perinatal period. Specific examples of strategies to promote the use of guidelines during the perinatal period, expand opportunities for professional and consumer education, increase dental insurance coverage, and integrate oral health care as a part of routine perinatal care are included. Evidence from the professional, peer-reviewed literature is cited throughout the document. The policy brief is available at http://www.mchoralhealth.org/PDFs/PerinatalBrief.pdf.
* Oral Health Care During Pregnancy: A Summary of Practice Guidelines summarizes the New York State Department of Health's publication, Oral Health Care During Pregnancy and Early Childhood: Practice Guidelines, which is geared toward prenatal and oral health professionals. The guidelines, intended to bring about changes in the health care delivery system and to improve the overall standard of care for pregnant women, are available at http://www.mchoralhealth.org/PDFs/Summary_PracticeGuidelines.pdf.
* Two Healthy Smiles: Tips to Keep You and Your Baby Healthy (brochure) is designed to educate women about the importance of oral hygiene and oral health care during pregnancy. Topics include brushing, flossing, eating healthy foods, and getting dental checkups and treatment. Additional topics include the impact of hormonal changes during pregnancy on gum health, caring for an infant's gums and teeth, and finding a dentist. The content is available electronically as an 8-1/2 x 11" fact sheet at http://www.mchoralhealth.org/PDFs/PregnancyBrochure.pdf.
Readers: The above-mentioned publications are also available in hard copy at no charge from the HRSA Information Center at http://www.ask.hrsa.gov. Additional resource materials on pregnancy and oral health are available from OHRC at http://www.mchoralhealth.org/materials/perinatal.html.
MCH Headlines - Judy Thierry HQE
New American Academy of Pediatrics (AAP) Resources for Vaccination
1. AAP offers new parent handouts on vaccine safety
The American Academy of Pediatrics is providing several new resources to help
you answer parents' questions about immunization. A one-page fact sheet on vaccine
safety and a back-and-front parent handout … for patient visits... These
documents have been reviewed for scientific accuracy by the AAP Committee on
Infectious Diseases. Facts for Parents about Vaccine Safety: http://www.cispimmunize.org/fam/facts/VaccineSafety_English.doc
Vaccine Safety: The Facts: http://www.cispimmunize.org/pro/pdf/VaccineSafety_parenthandout.pdf
2. AAP president speaks at national news conference today supporting
influenza and immunization
The National Foundation for Infectious Diseases hosted a news conference in Washington,
DC, to urge the public to follow the latest influenza and pneumococcal immunization
recommendations from the U.S. Centers for Disease Control and Prevention. The
newly expanded recommendations – endorsed by the AAP – include annual
influenza vaccination for all children between six months and 18 years of age.
AAP President Renee R. Jenkins, MD, FAAP, spoke at the news conference about
the impact of flu on school-age children and the benefits of vaccination in this
population. Dr. Julie Gerberding, director of the CDC, said it anticipates a
plentiful supply of 143 million doses of flu vaccine this year.
3. AAP statement on vaccines read on today's Oprah
The Oprah show today featured celebrity Jenny McCarthy, whose book "Mother
Warriors: A Nation of Parents Healing Autism Against All Odds" was released
this week. The producers declined an offer by the AAP to have a pediatrician
appear on the show or be in the audience. The producer did invite the Academy
to submit a statement (see below), part of which Oprah read on air. Statement
from American Academy of Pediatrics (AAP) to be read on Oprah:
“Pediatricians care for children with autism every day and understand how
devastating this diagnosis can be for a family. We know parents are seeking answers,
and we share their frustration over the undefined causes of autism and the lack
of an established treatment. The American Academy of Pediatrics is calling for
more studies to answer these questions.
About treatments:
The AAP urges parents to be cautious when choosing treatment options for autism.
The AAP recommends scientifically validated treatments for autism. Some alternative
treatments, such as chelation, are untested and can be dangerous.
About vaccines:
There is no valid scientific evidence that vaccines cause autism. But because
of unfounded fears about vaccines, the U.S. is suffering its biggest measles
outbreak in a decade. These are not benign diseases. Measles and other vaccine-preventable
infections can have serious consequences, including seizures, brain damage and
even death.
The AAP urges parents who have questions about vaccines or autism to talk to their pediatrician. For more information, visit www.aap.org.
4. Pediatricians to appear on ABC News Now Monday to discuss autism -- Lou Cooper, MD, FAAP, former AAP president, and Ari Brown, MD , FAAP, are scheduled to appear Monday, Sept. 29, 2008 on the cable news network ABC News Now in an hour-long autism special with host Dr. Tim Johnson. The guest list also includes actress Didi Conn, actor Joe Mantegna, and Ken Reibel, the parent of an autistic son, who supports immunization. The video will also be posted on the ABC Web site, where it will anchor a collection of patient resources on autism.
5. AAP Web site features graphic photos of vaccine-preventable diseases--Many young parents today have never seen a case of measles, chickenpox or polio. The AAP has assembled a collection of graphic photos of vaccine-preventable diseases that illustrate how serious these diseases can be. You will find a link to the photos, as well as additional parent-friendly materials, at http://www.cispimmunize.org.
6. Amanda Peet public service announcement on immunizations is available to members-- This PSA is part of a campaign by the AAP and Every Child by Two to promote the benefits of immunization. Pediatricians who would like copies can email info@ecbt.org with their name, mailing address, phone number and the number of DVDs they would like. The first DVD is free, and additional copies are $10 each. The videos can also be viewed free at http:// www.vaccinateyourbaby.org.
7. Immunization Alliance issues call to action--The Immunization Alliance last week issued a national call to Action urging policymakers, public health agencies, physicians and the public to work together to preserve the health of the nation’s children through immunization. Included are requests for a public information campaign by the government; a commitment to ongoing research to ensure the continued safety, efficacy and development of vaccines; balanced reporting by the media; continued efforts from doctors in working with parents; and confidence from parents themselves. You can see the full Call to Action and accompanying news release at http://www.aap.org/advocacy/releases/sept08Immunizationalliance.htm. The Call to Action generated widespread national media coverage, including a story by the Associated Press that appeared in an impressive 400 newspaper and broadcast outlets. The alliance is a coalition of 25 national organizations, including the AAP, that support immunizations.
8. Dr. Paul Offit's book on autism receives favorable review in Wall Street Journal --"Autism's False Prophets," a new book by Paul A. Offit, MD, FAAP, traces the history of autism research and how bad science put children's health at risk. The book has received favorable coverage in the media, including a positive review Tuesday in the Wall Street Journal. Read the review at http://online.wsj.com/article/SB122212979072465559.html.
Preventing Alcohol, Tobacco, and Other Substance-exposed Pregnancies:
A Community Affair
After this 2 day meeting on supported by NIAAA and the Legacy
Foundation and upon referencing some content for one of the breakout sessions,
I discovered this site and the state-by-state data on alcohol and tobacco’s
impact. Four aspects on the personal toll, state level data, crashes and
a series of editorials on the human costs in all of our lives are clickable.
Please take a moment to look at your respective state or states and pass this
on.
http://www.fdlreporter.com/apps/pbcs.dll/article?AID=/99999999/WIS0110/399990694/1979
The workgroup is proceeding to look at what effective messages are and how they are created. Which ones reach segmented audiences and promote screening, assessment, referral and appropriate treatment and management across the disease spectrum. Look for a web cast on media and marketing effective public health messages this season.
Medical Mystery Tour - Neil Murphy, Southcentral Foundation; ANMC
Fetal Heart Monitoring: The answers applied
As you have seen in the last two CCCC issues*, we have been defining terms in fetal heart monitoring so we have a common set of expectations. Now how do we use those terms in clinical practice?
Key Points from 2008 NICHD Electronic Fetal Monitoring Workshop
1. Uterine Contractions. The original report did not
have guidelines for the interpretation of uterine contractions. Uterine
contractions are quantified as the number of contractions present in a 10-minute
window, averaged over 30 minutes.
Normal: ≤5
contractions in 10 minutes
Tachysystole: >5
contractions in 10 minutes
*should
be qualified as to the presence or absence of FHR decelerations
*may
occur in spontaneous or stimulated labor
*hyperstimulation
and hypertonus and hypercontractility are discouraged / no longer
used.
2. Quantitation of Decelerations. Decelerations are defined as recurrent if they occur with ≥50% of uterine contractions in any 20-minute window and intermittent if they occur with <50% of uterine contractions in any 20-minute window.
3. General Considerations. The following general considerations
were emphasized:
a. FHR
response is a dynamic process and evolves over time; FHR patterns require frequent
assessment.
b. The FHR
tracing should be interpreted in its context and any categorization of a FHR
tracing is limited to the time period of that particular assessment
c. Presence
of accelerations reliably predicts the absence of fetal metabolic acidemia, but
their absence does not predict the presence of acidemia. Similarly, moderate
FHR variability reliably predicts the absence of fetal metabolic acidemia, but
its absence does not predict the presence of acidemia.
4. Interpretation System. A three-tier interpretation
system was proposed to aid in reading, communicating, and managing FHR tracings.
The best way to learn this system is to know categories I and II; all other FHR
patterns are category II:
a. Category I
(Normal): Strongly predictive of normal fetal acid-base
status; may be followed in a routine manner. These tracings include all of
the following:
- Baseline rate 110-160 bpm
- Moderate variability
- Absent late or variable decelerations
- Early decelerations may or may not be present
- Accelerations may or may not be present
b. Category II
(Indeterminate): Not predictive of abnormal fetal
acid-base status, require evaluation and continued surveillance and reevaluation,
taking into account the clinical context.
c. Category III
(Abnormal): Predictive of abnormal fetal acid base
status; require prompt evaluation and management. Include either:
- Absent baseline FHR variability and any of the following:
- Recurrent (see above) late decelerations
- Recurrent (see above) variable decelerations
- Bradycardia
- Sinusoidal
Editorial Comment
Please update your maternity care guidelines to include the above criteria
The Indian Health system have spent the last several years attempting to improve
the use of electronic fetal monitoring (EFM) by encouraging the use of the NICHD
EFM criteria from 1997. The criteria apply to all nurses, residents, midwives,
and attending physicians who are involved in maternity care. This is a major
accomplishment and is a key factor our efforts to improve the patient safety
climate in our system.
In April 2008 the NICHD directed a workshop to revisit the original 1997 NICHD recommendations; the updated definition and interpretation standards were published in September 2008 (see Hot Topics – Obstetrics). We encourage you to look over this publication, but in an effort to make you aware of the key changes. Continued adherence to the NICHD standards is critical to keep up our high levels of standards for care and communication.
In each of your Service Units and facilities you need to update your maternity care guidelines to include the above criteria.
Resources
Electronic fetal heart rate monitoring: research guidelines for interpretation.
National Institute of Child Health and Human Development Research Planning Workshop.
Am J Obstet Gynecol. 1997 Dec;177(6):1385-90. http://www.ncbi.nlm.nih.gov/pubmed/9423739
Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute
of Child Health and Human Development workshop report on electronic fetal monitoring:
update on definitions, interpretation, and research guidelines. Obstet Gynecol.
2008 Sep;112(3):661-6.
http://www.ncbi.nlm.nih.gov/pubmed/18757666
*Fetal
Heart Monitoring: The answers
http://www.ihs.gov/MedicalPrograms/MCH/M/ob.cfm?module=9_08ft#mmt
Medscape*
A sample of CME offerings on the Medscape website:
Atrophic Vaginitis: An Undertreated Epidemic, Part I and Part II
http://www.medscape.com/viewprogram/15704
http://www.medscape.com/viewprogram/14931
How to Assess Familial Risk of Cancer: Red Flags & Pearls
http://www.medscape.com/viewprogram/17063
Women's Health: Menopausal Patients and Insomnia -- A Case Study
http://www.medscape.com/viewprogram/17161
Women and Sleep: The Life Cycle
http://www.medscape.com/viewprogram/17198
Impact of Bodyweight and Lifestyle on IVF Outcome
http://www.medscape.com/viewprogram/17192
Gestational Diabetes Mellitus: Postpartum Opportunities For the Diagnosis
and Prevention of Type 2 Diabetes Mellitus
http://www.medscape.com/viewprogram/17234
General Links to Medscape:
Ask the Experts topics in Women's Health and OB/GYN Index, by specialty, Medscape
http://www.medscape.com/pages/editorial/public/ate/index-womenshealth
OB GYN & Women's Health Clinical Discussion Board Index, Medscape
http://boards.medscape.com/forums?14@@.ee6e57b
Clinical Discussion Board Index, Medscape
Hundreds of ongoing clinical discussions available
http://boards.medscape.com/forums?14@@.ee6e57b
Free CME: MedScape CME Index by specialty
http://www.medscape.com/cmecenterdirectory/Default
*NB: Medscape is free to all, but registration is required. It can be accessed from anywhere with Internet access. You just need to create a personal username and password.
Menopause Management
Cochrane Update: Oestrogens for Preventing Recurrent Urinary Tract Infection in Postmenopausal Women
BACKGROUND: Recurrent urinary tract infection (RUTI) is defined
as three episodes of urinary tract infection (UTI) in the previous 12 months
or two episodes in the last six months. The main factors associated with RUTI
in postmenopausal women are vesical prolapse, cystocoele, post-voidal residue,
and urinary incontinence, all associated with a decrease in estrogen. The use
of estrogens to prevent RUTI has been proposed.
OBJECTIVES: To estimate the efficacy and safety of oral or vaginal
estrogens for preventing RUTI in postmenopausal women.
SEARCH STRATEGY: We searched the Cochrane Renal Group's specialized
register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE
(from 1950), EMBASE (from 1980), and reference lists of articles without language
restriction. Date of last search: February 2007.
SELECTION CRITERIA: Randomized controlled trials (RCTs) in which
postmenopausal women (more than 12 months since last menstrual period) diagnosed
with RUTI received any type of estrogen (oral, vaginal) versus placebo or any
other intervention were included.
DATA COLLECTION AND ANALYSIS: Authors extracted data and assessed
quality. Statistical analyses were performed using the random effects model,
and the results were expressed as relative risk (RR) for dichotomous outcomes
or mean difference (WMD) for continuous data with 95% confidence intervals (CIs).
MAIN RESULTS: Nine studies (3,345 women) were included. Oral
estrogens did not reduce UTI compared to placebo (4 studies, 2,798 women: RR
1.08, 95% CI 0.88 to 1.33). Vaginal estrogens versus placebo reduced the number
of women with UTIs in two small studies using different application methods.
The RR for one was 0.25 (95% CI 0.13 to 0.50) and 0.64 (95% CI 0.47 to 0.86)
in the second. Two studies compared oral antibiotics versus vaginal estrogens
(cream [1], pessaries [1]). There was very significant heterogeneity and the
results could not be pooled. Vaginal cream reduced the proportion of UTIs compared
to antibiotics in one study and in the second study antibiotics were superior
to vaginal pessaries. Adverse events for vaginal estrogens were breast tenderness,
vaginal bleeding or spotting, nonphysiologic discharge, vaginal irritation, burning,
and itching.
AUTHORS' CONCLUSION: Based on only two studies comparing vaginal estrogens to
placebo, vaginal estrogens reduced the number of UTIs in postmenopausal women
with RUTI; however, this varied according to the type of estrogen used and the
treatment duration.
Oestrogens for preventing recurrent urinary tract infection in postmenopausal
women. Obstet Gynecol. 2008 Sep;112(3):689-90. http://www.ncbi.nlm.nih.gov/pubmed/18757671
Midwives Corner - Lisa Allee, CNM, 4 Corners Regional Health Center, Red Mesa, AZ
The conflict and compromises between how we want to practice midwifery and how we do practice midwifery in the hospital setting
Hunter’s qualitative research from the United Kingdom gives voice to something I would guess many midwives have experienced consciously or unconsciously when working in the hospital setting. She looked at the emotional work of midwives and found that rather than it being located in the midwife-patient relationships as found in other studies, it was generated by the conflict between a strong belief in the “with woman” ideology of the midwifery model and the pressures within a hospital setting to adhere to the ideology of “with institution” which frequently means providing care in prescribed ways rather than in ways that are individualized to the woman being cared for. This in congruency between how midwives truly want to practice and how they are pressured to practice in institutions creates emotional work. She found that midwives working in community-based practices, however, had very little of this conflict. Hunter makes suggestions for dealing with this conflict in the hospital-based setting. First and foremost she suggests the prime importance of explicitly recognizing this conflict and recognizing it as a universal issue rather than what is done currently which is interpreting it as a personal dilemma which often leads to guilt and self-blame—the individual midwife feels she is failing her patients instead of recognizing this as a system problem. She suggests short-term strategies of acknowledging the with-woman model of care ideals and the current realities of practice and providing education and support about the dilemmas that can arise. Then she discusses more long term strategies such as moving normal births to community-based midwifery with birth occurring either at home or in midwifery-led birth centers. She also discusses recognizing the hospital-based midwife as an expert in abnormal midwifery skilled in managing technology, caring for women with complications and still providing midwifery-model based care.
Hunter B. Conflicting ideologies as a source of emotion work in midwifery. Midwifery. 2004 Sept;20(3):261-72. http://www.ncbi.nlm.nih.gov/pubmed/15337282
Navajo News - John Balintona, Shiprock
Navajo Area Women’s Health Provider Meeting
The Navajo Area Women’s Health Provider Meeting was held at Chinle, Arizona on September 12, 2008. Women’s health care providers from the Navajo Nation attended the annual meeting to discuss pertinent topics, earn CME, and renew friendships and associations. Over the next several issues, selected summaries from the meeting will be published.
Annual Four Corners CNM Meeting
The annual meeting of the Four Corners Certified Nurse Midwives was held in conjunction with the Navajo Area Women’s Health Provider Meeting in Chinle, Arizona. Certified Nurse Midwives from Chinle, Fort Defiance, Gallup, Shiprock, Kayenta, and Tsaile were present. Several topics were presented including promotion of breastfeeding on the Navajo Nation and support for pending legislation at the Navajo Nation Council aimed at eliminating barriers for breastfeeding at the workplace. Discussion took place as to the action by area CNMs to counteract the advertising pressure from formula companies and their affect on the feeding practices of newborns. The Four Corners chapter re-affirmed the close relationship between midwifery and the Navajo Area Indian Health Service. The midwives discussed the Centering Pregnancy Program for group prenatal care. Several sites noted preparations for starting programs at the service units starting in 2009. The group discussed several barriers and solutions for starting a Centering Pregnancy Program to include; space issues, scheduling of care conferences, maintaining cultural relevance, and provider concerns.
Continuing Medical Education: Preterm Labor Management
Attendees at the meeting were fortunate to earn CME credits by attending a presentation by Monique Lin, MD, perinatologist from the Phoenix Perinatal Associates. The topic of Dr. Lin’s presentation concerned current information regarding several aspects of preterm labor management.
Highlights:
- Magnesium Sulfate may be losing favor as a primary tocolytic. (Obstet Gynecol. 2006 Oct;108(4):986-9)
- Magnesium Sulfate may reduce the risk of cerebral palsy in fetuses at risk for preterm birth. (N Engl J Med. 2008 Aug 28;359(9):895-905)
- Phoenix Perinatal Associates recommends continued use of Magnesium Sulfate for maternal transport.*
- Antepartum Progesterone for preterm labor
- 17 hydroxyprogesterone caproate has been shown to reduce preterm birth for those at risk for preterm delivery.
- The ideal formulation and delivery of medication is unknown.
- Availability may be an issue in the NAIHS.
* The statement in no way suggests that this method of care is uniform for all cases consulted by the Phoenix Perinatal Associates. The author advises interpretation and consultation for each individual case.
Nurses Corner - Sandra Haldane, HQE
Nursing Leadership; An Excerpt from the IHS CEO
Brief, Volume 2, Issue 4
The Office of Public Health Support presents the fourth IHS CEO Brief in
our email series designed to help you address the challenge of retaining our
professional and clinical staff.
In this issue, a nurse executive makes a bold move with across-the-board salary bonuses to recruit and retain nurses. She believes this will help the facility ensure the best possible care for its patients. The best practice demonstrated by this case study is the importance of providing resourceful leadership.
Case Study:
Nurse Executive
Location: Small IHS Hospital
Background: In 2004, the hospital's leadership team successfully
instituted a 3-R bonus program to provide relocation, recruitment and retention
bonuses in the Family Care and Obstetrics units. Two years later, a new appointee
to the nurse executive position found that there were still a number of vacant
positions on her nursing teams in all departments. There were even positions
available in Emergency, Surgery, and especially Outpatient, departments for which
she knew nurses were easier to recruit and retain. This concerned the nurse executive.
She knew that continuity of care is essential to enabling a hospital to provide
the best possible patient outcomes.
Challenge: Exit interviews and staff interactions with the nurse
executive indicated that the nurses were interested in earning more money. Many
were leaving IHS in order to work at a local private hospital that was offering
them a $10,000 recruitment bonus plus an additional $10,000 bonus at the end
of their second year. For staff members at the GS-9 and GS-10 levels, this meant
a 19 percent to 22 percent increase in pay over two years.
Solution: Realizing that a large part of her budget was spent
on contract nurses and that the facility's CEO had the authority to offer 3-R
bonuses, the nurse executive met with her governing board to discuss reallocating
money spent on hiring contract employees to retaining staff nurses. She proposed
a plan to apply the money to a 10 percent recruitment and relocation bonus and
a 15 percent across-the-board performance retention bonus for all nurses.
Lesson Learned: The nurse executive realized that recruitment
and retention incentives would encourage her dedicated nurses to stay with the
organization and remain committed to both the IHS mission and the hospital's
patient population. The incentives provided a practical way to ensure that a
strong sense of continuity would be maintained on behalf of patients.
Best Practices in Action C T I O N
One of
the most essential leadership responsibilities is supporting
and protecting the interests of staff members. When staff are being drawn away
for reasons such as salary, it is important to reinforce their sense of importance
to the overall success of the organization and the high level of care provided
to patients by re-establishing effective support for pay incentives.
We recognize that you may have
successful retention strategies and your own best practices and we value your
input. Send your stories or comments. Add a colleague to
the mailing list or update us with changes in your email address. Email us at:
MEDCOM has added Obstetrical Series to the Course Catalog
This series has been added to our course curriculum at no additional charge. You may access all of MedCom’s courses online. The new Obstetric series includes:
Caring for the Antepartum Patient
Electronic Fetal Monitoring
Labor and Pain Control
Assisted Delivery and Cesarean Section
Newborn Stabilization and Care
Caring for the Postpartum Patient
Obstetrical Nursing: Caring for the Antepartum Patient
Obstetrical Nursing: Electronic Fetal Monitoring
Obstetrical Nursing: Labor and Delivery
Obstetrical Nursing: Assisted Delivery and Cesarean Section
Obstetrical Nursing: Newborn Stabilization and Care
Obstetrical Nursing: Caring for the Postpartum Patient
Please register with MedCom at: http://www.medcomrn.com/ihs/
Office of Women's Health, CDC
Emergency Planning Tips If You're Pregnant or Have Young Children
This
CDC website has resources to help pregnant women and families with young children
plan for an emergency or disaster.
Resources include what to do:
- If you are asked to evacuate;
- If you have to stay in a shelter or place other than your home;
- During and just after a disaster; or
- If you are recovering from a disaster
There is also a list of preparedness/disaster
resources and a link to the ACNM “Emergency Childbirth” section.
http://www.cdc.gov/Features/Emergencies/Pregnancy-Infants.html
Electronic Media and Youth Violence: A CDC Issue Brief for Educators and Caregivers
Electronic Media and Youth Violence: A CDC Issue Brief
for Educators and Caregivers focuses on the phenomena of electronic aggression.
Electronic aggression is defined as any kind of harassment or bullying that occurs
through email, chat rooms, instant messaging, websites, blogs, or text messaging.
The brief summarizes what is known about young people and electronic aggression,
provides strategies for addressing the issue with young people, and discusses
the implications for school staff, education policy makers, and parents and caregivers.
http://www.cdc.gov/ncipc/dvp/YVP/electronic_aggression.htm
Increasing Prevalence of Gestational Diabetes and Pregnancy-Related Hypertension in Los Angeles County, California, 1991–2003
INTRODUCTION: Gestational
diabetes and pregnancy-related hypertension can lead to adverse health effects
in mothers and infants. We assessed recent trends in the rates of these conditions
in Los Angeles County, California.
METHODS: Hospital discharge data were used to identify all women aged 15–54
years who resided in the county, had a singleton delivery from 1991 through 2003,
and had gestational diabetes or pregnancy-related hypertension listed as a discharge
diagnosis at the time of delivery. The prevalence of each condition was calculated
by calendar year, race/ethnicity, and age group. Temporal trends in the rates
were assessed by using negative binomial regression models, controlling for race/ethnicity
and age. Separate models were run for each racial/ethnic and age group.
RESULTS: The age-adjusted prevalence of gestational diabetes increased more than
threefold (from 14.5 cases per 1000 women in 1991 to 47.9 cases per 1000 in 2003).
The age-adjusted prevalence of pregnancy-related hypertension also increased
(from 40.5 cases per 1000 in 1991 to 54.4 cases per 1000 in 2003). In the multivariable
regression analysis, the annual rate increase for gestational diabetes was 8.3%
overall and was highest among Hispanics (9.9%). The annual rate increase for
pregnancy-related hypertension was 2.8% overall and was highest among blacks
(4.8%).
CONCLUSION: The rates of gestational diabetes and pregnancy-related hypertension
are increasing in Los Angeles County. Further research is needed to determine
the causes of the observed increases and the growing racial/ethnic disparities
in those rates.
Baraban E, McCoy L, Simon P. Increasing prevalence of gestational diabetes
and pregnancy-related hypertension in Los Angeles County, California, 1991–2003.
Prev Chronic Dis 2008;5(3).
http://www.cdc.gov/pcd/issues/2008/jul/07_0138.htm
Osteoporosis
Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians
DESCRIPTION: The American College of Physicians (ACP)
developed this guideline to present the available evidence on various pharmacologic
treatments to prevent fractures in men and women with low bone density or osteoporosis.
METHODS: Published literature on this topic was identified by using MEDLINE (1966
to December 2006), the ACP Journal Club database, the Cochrane Central Register
of Controlled Trials (no date limits), the Cochrane Database of Systematic Reviews
(no date limits), Web sites of the United Kingdom National Institute of Health
and Clinical Excellence (no date limits), and the United Kingdom Health Technology
Assessment Program (January 1998 to December 2006). Searches were limited to
English-language publications and human studies. Keywords for search included
terms for osteoporosis, osteopenia, low bone density, and the drugs listed in
the key questions. This guideline grades the evidence and recommendations according
to the ACP's clinical practice guidelines grading system.
RECOMMENDATION 1: ACP recommends that clinicians offer pharmacologic treatment
to men and women who have known osteoporosis and to those who have experienced
fragility fractures (Grade: strong recommendation; high-quality evidence).
RECOMMENDATION 2: ACP recommends that clinicians consider pharmacologic treatment
for men and women who are at risk for developing osteoporosis (Grade: weak recommendation;
moderate-quality evidence).
RECOMMENDATION 3: ACP recommends that clinicians choose among pharmacologic treatment
options for osteoporosis in men and women on the basis of an assessment of risk
and benefits in individual patients (Grade: strong recommendation; moderate-quality
evidence). RECOMMENDATION 4: ACP recommends further research to evaluate treatment
of osteoporosis in men and women.
Qaseem A, Snow V, Shekelle P, Hopkins R Jr,
Forciea MA, Owens DK; Clinical Efficacy Assessment Subcommittee of the American
College of Physicians. Pharmacologic treatment of low bone density or osteoporosis
to prevent fractures: a clinical practice guideline from the American College
of Physicians. Ann Intern Med. 2008 Sep 16;149(6):404-15.
http://www.ncbi.nlm.nih.gov/pubmed/18794560?dopt=Abstract
Free Full Text http://www.annals.org/cgi/content/full/149/6/404
A WHO Fracture Risk Assessment tool is available at: http://www.shef.ac.uk/FRAX/index.htm
Patient Information
The Eagle Book Animation Series
The Centers for Disease Control and Prevention (CDC) Native Diabetes Wellness Program (NDWP) in the Division of Diabetes Translation (DDT) announces the newest Eagle Book product: The Eagle Book animation series. The animation series of the Eagle Books is available as a full featured DVD and with the basic animation appearing on the internet http://www.cdc.gov/cdctv where it currently holds a spotlight feature http://www.cdc.gov/Features/VideoBooks/EagleBooks.html
The Eagle Books are a set- of- four books for children ages 4 – 9.
- Through the Eyes of the Eagle,
- Knees Lifted High,
- Plate Full of Color, and
- Tricky Treats
They show healthy lifestyles through physical activity and healthy eating with practicing traditional ways and looking to the wisdom of their elders.
The books were originally created for American Indian children to prevent type 2 diabetes but have had remarkable cross cultural appeal and application to promoting health literacy in multiple health promotion and disease prevention programs.
The books have had an amazing outgrowth with the following
support materials:
Eagle Books Teachers/Community Guide http://www.cdc.gov/diabetes/pubs/eagleguide.htm
Eagle Books Coloring Books http://www.cdc.gov/diabetes/pubs/eagle.htm
Eagle Books animation full featured DVD
Eagle Books animation on the Internet - www.CDC.GOV/CDCTV/
Indian health Service (I H S), Urban Indian Health and Tribal Programs can order the Eagle Books and DVD’s at no charge from the IHS online ordering system at http://www.ihs.gov/MedicalPrograms/diabetes/resources/rde/index.cfm?module=catalog
All others will need to order their bulk orders of the Eagle Books through the the Public Health Foundation http://bookstore.phf.org or call them (phone) 202.218.4400 (Bulk orders $85 for 68 books, plus shipping).
One complimentary set of the Eagle Books and a DVD are available from: http://wwwn.cdc.gov/pubs/diabetes.aspx
Perinatology Picks
Correlation of catheterized and clean catch urine protein/creatinine ratios in preeclampsia evaluation
OBJECTIVE: To examine
whether clean catch urine specimens correlate with catheterized specimens for
determination of protein/creatinine ratios in pregnant women being evaluated
for preeclampsia. METHODS: Sixty pregnant women who were at least at 20 weeks
of gestation were enrolled. Patients with ruptured membranes, vaginal bleeding,
or urinary tract infections were excluded. Midstream clean catch urine specimens
were collected. Catheterized specimens were then collected and used for clinical
management. The specimens were analyzed for protein, creatinine, urinalysis,
and culture. Based on sample size calculations, 60 participants were needed to
detect a correlation of 0.90 with 80% power and alpha=0.05.
RESULTS: Mean gestational age at enrollment was 35.9 weeks (range 23.1-41.7 weeks).
Median (range) clean catch and catheterized protein/creatinine ratios were 0.204
(0.089-3.465) and 0.181 (0.067-3.335), respectively, with a correlation coefficient
of 0.897 (P<.001). When results were categorized by degree of proteinuria
using a cutoff of 0.3, sensitivity and specificity of the clean catch protein/creatinine
ratios were 95.2% and 97.4%. When using a more conservative cutoff of 0.19, sensitivity
and specificity of the clean catch protein/creatinine ratios were 96.4% and 75.0%.
CONCLUSION: Clean catch and catheterized urine specimens correlate well in women
with suspected preeclampsia. Routine catheterization of pregnant women is not
necessary in the evaluation of preeclampsia.
Chen BA, Parviainen K, Jeyabalan A. Correlation of catheterized and clean catch urine protein/creatinine ratios in preeclampsia evaluation. Obstet Gynecol. 2008 Sep;112(3):606-10. http://www.ncbi.nlm.nih.gov/pubmed/18757659
A randomised controlled trial of early versus delayed oxytocin augmentation to treat primary dysfunctional labour in nulliparous women
BACKGROUND:
Oxytocin is widely used to speed up slow labour, especially in nulliparous women,
but randomised trials, apart from one reported only in abstract, have been too
small to exclude important effects.
OBJECTIVE: To test the hypothesis that early use of oxytocin reduces the need
for caesarean delivery.
DESIGN: A randomised controlled trial.
SETTING: Twelve obstetric units within the Northern and Yorkshire regions in
the North East of England.
PARTICIPANTS: A total of 412 low-risk nulliparous women in spontaneous labour
at term, who had been diagnosed with primary dysfunctional labour were recruited
from January 1999 to December 2001.
INTERVENTION: Immediate oxytocin administration (active group) or oxytocin withheld
for up to 8 hours (conservative group).
MAIN OUTCOME MEASURES: Caesarean section and operative vaginal delivery rates.
The length of labour measured from the time of randomisation to delivery. The
rate of maternal Edinburgh Postnatal Depression Scale (EPDS) greater than 12
(major depression) within 48 hours of delivery. RESULTS: The caesarean section
rates were 13.5% active versus 13.7% controls (OR 0.98, 95% CI 0.6-1.7). Operative
delivery, 24.5% versus 30.9% (OR 0.73, 95% CI 0.5-1.1). The median (interquartile
range) randomisation to delivery interval in the active group was 5 hours 52
minutes (3:57-8:28) and in the conservative group 9 hours 8 minutes (5:06-13:16)
(P < 0.001). The rate of EPDS >12 was 20% in the active arm versus 15%
among controls (OR 1.26, 95% CI 0.7-2.2). There was one perinatal death in each
group and no major differences in perinatal outcomes.
CONCLUSIONS: Among nulliparous women with primary dysfunctional labour, early
use of oxytocin does not reduce caesarean section or short-term postnatal depression.
However, it shortens labour considerably and may reduce operative vaginal deliveries.
Hinshaw K, Simpson S, Cummings S, Hildreth A, Thornton J. A randomised controlled trial of early versus delayed oxytocin augmentation to treat primary dysfunctional labour in nulliparous women. BJOG. 2008 Sep;115(10):1289-95; discussion 1295-6. http://www.ncbi.nlm.nih.gov/pubmed/18715415
Do Mechanical Methods of Cervical Ripening Increase Infectious Morbidity? A Systematic Review
The purpose of this study was to review systematically randomized controlled trials that were associated with cervical ripening. We identified randomized controlled trials that compared the use of Foley catheter, with or without extraamniotic saline solution infusion, Laminaria, or hygroscopic dilators for cervical ripening or induction with pharmacologic agents or placebo. Randomized controlled trials that evaluated maternal or neonatal infection were selected. The outcomes that were assessed were maternal and neonatal infection, chorioamnionitis, and endomyometritis. Thirty studies met inclusion criteria. Compared with the use of pharmacologic methods alone, patients who underwent cervical ripening with mechanical agents had a significantly higher rate of maternal infection rates. Similar results were noted for patients who underwent ripening with Foley catheter alone in comparison with pharmacologic agents. No difference was noted in maternal infection rates for patients who underwent ripening with extraamniotic saline solution infusion, Laminaria, or hygroscopic dilators. Compared with the use of pharmacologic agents alone, maternal and neonatal infectious morbidity appears to be increased when mechanical agents are used for cervical ripening.
Heinemann J, Gillen G, Sanchez-Ramos L, Kaunitz AM. Do Mechanical Methods of Cervical Ripening Increase Infectious Morbidity? A Systematic Review. Am J Obstet Gynecol. 2008 Aug;199(2):177-87; discussion 187-8. http://www.ncbi.nlm.nih.gov/pubmed/18674661
QuickStats: Rates* of Cesarean Deliveries --- Selected Countries,† 2005
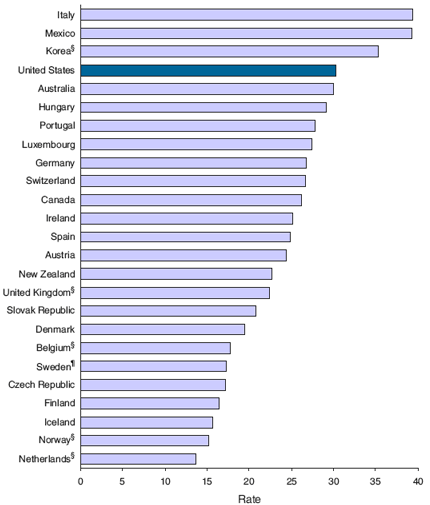
* Per 100 live births.
† Includes rates from 25 of 30 Organisation for Economic Co-operation
and Development member countries; recent data on cesarean deliveries were not
available from France, Greece, Japan, Poland, and Turkey.
§ Based on 2004 data.
¶ Based on 2003 data.
In 2005, cesarean deliveries accounted for more than 25% of all live births in
12 industrialized countries, including the United States (30%). Nearly 40% of
births were by cesarean delivery in Italy and Mexico. The Netherlands had the
lowest rate of cesarean deliveries (14%), and four of the six lowest rates were
in Nordic countries.
Centers for Disease Control. QuickStats: Rates* of Cesarean Deliveries --- Selected Countries,† 2005. MMWR. September 19, 2008 / 57(37);1019.
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5737a7.htm?s_cid=mm5737a7_e
Primary Care Discussion Forum - Ann Bullock, Cherokee, NC
Topic: Geriatric Medication Issues
Starts: November 3, 2008, Monday
Moderators: Chris Lamer, PharmD and
Bruce Finke, MD
Contact: Ann Bullock
How to subscribe / unsubscribe to the Primary Care Discussion Forum?
Subscribe to the Primary Care listserv
http://www.ihs.gov/cio/listserver/index.cfm?module=list&option=list&num=46&startrow=51
Unsubscribe from the Primary Care listserv
http://www.ihs.gov/cio/listserver/index.cfm?module=list&option=list&num=46&startrow=51
Questions on how to subscribe, contact ANNBULL@nc-cherokee.com
STD Corner - Lori de Ravello, National IHS STD Program
Trends in HIV- and STD-Related Risk Behaviors Among High School Students --- United States, 1991--2007
Persons who engage in unprotected sexual intercourse or use injection drugs are at increased risk for human immunodeficiency virus (HIV) infection and sexually transmitted diseases (STDs). Changes in HIV- and STD-related risk behaviors among high school students in the United States during 1991--2005 were reported previously (1). To update these analyses through 2007, CDC analyzed data from nine biennial national Youth Risk Behavior Surveys (YRBS). This report summarizes the results of that analysis, which indicated that, during 1991--2007, the percentage of U.S. high school students who ever had sexual intercourse decreased 12%, the percentage who had sexual intercourse with four or more persons during their lifetime decreased 20%, and the percentage who were currently sexually active decreased 7%. Among students who were currently sexually active, the prevalence of condom use increased 33%. However, these changes in risk behaviors were not observed in some subgroups. In addition, no changes were detected in the prevalence of sexual risk behaviors from 2005 to 2007, and many students still engaged in behaviors that place them at risk for HIV infection and STDs. Additional efforts to reduce sexual risk behaviors, particularly among black, Hispanic, and male students, must be implemented to meet the Healthy People 2010 national health objective for adolescent sexual behaviors (objective no. 25-11) (2) and to decrease rates of HIV infection and STDs.
In an editorial comment, the CDC observed:
A Healthy People 2010 national health objective (no. 25-11) is to increase
to 95% the proportion of adolescents in grades 9--12 who abstain from sexual
intercourse or use condoms if currently sexually active (2). CDC reported
previously that, in 2007, 87% of high school students reported abstaining from
sexual intercourse or using condoms if currently sexually active (4),
compared with 80% in 1991. Despite this progress, the analyses in this report
indicate that no changes were detected in the prevalence of sexual risk behaviors
from 2005 to 2007, and some subgroups did not experience the overall changes
observed during 1991--2007. For example, among black students, the prevalence
of sexual experience, multiple sex partners, and current sexual activity remained
higher than among any other subgroup of high school students, the prevalence
of sexual experience did not decrease during 2001--2007, and the prevalence of
condom use did not increase during 1999--2007. Among Hispanic students, the prevalence
of sexual experience, multiple sex partners, and current sexual activity did
not change during 1991--2007. Among male students, the prevalence of sexual experience
and multiple sex partners did not decrease after 1997, and current sexual activity
did not change during 1991--2007. Therefore, renewed efforts to delay onset of
sexual activity and increase condom use among students who are sexually active
are warranted, especially among black, Hispanic, and male students.
Centers for Disease Control. Trends in HIV- and STD-Related Risk Behaviors Among High School Students --- United States, 1991—2007. MMWR. August 1, 2008 / 57(30);817-822
The full report is available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5730a1.htm?s_cid=mm5730a1_e
Barbara Stillwater, Alaska State Diabetes Program
Small Steps, Big Rewards for Families with a History of Gestational Diabetes
The National Diabetes Education Program offers teaching sheets for families:
Gestational diabetes affects women during pregnancy and causes a lifelong risk for diabetes. Children of women with a history of gestational diabetes are also at risk, especially if they are overweight. But families can take small steps to prevent or delay type 2 diabetes.
If you had gestational diabetes, you can prevent type 2 diabetes by losing a small amount of weight by being more physically active and making healthy food choices. And your children can lower their risk for type 2 diabetes if they do not become overweight. The National Diabetes Education Program (NDEP) suggests taking these small steps to prevent diabetes:
Tips for Mothers:
- If you have been diagnosed with gestational diabetes, get tested for diabetes six to 12 weeks after your baby is born, then every one to two years.
- Breastfeed your baby. It may lower your child’s risk for type 2 diabetes.
- Try to reach your pre-pregnancy weight six to 12 months after your baby is born. Then, if you still weigh too much, work to lose at least 5 to 7 percent (10 to 14 pounds if you weigh 200 pounds) of your body weight.
- Be physically active at least 30 minutes a day, 5 days a week and eat smaller portions of healthy foods to help you reach and stay at a healthy weight.
Tips for the Family:
- Tell your child’s doctor that you had gestational diabetes and ask for an eating plan for your child.
- Follow a healthy lifestyle as a family. Serve your children healthy foods such as fruits and vegetables, fish, lean meats, dry beans and peas, whole grains, and low-fat or skim milk and cheese. Choose water to drink.
- Help your children be physically active at least 60 minutes a day and limit TV and inactive video and computer game time to an hour or two a day.
Read NDEP’s Its Never Too Early to Prevent Diabetes. A Lifetime of Small Steps for a Healthy Family tip sheet (also available in Spanish) and download or order free diabetes prevention materials by visiting www.YourDiabetesInfo.org or calling 1-888-693-NDEP (6337). For more information about helping children maintain a healthy weight, contact the Weight-control Information Network at www.win.niddk.nih.gov or 1-877-946-4627.
http://ndep.nih.gov/diabetes/pubs/gestational-diabetes-article.pdf
Healthcare Cost and Utilization Project
The HCUP
Facts and Figures report showcases the wealth of statistics available
from the HCUP NIS database. It features an overview of numerous hospital-related
topics, including general characteristics of U.S. hospitals and the patients
being treated; the most common diagnoses, conditions, and procedures associated
with inpatient stays; the costs and charges associated with hospitalizations;
and a special section on selected priority health conditions designated by the
U.S. Department of Health and Human Services.
Highlights of hospital care in 2006 include the finding that six of the 20 most
costly conditions associated with hospitalizations were related to the circulatory
system. Though these stays accounted for 18 percent of all hospitalization costs—with
three diagnoses (coronary artery disease, heart attack, and congestive heart
failure) among the most costly in 2006—the growth in costs for these conditions
has slowed dramatically since 2003. Examples of trend information presented in
the report include findings that, between 1993 and 2006, the total number of
Cesarean sections grew nearly 69 percent, while vaginal births remained steady.
Maternal complications including hypertension, diabetes, and anemia increased
for pregnant women, regardless of whether they were undergoing C-sections or
vaginal deliveries, but were more common in women who had C-sections. Infant
complications also increased during this period, including respiratory problems, "infant
of a diabetic mother" syndrome, jaundice, and feeding problems, and were
also more common in babies delivered via C-section than vaginally.
http://www.hcup-us.ahrq.gov/reports/factsandfigures/HAR_2006.pdf
Women's Health Headlines, Carolyn Aoyama, HQE
Differentiation among Types of Intimate Partner Violence: Research Update and Implications for Interventions.
A growing body of empirical research has demonstrated that intimate partner violence is not a unitary phenomenon and that types of domestic violence can be differentiated with respect to partner dynamics, context, and consequences. Four patterns of violence are described: Coercive Controlling Violence, Violent Resistance, Situational Couple Violence, and Separation-Instigated Violence. The controversial matter of gender symmetry and asymmetry in intimate partner violence is discussed in terms of sampling differences and methodological limitations. Implications of differentiation among types of domestic violence include the need for improved screening measures and procedures in civil, family, and criminal court and the possibility of better decision making, appropriate sanctions, and more effective treatment programs tailored to the characteristics of different types of partner violence. In family court, reliable differentiation should provide the basis for determining what safeguards are necessary and what types of parenting plans are appropriate to ensure healthy outcomes for children and parent–child relationships.
Courts, 2008
Kelly JB, Johnson MP. Differentiation among Types of Intimate Partner Violence:
Research Update and Implications for Interventions. Family Court Review, Vol.
46 No. 3, July 2008 476 –499.
No URL is available for the above article. Contact Carolyn Aoyama for additional information. Carolyn.Aoyama@ihs.gov.
Save the dates
Medical Providers’ Best Practices Conference, 3rd Annual
- November 18-19, 2008; Sacramento, CA
- California Area Indian Health Service
- More information: 916.930.3937 or IHS-CAOGPRA@ihs.gov
2008 Indian Health Information Management Conference, “Managing Health Information Technology to Improve Performance and Outcomes”
- December 15 – 19, 2008 ; Phoenix, Arizona
- Information at: http://www.ihs.gov/cio/ihimc/
First International Meeting on Indigenous Women’s Health/Third International Meeting on Indigenous Child Health Conference; Many Voices into One Song
- Women’s Health March 4-6, 2009
- Child Health March 6-8, 2009
- Albuquerque , NM
- Joint conference of Women’s Health and Children’s Health Providers from Canada and the United States
Advances in Indian Health Conference
- April 21-24, 2009 in Albuquerque, NM
- Indian Health's conference for primary care providers and nurses
- 28 hours of CME/CE credit
- Optional Diabetes track
- Contact the Course Director, Dr. Ann Bullock, at annbull@nc-cherokee.com for more information.
What's new on the ITU MCH web pages?
There are several upcoming Conferences
and Online CME/CEU resources, etc….
and the latest Perinatology Corners (free online CME from IHS)
…or just take a look at the What’s New page
Did you miss something in the last OB/GYN Chief Clinical Consultant Corner?
The September 2008 OB/GYN CCC Corner is available.
Hot Topics ‹ Previous
OB/GYN
Jean Howe, MD, MPH is the Obstetrics and Gynecology Chief Clinical Consultant (OB/GYN C.C.C.). Dr. Howe is very interested in establishing a dialogue and/or networking with anyone involved in women's health or maternal child health, especially as it applies to American Indian and Alaska Native women and also indigenous peoples around the world. Please don't hesitate to contact her by e-mail (jean.howe@ihs.gov) or phone at (928) 674-7422.

