Hepatitis B
 FAQs
for Health Professionals
FAQs
for Health Professionals
Overview and Statistics
Case definitions have been developed by CDC, in collaboration with the Council of State and Territorial Epidemiologists, to provide uniform clinical and laboratory-testing criteria for the identification and reporting of nationally notifiable infectious diseases. The case definitions for acute, chronic, and perinatal hepatitis B are available at the following links:
Perinatal Hepatitis B (Acquired in the United States or U.S. Territories)
Additional guidance on viral hepatitis surveillance and case management is available at http://www.cdc.gov/hepatitis/SurveillanceGuidelines.htm.
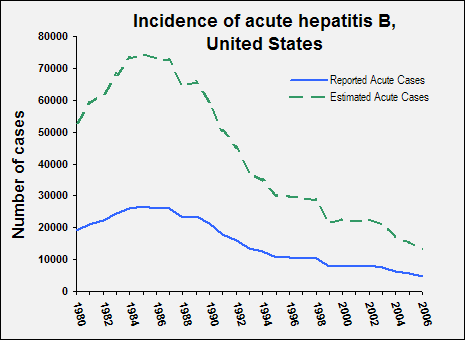
In 2006, 4,758 cases of acute hepatitis B in the United States were reported to CDC; the overall incidence of reported acute hepatitis B was 1.6 per 100,000 population, the lowest ever recorded. However, because many HBV infections are either asymptomatic or never reported, the actual number of new infections is estimated to be approximately tenfold higher. In 2006, an estimated 46,000 persons in the United States were newly infected with HBV. Rates are highest among adults, particularly males aged 25–44 years.
The rate of new HBV infections has declined by approximately 80% since 1991, when a national strategy to eliminate HBV infection was implemented in the United States. The decline has been greatest among children born since 1991, when routine vaccination of children was first recommended.
An estimated 800,000–1.4 million persons in the United States have chronic HBV infection. Chronic infection is an even greater problem globally, affecting approximately 350 million persons. An estimated 620,000 persons worldwide die from HBV-related liver disease each year.
Viral hepatitis surveillance reports and guidelines are available at http://www.cdc.gov/hepatitis/statistics.htm.
HBV is transmitted through activities that involve percutaneous (i.e., puncture through the skin) or mucosal contact with infectious blood or body fluids (e.g., semen, saliva), including
- Sex with an infected partner
- Injection drug use that involves sharing needles, syringes, or drug-preparation equipment
- Birth to an infected mother
- Contact with blood or open sores of an infected person
- Needle sticks or sharp instrument exposures
- Sharing items such as razors or toothbrushes with an infected person
HBV is not spread through food or water, sharing eating utensils, breastfeeding, hugging, kissing, hand holding, coughing, or sneezing.
HBV can survive outside the body at least 7 days and still be capable of causing infection.
Any blood spills — including dried blood, which can still be infectious — should be cleaned using 1:10 dilution of one part household bleach to 10 parts of water for disinfecting the area. Gloves should be used when cleaning up any blood spills.
The following populations are at increased risk of becoming infected with HBV:
- Infants born to infected mothers
- Sex partners of infected persons
- Sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., >1 sex partner during the previous 6 months)
- Men who have sex with men
- Injection drug users
- Household contacts of persons with chronic HBV infection
- Healthcare and public safety workers at risk for occupational exposure to blood or blood-contaminated body fluids Hemodialysis patients
- Residents and staff of facilities for developmentally disabled persons
- Travelers to countries with intermediate or high prevalence of HBV infection
The risk for HBV infection in international travelers is generally low, except for certain travelers to regions where the prevalence of chronic HBV infection is high or intermediate (i.e., hepatitis B surface antigen prevalence of ≥2%). Hepatitis B vaccination should be administered to unvaccinated persons traveling to those countries.
More information about hepatitis B and travel is available from CDC's Travelers' Health site.
The presence of signs and symptoms varies by age. Most children under age 5 years and newly infected immunosuppressed adults are asymptomatic, whereas 30%–50% of persons aged ≥5 years have initial signs and symptoms. When present, signs and symptoms can include
- Fever
- Fatigue
- Loss of appetite
- Nausea
- Vomiting
- Abdominal pain
- Dark urine
- Clay-colored bowel movements
- Joint pain
- Jaundice
Persons with chronic HBV infection might be asymptomatic, have no evidence of liver disease, or have a spectrum of disease ranging from chronic hepatitis to cirrhosis or hepatocellular carcinoma (a type of liver cancer).
Symptoms begin an average of 90 days (range: 60–150 days) after exposure to HBV.
Symptoms typically last for several weeks but can persist for up to 6 months.
Acute infection ranges from asymptomatic or mild disease to — rarely — fulminant hepatitis. Disease is more severe among adults aged >60 years. The fatality rate among acute cases reported to CDC is 0.5%–1%.
Approximately 25% of those who become chronically infected during childhood and 15% of those who become chronically infected after childhood die prematurely from cirrhosis or liver cancer, and the majority remain asymptomatic until onset of cirrhosis or end-stage liver disease. In the United States, chronic HBV infection results in an estimated 2,000–4,000 deaths per year.
The risk for chronic infection varies according to the age at infection and is greatest among young children. Approximately 90% of infants and 25%–50% of children aged 1–5 years will remain chronically infected with HBV. By contrast, approximately 95% of adults recover completely from HBV infection and do not become chronically infected.
For acute infection, no medication is available; treatment is supportive.
For chronic infection, several antiviral drugs (adefovir dipivoxil, interferon alfa-2b, pegylated interferon alfa-2a, lamivudine, entecavir, and telbivudine) are available. Persons with chronic HBV infection require medical evaluation and regular monitoring to determine whether disease is progressing and to identify liver damage or hepatocellular carcinoma.
Hepatitis B surface antigen (HBsAg): A protein on the surface of HBV; it can be detected in high levels in serum during acute or chronic HBV infection. The presence of HBsAg indicates that the person is infectious. The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make hepatitis B vaccine.
Hepatitis B surface antibody (anti-HBs): The presence of anti-HBs is generally interpreted as indicating recovery and immunity from HBV infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B.
Total hepatitis B core antibody (anti-HBc): Appears at the onset of symptoms in acute hepatitis B and persists for life. The presence of anti-HBc indicates previous or ongoing infection with HBV in an undefined time frame.
IgM antibody to hepatitis B core antigen (IgM anti-HBc): Positivity indicates recent infection with HBV (≤6 months). Its presence indicates acute infection.
Hepatitis B e antigen (HBeAg): A secreted product of the nucleocapsid gene of HBV that is found in serum during acute and chronic hepatitis B. Its presence indicates that the virus is replicating and the infected person has high levels of HBV.
Hepatitis B e antibody (HBeAb or anti-HBe): Produced by the immune system temporarily during acute HBV infection or consistently during or after a burst in viral replication. Spontaneous conversion from e antigen to e antibody (a change known as seroconversion) is a predictor of long-term clearance of HBV in patients undergoing antiviral therapy and indicates lower levels of HBV.
The following table provides interpretations for hepatitis B serologic markers. A PDF version is also available.
| Interpretation of Hepatitis B Serologic Test Results | ||
|---|---|---|
| Tests | Results | Interpretation |
| HBsAg anti-HBc anti-HBs |
negative negative negative |
Susceptible |
| HBsAg anti-HBc anti-HBs |
negative positive positive |
Immune due to natural infection |
| HBsAg anti-HBc anti-HBs |
negative negative positive |
Immune due to hepatitis B vaccination |
| HBsAg anti-HBc IgM anti-HBc anti-HBs |
positive positive positive negative |
Acutely infected |
| HBsAg anti-HBc IgM anti-HBc anti-HBs |
positive positive negative negative |
Chronically infected |
| HBsAg anti-HBc anti-HBs |
negative positive negative |
Interpretation unclear; four possibilities:
|
| Hepatitis
B surface antigen (HBsAg): A protein on the surface
of HBV; it can be detected in high levels in serum during acute
or chronic HBV infection. The presence of HBsAg indicates that the
person is infectious. The body normally produces antibodies to HBsAg
as part of the normal immune response to infection. HBsAg is the
antigen used to make hepatitis B vaccine.
Hepatitis B surface antibody (anti-HBs):
The presence of anti-HBs is generally interpreted as indicating
recovery and immunity from HBV infection. Anti-HBs also develops
in a person who has been successfully vaccinated against hepatitis
B. Adapted from: A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. Part I: Immunization of Infants, Children, and Adolescents. MMWR 2005;54(No. RR-16). |
||
HBsAg will be detected in an infected person’s blood an average of 4 weeks (range: 1–9 weeks) after exposure to the virus. About 1 of 2 patients will no longer be infectious by 7 weeks after onset of symptoms, and all patients who do not remain chronically infected will be HBsAg-negative by 15 weeks after onset of symptoms.
CDC offers an online training that covers the serology of hepatitis B and other types of viral hepatitis, available at http://www.cdc.gov/hepatitis/ResourceCntr/Professionals/Training/SerologyStart.htm.
The Advisory Committee on Immunization Practices recommends that the following persons be vaccinated against hepatitis B:
- All infants, beginning at birth
- All children aged <19 years who have not been vaccinated previously
- Susceptible sex partners of hepatitis B surface antigen (HBsAg)-positive persons
- Sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., >1 sex partner during the previous 6 months)
- Persons seeking evaluation or treatment for a sexually transmitted disease
- Men who have sex with men
- Injection drug users
- Susceptible household contacts of HBsAg-positive persons
- Healthcare and public safety workers at risk for exposure to blood or blood-contaminated body fluids
- Persons with end-stage renal disease, including predialysis, hemodialysis, peritoneal dialysis, and home dialysis patients
- Residents and staff of facilities for developmentally disabled persons
- Travelers to regions with intermediate or high rates of endemic HBV infection
- Persons with chronic liver disease
- Persons with HIV infection
- All other persons seeking protection from HBV infection — acknowledgment of a specific risk factor is not a requirement for vaccination
Yes. In certain healthcare, evaluation, or treatment settings, a high proportion of clients have known risk factors for HBV infection. The Advisory Committee on Immunization Practices recommends universal vaccination of adults who receive care in those settings, including
- Sexually transmitted disease treatment facilities
- HIV testing and treatment facilities
- Facilities providing drug-abuse treatment and prevention services
- Healthcare settings targeting services to injection drug users
- Correctional facilities
- Healthcare settings targeting services to men who have sex with men
- Chronic hemodialysis facilities and end-stage renal disease programs
- Institutions and nonresidential day care facilities for developmentally disabled persons
Two single-antigen vaccines and three combination vaccines are currently licensed in the United States.
Single-antigen hepatitis B vaccines
- ENGERIX-B®
- RECOMBIVAX HB®
Combination vaccines
- COMVAX®: Combined hepatitis B-Haemophilus influenzae type b (Hib) conjugate vaccine. Cannot be administered before age 6 weeks or after age 71 months.
- PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years.
- TWINRIX®: Combined hepatitis A and hepatitis B vaccine. recommended for persons aged ≥18 years who are at increased risk for both hepatitis A virus and HBV infections.
The vaccination schedule most often used for children and adults is 3 intramuscular injections, the second and third doses administered 1 and 6 months, respectively, after the first dose. Alternate schedules have been approved for certain vaccines and/or populations.
|
Recommended doses of
currently licensed formulations of hepatitis B vaccine, by age group and vaccine type |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | Single-antigen vaccine | Combination vaccine | |||||||||
| Recombivax HB | Engerix-B | Comvax* | Pediarix† | Twinrix§ | |||||||
| Dose (μg)¶ |
Vol(mL) | Dose (μg)¶ |
Vol(mL) | Dose (μg)¶ |
Vol (mL) | Dose (μg)¶ |
Vol (mL) | Dose (μg)¶ |
Vol (mL) | ||
| Infants (<1 yr) | 5 | 0.5 | 10 | 0.5 | 5 | 0.5 | 10 | 0.5 | NA** | NA | |
| Children (1–10 yrs) | 5 | 0.5 | 10 | 0.5 | 5* | 0.5 | 10† | 0.5 | NA | NA | |
| Adolescents | 11–15 yrs | 10†† | 1.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| 11–19 yrs | 5 | 0.5 | 10 | 0.5 | NA | NA | NA | NA | NA | NA | |
| Adults (≥20 yrs) | 10 | 1.0 | 20 | 1.0 | NA | NA | NA | NA | 20§ | 1.0 | |
|
Hemodialysis patients and other immuno- compromised persons |
<20 yrs§§ | 5 | 0.5 | 10 | 0.5 | NA | NA | NA | NA | NA | NA |
| ≥20 yrs | 40¶¶ | 1.0 | 40*** | 2.0 | NA | NA | NA | NA | NA | NA | |
* Combined hepatitis B–Haemophilus influenzae type b conjugate
vaccine. This vaccine cannot be administered at birth, before age 6 weeks,
or after age 71 months.
† Combined hepatitis B, diphtheria, tetanus, acellular pertussis
adsorbed, inactivated poliovirus vaccine. This vaccine cannot be administered
at birth, before age 6 weeks, or at age >7 years.
§ Combined hepatitis A and hepatitis B vaccine. This vaccine
is recommended for persons aged ≥18 years who are at increased risk for
both hepatitis B virus and hepatitis A virus infections.
¶ Recombinant hepatitis B surface antigen protein dose.
** Not applicable.
†† Adult formulation administered on a 2-dose schedule.
§§ Higher doses might be more immunogenic, but no specific recommendations
have been made.
¶¶ Dialysis formulation administered on a 3-dose schedule at 0, 1,
and 6 months.
*** Two 1.0-mL doses administered at one site, on a 4-dose schedule at 0,
1, 2, and 6 months.
Anyone who has had a serious allergic reaction to a prior dose of hepatitis B vaccine, a component of the hepatitis B vaccine, or yeast should not receive hepatitis B vaccine.
Yes. No differences in immune response are observed when vaccines from different manufacturers are used to complete the vaccine series.
No, the series does not need to be restarted.
- If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible.
- The second and third doses should be separated by an interval of at least 8 weeks.
- If only the third dose is delayed, it should be administered as soon as possible.
No. If necessary, administering extra doses of hepatitis A or hepatitis B vaccine is not harmful.
Yes. When hepatitis B vaccine has been administered at the same time as other vaccines, no interference with the antibody response of the other vaccines has been demonstrated. Separate body sites and syringes should be used for simultaneous administration of injectable vaccines.
Studies indicate that immunologic memory remains intact for at least 20 years among healthy vaccinated individuals who initiated hepatitis B vaccination >6 months of age. The vaccine confers long-term protection against clinical illness and chronic hepatitis B virus infection. Cellular immunity appears to persist even though antibody levels might become low or decline below detectable levels.
Among vaccinated cohorts who initiated hepatitis B vaccination at birth, long-term follow-up studies are ongoing to determine the duration of vaccine-induced immunity.
Infants born to HBV-infected mothers require hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth to protect them from infection. However, because errors or delays in documenting, testing, and reporting maternal HBsAg status can and do occur, administering the first dose of hepatitis B vaccine soon after birth to all infants acts as a safety net, reducing the risk for perinatal infection when maternal HBsAg status is either unknown or incorrectly documented at delivery. Also, initiating the hepatitis B vaccine series at birth has been shown to increase a child's likelihood of completing the vaccine series on schedule.
Yes. Hepatitis B vaccine contains no live virus, so neither pregnancy nor lactation should be considered a contraindication to vaccination of women. On the basis of limited experience, there is no apparent risk of adverse effects to developing fetuses when hepatitis B vaccine is administered to pregnant women. Meanwhile, new HBV infection in a pregnant woman might result in severe disease for the mother and chronic infection for the newborn.
Yes, although a larger vaccine dose is required to induce protective antibody in hemodialysis patients. Larger doses or additional doses might also be necessary for other immunocompromised persons. Serologic testing of hemodialysis patients and other immunocompromised persons is recommended 1–2 months after administration of the final dose of the primary vaccine series to determine the need for revaccination. Detailed guidance on vaccination of hemodialysis patients and other immunocompromised persons is available from the Advisory Committee on Immunization Practices recommendations on adult hepatitis B vaccination (available at http://www.cdc.gov/mmwr/PDF/rr/rr5516.pdf).
Yes. After a person has been exposed to HBV, appropriate prophylaxis, given as soon as possible but preferably within 24 hours, can effectively prevent infection. The mainstay of postexposure immunoprophylaxis is hepatitis B vaccine, but in certain circumstances the addition of HBIG will provide increased protection.
Historically, routine prevaccination testing has not been recommended because it has not generally been found to be cost-effective with regard to vaccination. However, with the availability of antiviral agents to treat chronic HBV infection, new recommendations for identifying persons with chronic HBV infection are being developed. CDC currently recommends that certain populations undergo testing for HBV infection, including
- Hemodialysis patients
- Pregnant women
- Persons with known or suspected exposure to HBV including:
- infants born to HBV-infected mothers
- household contacts of HBV-infected persons
- persons with known occupational or other exposures to infectious blood or body fluids
- Foreign-born persons from countries of high HBV endemicity
- HIV-positive persons
For these populations, serologic assays for HBsAg and anti-HBs should be used to determine infection or immunity prior to vaccination.
Persons who have already been infected with HBV will receive no benefit from vaccination. However, there is no risk to a previously infected person who receives vaccination.
Testing for immunity is advised only for persons whose subsequent clinical management depends on knowledge of their immune status, including
- Infants born to HBsAg-positive mothers
- Healthcare workers and public safety workers at high risk for continued percutaneous or mucosal exposure to blood or body fluids
- Chronic hemodialysis patients, HIV-infected persons, and other immunocompromised persons (e.g., hematopoietic stem-cell transplant recipients or persons receiving chemotherapy)
- Sex partners of persons with chronic HBV infection
When necessary, postvaccination testing for antibody to hepatitis B surface antigen (anti-HBs) should generally be performed 1–2 months after completion of the vaccine series.
For infants born to HBsAg-positive mothers, postvaccination testing should be performed 1–2 months after completion of ≥3 doses of a licensed hepatitis B vaccine series (i.e., at age 9–18 months, generally at the next well-child visit). To avoid detection of anti-HBs from hepatitis B immune globulin administered during infancy and to maximize detection of late HBV infection, testing should not be performed before age 9 months nor within 4 weeks of the most recent vaccine dose.
Booster doses of hepatitis B vaccine are recommended only in certain circumstances:
- For hemodialysis patients, the need for booster doses should be assessed by annual testing for antibody to hepatitis B surface antigen (anti-HBs). A booster dose should be administered when anti-HBs levels decline to <10 mIU/mL.
- For other immunocompromised persons (e.g., HIV-infected persons, hematopoietic stem-cell transplant recipients, and persons receiving chemotherapy), the need for booster doses has not been determined. When anti-HBs levels decline to <10 mIU/mL, annual anti-HBs testing and booster doses should be considered for those with an ongoing risk for exposure.
For persons with normal immune status who have been vaccinated, booster doses are not recommended.
Page last modified: July 8, 2008
Content source:
Division of Viral Hepatitis
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
