 |
|
 |
 |
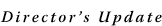

Advances in Imaging Reveal New and Better Ways to Fight Cancer
Imaging may be the most rapidly advancing technology in oncology today. Far beyond the traditional uses to screen, stage disease, and follow patients for recurrence or progression, we now have the ability to image across various levels of biological organization, not just organs or tumors, but also molecules, single cells, and tissues. Such information - obtained in real time and noninvasively - can provide important details about whether patients may be candidates for certain therapies or provide a rapid assessment of whether they are responding to treatment.
This special issue of the NCI Cancer Bulletin provides a window into just some of the exciting work being done in the field of cancer imaging, from individual research projects to NCI's support of companies developing new imaging technologies through our Small Business Innovation Research Program or through collaborations with private-sector partners as part of NCI's recently launched Advanced Technology Partnerships Initiative.
Read more 


Clinic Will Speed Drug Development
When NCI's new Molecular Imaging Clinic opens in January 2009, it will be one of the few places in the world where state-of-the-art imaging tools are dedicated to understanding how drugs behave in people with cancer. What is learned there could help investigators determine how best to use existing drugs and determine the properties of new molecularly targeted drugs that are just entering the clinic.
"Our goal is to use advanced imaging technologies to accelerate the development of therapies for cancer," said Dr. Peter Choyke, who directs the Molecular Imaging Program in NCI's Center for Cancer Research (CCR). He has assembled a multidisciplinary team that includes imaging scientists, chemists, physicists, engineers, oncologists, and molecular biologists. They have many collaborators inside and outside NCI.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Advances in Imaging Reveal New and Better Ways to Fight Cancer
Imaging may be the most rapidly advancing technology in oncology today. Far beyond the traditional uses to screen, stage disease, and follow patients for recurrence or progression, we now have the ability to image across various levels of biological organization, not just organs or tumors, but also molecules, single cells, and tissues. Such information - obtained in real time and noninvasively - can provide important details about whether patients may be candidates for certain therapies or provide a rapid assessment of whether they are responding to treatment.
This special issue of the NCI Cancer Bulletin provides a window into just some of the exciting work being done in the field of cancer imaging, from individual research projects to NCI's support of companies developing new imaging technologies through our Small Business Innovation Research Program or through collaborations with private-sector partners as part of NCI's recently launched Advanced Technology Partnerships Initiative.
The extent of the activity in this field is truly remarkable and too broad for us to do it justice in 8 pages. Researchers from the Siteman Cancer Center in St. Louis, part of the NCI Alliance for Nanotechnology in Cancer, for instance, are using nanoparticles as part of an MRI scan to construct three-dimensional maps of tumor-induced angiogenesis and monitor the effects of drug therapies on those new blood vessels. Other researchers are modifying targeted therapies so that they can be used as imaging agents.
Imaging advances may be able to vastly improve standard treatments like surgery. For instance, a team in NCI's Center for Cancer Research (CCR), led by Dr. Hisataka Kobayashi, has developed a fluorescently tagged cancer-specific antibody that can put a spotlight on micrometastases. Although still at the preclinical stage, this work has the potential to allow cancer surgeons to eradicate insidious tumor "seedlings" well before
they have the chance to threaten a patient's life.
Advances in imaging technology are also providing investigators with the means to explore entirely new realms of molecular biology. In CCR, Dr. Tom Misteli is using live-cell imaging and high-resolution microscopy to map the position of chromosomes within a cell's nucleus, something Dr. Misteli and his team have found can influence how cells function during processes like early tumorigenesis. Their work may eventually point to new possibilities for early detection.
My belief is that every clinical trial should have an imaging component to help answer important research questions. Along those lines, NCI's new Molecular Imaging Clinic at the NIH Clinical Center will play a pivotal role in allowing researchers to quickly assess whether a drug is likely to have its intended biological effects. Such information will speed the development of new drugs into more advanced trials, saving both time and valuable resources.
I hope you find this special issue informative. It underscores the fact that translating this research into the everyday care of patients and the conduct of clinical trials is a high priority for NCI. The progress to date has been remarkable, and I'm confident that its impact on the cancer burden will be substantial.
Dr. John E. Niederhuber
Director, National Cancer Institute |
 |
 |

When NCI's new Molecular Imaging Clinic opens in January 2009, it will be one of the few places in the world where state-of-the-art imaging tools are dedicated to understanding how drugs behave in people with cancer. What is learned there could help investigators determine how best to use existing drugs and determine the properties of new molecularly targeted drugs that are just entering the clinic.
 "Our goal is to use advanced imaging technologies to accelerate the development of therapies for cancer," said Dr. Peter Choyke, who directs the Molecular Imaging Program in NCI's Center for Cancer Research (CCR). He has assembled a multidisciplinary team that includes imaging scientists, chemists, physicists, engineers, oncologists, and molecular biologists. They have many collaborators inside and outside NCI.
"Our goal is to use advanced imaging technologies to accelerate the development of therapies for cancer," said Dr. Peter Choyke, who directs the Molecular Imaging Program in NCI's Center for Cancer Research (CCR). He has assembled a multidisciplinary team that includes imaging scientists, chemists, physicists, engineers, oncologists, and molecular biologists. They have many collaborators inside and outside NCI.
The clinic was created in part because the existing imaging tools in the NIH Clinical Center were needed for patient studies, leaving little time for translational research. As molecularly targeted therapies were emerging several years ago, NCI officials saw the potential importance of imaging studies in drug development.
"Imaging can answer three important questions," said Dr. Choyke. "Does a patient have the target of a particular drug? Does the drug hit the target? And if so, does it do anything helpful for the patient?" When imaging shows that a drug has not reached its destination, researchers can modify the drug or its delivery. Or, they can abandon the project and redirect precious resources elsewhere.
The new facility has undergone extensive renovation and will have the latest scanners for detecting cancer and tracking drugs in the body, including a PET/CT unit, which can simultaneously capture PET and CT images, and 3 Tesla whole-body MRI.
Imaging studies are underway to study the behavior of drugs such as trastuzumab (Herceptin). Experimental imaging agents also will be tested in phase 0 trials - small studies in which patients receive a very low dose of an experimental drug to determine whether it behaves in people as it does in animal or cell models.
Many studies will be partnerships with investigators at NCI, other
institutions, pharmaceutical companies, and the imaging-equipment industry. NCI's Division of Cancer Treatment and Diagnosis played an integral role in the clinic's development and provides critical materials such as radioactive substances that are attached to drugs.
Radiolabeled drugs are given at low doses so they do not result in physiologic effects, but they still go to the same places in the body as drugs given at therapeutic doses. Investigators may eventually make "go" or "no-go" decisions about new drugs based in part on whether they hit their target and whether they have off-target (side) effects.
"Our mission is to use the cameras to do translational studies that will make drug development faster," said Dr. Karen Kurdziel, who directs the new clinic. "There are very few imaging centers dedicated to drug discovery and research. We have an incredible opportunity here."
|
 |
 |

 Dr. Sriram Subramaniam and his team in CCR's Laboratory of Cell Biology develop tools and strategies for capturing images of cells and viruses, with a focus on HIV and cancer. His group recently won an award for the image of a melanoma cell (below) and published three-dimensional (3D) images of the structures HIV uses to enter cells, created with a technique called electron tomography. Dr. Sriram Subramaniam and his team in CCR's Laboratory of Cell Biology develop tools and strategies for capturing images of cells and viruses, with a focus on HIV and cancer. His group recently won an award for the image of a melanoma cell (below) and published three-dimensional (3D) images of the structures HIV uses to enter cells, created with a technique called electron tomography.
 What is your mission?
What is your mission?
Our mission is to develop imaging technologies that can begin to zero in on cells and viruses at progressively higher resolutions. It seems like science fiction, but by using advanced imaging methods and computational tools, one can, in essence, walk into a cell and "see" the structures of specific molecules. This information is critical to understanding how cells are organized, and it also advances our efforts at NCI to discover what makes cancer cells different from normal cells and to design more effective vaccines strategies against HIV. Our technologies are especially powerful for addressing questions in structural biology that have not been tackled previously because the necessary tools did not exist.
What are your interests?
In the spectrum of imaging that runs from small molecules all the way up to humans, there are many gaps in what can be captured. We are most interested in the gap between molecules and cells, which includes structures such as retroviruses and mitochondria, the energy producers of cells. These structures carry out important work, yet they cannot be easily studied at molecular resolution by conventional imaging tools.
Have your melanoma studies revealed surprises?
Yes. These studies have provided fascinating glimpses of the interior of the cell. We have seen shapes that don't look anything like what you might expect based on textbook pictures from electron microscopic imaging of thin sections through these cells. We have also figured out how to translate these images into three dimensions and are developing automated tools to extract the wealth of structural information in these 3D cellular images.
How is this information relevant to patients?
Our technology allows you to mine the cell images
for "markers" that may be related to the fate of the cell or the distribution of antigenic markers and drugs.
For instance, we can quickly determine if there are changes in the proportion of the volume of a melanoma cell that is occupied by mitochondria and connect this information with biochemical findings. These are still early days, but this or a similar quantitative marker based on novel imaging tools may provide diagnostic or prognostic information on cancer. We are essentially charting new territory in documenting what happens within the cell.
|
 |
 |

"Imaging technology is used across the entire spectrum, from very basic research to clinical care," says Dr. James Tatum, associate director of NCI's Cancer Imaging Program (CIP), which is part of the Division of Cancer Treatment and Diagnosis and currently funds a portfolio of over 400 grant projects.
Part of CIP's mandate has been to support areas of cancer-imaging research considered too high-risk for commercial investment. Through its Imaging Development Group, it helps shepherd promising new imaging compounds from discovery through early clinical testing. Several of these new agents are now in clinical trials across the
country, with more in the pipeline.
To speed up and improve early clinical trials of these and other promising new imaging agents, CIP has been working closely with the American College of Radiology Imaging Network (ACRIN), an NCI-funded cooperative group.
Best known for performing large trials of mature imaging technology, such as the National CT Colonography Trial, ACRIN has received assistance from CIP to conduct early multicenter trials as well, explains Dr. David Mankoff, professor of radiology at the University of Washington and an ACRIN investigator. Examples of agents being tested in these smaller trials, coordinated through ACRIN's Experimental Imaging Sciences Committee, include 64Cu-ATSM to measure treatment response in cervical cancer, and 18F-fluoromisonidazole to monitor brain-tumor hypoxia (decreased oxygen supply).
A huge challenge in trials of these and other imaging radioisotopes is that "many of these probes have extremely short half lives, about two hours or so," explained Dr. Mankoff. "You can't just make them in one place and ship them all over the country - you have to have a distribution network." Fortunately, the clinical success of FDG-PET imaging has led to a commercial distribution network to make and distribute the fragile FDG isotope to clinical sites across the country. CIP "has been key" in convincing members of this network to also manufacture experimental probes for ACRIN, said Dr. Mankoff.
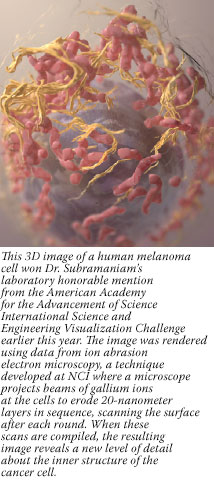
CIP also helps fund advanced imaging research laboratories around the country, including one that has the first dual MRI-PET device in the country, commissioned by Dr. Gregory Sorensen and colleagues at Massachusetts General Hospital.
"We are now able to combine the high spatial resolution and functional information from MRI with the metabolic and receptor information available from PET, to more carefully study tumor hypoxia, angiogenesis, and the link between [tumor] metabolism and response to therapies," explained Dr. Sorensen, who will soon begin the first clinical trial (funded in part by CIP) of dual MRI-PET to monitor patients with brain tumors during treatment.
Imaging technology is also poised to help researchers visualize the intricate inner workings of cancer cells. To this end, CIP has funded eight In vivo Cancer Molecular Imaging Centers that will help advance cellular and molecular imaging related to cancer.
"There is a compelling need to understand cancer from a systems biology perspective, and tumor biology is the most complex system you could possibly imagine," says Dr. Tatum. "It is my own belief that the only way you can understand what goes on in these complex systems in vivo is to be able to monitor or interrogate the system non-invasively employing advanced imaging methods across the resolution from cells to patients."
|
 |
 |

After experiments show that a new imaging technology can work, it's also important to confirm that its use in practice lives up to its promise. This effort is underway through the Division of Cancer Control and Population Sciences' Applied Research Program (ARP).
"We use physician surveys to understand attitudes about new technologies, but we also need to collect data in the course of delivering care to understand whether the new test is performing as it did in the efficacy studies…studies that are, after all, performed with staffing and coordination that may not be present in practice," Dr. Stephen Taplin, senior scientist in ARP, explains.
Dr. Taplin oversees the Breast Cancer Surveillance Consortium, a research network focused on understanding how current breast cancer screening methods operate in practice - how breast density, for example, affects cancer detection and whether new training software can improve radiologists' performance and make mammogram interpretation more accurate.

Mammography is a mature technology, notes Dr. Taplin. "We've had decades to evaluate its strengths and the consequences of its deficiencies." But as new technologies debut at a faster pace, the time to anticipate challenges and ask questions - and the resources available to address them - shrink. One solution for mammography evaluation was a resource-sharing partnership between federal and nonprofit agencies like the American Cancer Society.
Dr. Carrie Klabunde, an epidemiologist in ARP's Health Services and Economics Branch, manages a portfolio of research projects aimed at understanding the uptake of colorectal cancer screening in community practice. She is leading an effort to begin monitoring the use of virtual colonoscopy in the United States.
Virtual colonoscopy is a new technology to screen for colorectal cancer that uses external imaging devices to examine the lining of the colon. "There is still a need for high-quality data on ways of efficiently delivering virtual colonoscopy in practice, and on the procedure's outcomes," she notes.
One issue, for example, is suspicious findings that are inadvertently detected outside of the colon. "These findings could be anything from a tumor on a kidney to something that's completely benign, a lump of fat or normal variation on an organ," explained Dr. Klabunde. "There will be questions about who is responsible for finding out what that lump or bump may be, and who will pay for it. That will be one challenge ahead."
"There is a tremendous need for objective parties to look at issues such as these, because there are so many competing interests involved and because the analysis requires scientific rigor," said Dr. Taplin. "It's important for us to fund investigators who can look carefully and make sure that the technology is achieving the ends that we expect."
To build national capacity for monitoring the performance of colorectal cancer screening modalities, Dr. Klabunde and collaborators at the Agency for Healthcare Research and Quality have sponsored studies to develop and evaluate data systems for tracking colorectal cancer screening performance. A recent supplement to the journal Medical Care describes this research and can be ordered at http://healthservices.cancer.gov/publications/improv_deliv.html.
|
 |
 |

When used in appropriate situations and with careful attention to dosage, leading experts agree that the potential benefit of cancer imaging outweighs the risk. However, there has been mounting concern that several increasingly common imaging modalities may expose patients and health care workers to potentially unsafe radiation doses, the consequences of which only become evident with long-term population-based research.
 |
 |
An Integrative Approach to Cancer Imaging |
 |
|
The Division of Cancer Biology's Integrative Cancer Biology Program supports nine centers that are developing new models and tools to study cancer, with expertise in the fields of chemistry, physics, mathematics, and the computer sciences, as well as biology and medicine. At Massachusetts General Hospital and Harvard University, for example, they have a Center for the Development of a Virtual Tumor, where biomedical images are being used to help create computational models of gliomas that may predict cell behavior, guiding the development of new treatments.
Similarly, NCI has hosted a series of think tanks this year to convene physical scientists and oncologists on the topic of cancer. More workshops will be organized through NCI's Office of Technology and Industrial Relations. |
|
Computed tomography (CT) has come under intense scrutiny because the number of procedures has skyrocketed to more than 60 million annually, a nearly threefold increase since the early 1990s, and the average number of scans per patient is also increasing. The wider use of CT can be traced in large part to its speed and its remarkably clear images of organs and tissues. But the downside, explains Dr. Elaine Ron, a senior investigator in NCI's Division of Cancer Epidemiology and Genetics (DCEG), is that CT requires significantly greater doses of radiation compared to a conventional x-ray. In the case of abdominal CT, for example, the dose is 50 times higher.
Among the chief concerns is use of CT in children. With their still-developing bodies, experts warn, they are more prone to radiation-induced effects that can increase cancer risk. Such concerns led NCI to partner with the Society for Pediatric Radiology to develop a brochure, recently updated, to help educate health care workers about appropriate CT use in children and the potential risks of CT-emitted ionizing radiation. DCEG researchers are also collaborating with researchers from Newcastle University in the United Kingdom and Maccabi Healthcare Services in Israel to investigate the risk of cancer in children and adolescents who have undergone CT scans.
CT is not the only radiologic procedure that NCI researchers are studying. Other diagnostic techniques and treatments that rely on a type of x-ray technology called fluoroscopy, especially coronary angiograms and angioplasty, are proliferating. Because of the length of these procedures, the cumulative exposure to radiation is considerable. In conjunction with five medical societies, NCI has launched a study to determine whether radiologists and cardiologists, among others, who are involved in fluoroscopy-guided procedures have a higher risk of cancer.
The research began because of reports of certain cancers that appear to arise more often among physicians who perform fluoroscopy, explained Dr. Amy Berrington de Gonzalez, an investigator in DCEG's Radiation Epidemiology Branch. There have been efforts by NCI and other medical societies to educate medical personnel about the potential radiation-related risks associated with these procedures. However, the available evidence, including some surveys conducted by the FDA, suggests the message still isn't getting through, particularly to those without extensive radiology training, she stressed.
The long-term study will compare cancer-related deaths between
physicians regularly involved in radiation-related procedures and those who are not involved in such procedures. "Then we'll be able to determine whether increasing use of these new imaging technologies are associated with an elevated cancer risk," she says.
|
 |
 |

NCI recently completed the retooling of its Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR)
Programs to enhance their role as an engine of innovation, and cancer imaging technology is one of the
top priorities for the programs.
In 2007 at the request of former NIH Director Dr. Elias Zerhouni, NCI took the leadership role in developing new initiatives for the small business set-aside research programs across all NIH institutes. One of the major changes was to consolidate the management of NCI's more than 300 small business projects - previously scattered across the Institute's numerous research divisions - into a new NCI SBIR Development Center, staffed by professionals with specific industry and entrepreneurial expertise. Cancer imaging technologies are the largest single component of NCI's small business portfolio, making up about 30 percent of all such projects.
Dr. David Beylin was recently hired for NCI's small business center. He came to NCI from Naviscan, Inc., a medical device company which used SBIR funds to take its PEM Flex PET Scanner - a device that combines PET technology with mammography, revealing with high accuracy and sensitivity breast tumors as small as a grain of rice - through the stages of development, prototype building, and clinical trials. The SBIR funds enabled Naviscan to raise more than $20 million of venture capital, obtain FDA clearances, and start selling the device in the United States.
To avoid the "valley of death" that many former SBIR projects encounter after the NCI funding for their projects ends and before private investors are willing to pledge support, another new NCI initiative offers small businesses the opportunity to compete for SBIR Bridge Awards to extend funding up to a total of $3 million over an additional 3 years.
Cancer imaging and therapeutics are the two priority areas for the Bridge Awards, noted NCI SBIR Development Center Director Michael Weingarten. Recently for
the first time, the SBIR peer-review panels for the Bridge Awards included panelists with venture capital and entrepreneurial backgrounds. The reviewers' discussions "broke important new ground by evaluating both the science and the commercialization strategies of the companies' proposals," he noted. "The salient feature of this award is that it encourages partnerships between NIH's SBIR Phase II awardees and third-party investors or strategic partners that have significant prior experience in the commercialization of emerging technologies."
 |
 |
| Showcasing CCR's Technology Advances |
NCI co-hosted a technology fair (pictured above) at the Bethesda Marriott Conference Center in 2007 with several Maryland State and Montgomery County organizations, to showcase CCR's technology advances that are available for commercial licensing and to profile intramural research projects for potential industrial collaborators.
Meeting attendees also learned how to license technology, collaborate with intramural researchers, and obtain funding |
for small businesses during sessions led by members of the NIH Office of Technology Transfer, the NCI Technology Transfer Center, and NCI's SBIR/STTR Program.
Another technology event may be scheduled for late 2009 with future industry outreach initiatives being planned on a regular basis thereafter. For more information, contact Eric Hale, associate director of CCR's Office of Policy and Intellectual Property, at 301-594-9254. |
|
 |
 |

| For Researchers and Industry |
|
 |
 |
 |
 |
 |
 |
U.S. Cancer Incidence and Mortality Drop |
 |
|
Both overall incidence of cancer and cancer deaths dropped for the first time in U.S. men and women, according to a report on current cancer trends published by the American Cancer Society, CDC, NCI, and North American Association of Central Cancer Registries. The trends are based on data from 1975 through 2005 and were reported online November 25 in the Journal of the National Cancer Institute.
The overall decline in incidence and mortality is due largely to decreases in the three most common cancers in men - prostate, lung, and colorectal - and in two of the three most common cancers in women - breast and colorectal.
This year's report also includes state-by-state information on lung cancer incidence and mortality, as well as trends of tobacco use and control. Although overall lung cancer mortality in women has stabilized since 2003, California was the only state to show a statistically significant decline in both lung cancer incidence and death for men and women. By contrast, lung cancer incidence and/or mortality increased in 18 states, most of which are in the Midwest or South. Ten states did not have incidence data available.
|
 |
|
Tumor Environment Linked to Survival in Lymphoma
In diffuse large B-cell lymphoma (DLBCL), researchers have identified three patterns of gene activity in and around tumors that may determine survival following therapy. These patterns, or gene signatures, were associated with survival in three independent populations of patients treated with standard therapies for the disease.
The results suggest that DLBCL, like other cancers, is not a single disease but rather consists of multiple, biologically distinct subtypes. In addition, the study highlights the importance of the tumor microenvironment - the nonmalignant host cells adjacent to tumors - in some forms of the disease.
Dr. Louis M. Staudt of NCI's Center for Cancer Research (CCR) and his colleagues in the Lymphoma/Leukemia Molecular Profiling Project published their findings in the November 27 New England Journal of Medicine.
"Interestingly, we found that it's not just the characteristics of the malignant cells that influence patient survival, but also features of the tumor microenvironment in the lymph node," said Dr. Georg Lenz of CCR, the study's first author.
The team analyzed 414 pretreatment biopsy specimens from two groups of patients and linked the results to clinical outcomes. This revealed a previously unknown gene signature and confirmed two signatures identified in an earlier study. One of these signatures, called germinal-center B-cell, is based on gene activity in malignant lymphoma cells; it distinguishes two forms of the disease, known as germinal-center B-cell-like and activated B-cell-like DLBCL.
All three signatures were validated in a third patient population; each was also found to predict survival after treatment with combination chemotherapy called CHOP or CHOP plus rituximab (Rituxan).
The researchers suggest that their results have implications for clinical trials. Some patients have a better prognosis regardless of the therapy, and this could make a drug seem better than it actually is. "When you are enrolling patients on clinical trials, you need to know which kind of DLBCL is being treated so that the results can be assessed accurately and compared across trials," said Dr. Staudt.
For instance, patients with a tumor-microenvironment signature called stromal 1 tended to respond to treatment. But those with a microenvironment signature called stromal 2 had an inferior prognosis.
The stromal 2 signature includes genes linked to angiogenesis, the growth of tumor blood vessels. Based on this finding, the researchers propose that these patients may benefit from antiangiogenic drugs, such as bevacizumab (Avastin), while other patients may see little benefit from these agents.
"One wonders whether an antiangiogenic agent should be added to the treatment of patients with a lymphoma containing the stromal 2 signature," writes Dr. Charis Eng of the Genomic Medicine Institute at the Cleveland Clinic in an accompanying editorial.
Dr. Eng praised the study, noting that the results are consistent with recent studies showing a connection between the tumor microenvironment and clinical outcomes in other cancers.
"These findings give us a completely new way of thinking about this lymphoma," said Dr. Staudt. Researchers may now shift some attention away from malignant cells and toward developing a better understanding of tumor microenvironment, he added.
After Menopause, Weight Affects Breast Cancer Rates More than Mammography Use
Women who are overweight or obese after menopause face an increased risk of breast cancer, but a large prospective cohort study indicates that the frequency of mammography use and screening accuracy are not the primary explanations for higher rates of breast cancer in these women. The same is true of large, invasive breast cancer tumors and advanced stage disease; risk increases with weight, but higher rates are not explained by the frequency or accuracy of screening mammography before breast cancer was diagnosed. The study appears in the December 3 Journal of the National Cancer Institute.
Dr. Karla Kerlikowske of the San Francisco Veterans Affairs Medical Center and colleagues gathered data on 287,115 postmenopausal women who were registered in the Breast Cancer Surveillance Consortium database. Reflecting a trend in the U.S. population, 58 percent of the women in the study were overweight or obese. The women were not using postmenopausal hormone therapy when they received their mammograms between 1996 and 2005; 4,446 of them were diagnosed with breast cancer within a year of getting a mammogram.
Compared to women with a normal body mass index (BMI 18.5-24.9), risk for the first year after a mammogram was about 12 percent higher in overweight women (BMI between 25-29.9), 20 percent higher with a BMI between 30-34.9, and 30 percent higher when their BMI was higher still. Similar but independent patterns were found for high-grade, advanced-stage, estrogen receptor-positive, and large invasive disease. Factoring in the frequency of the women's previous mammogram use did not change these results.
Though under-using mammography did not entirely account for the higher rate, the authors cite its value as "the only secondary prevention measure that has been proven to decrease breast cancer mortality" by detecting disease at an earlier, more treatable stage. And though the link found here between higher BMI and advanced stage disease was moderate, it was also conclusive. "Our results suggest that postmenopausal women who are overweight or obese…should be encouraged to lose weight and to undergo routine screening mammography, two factors that may decrease the number of women who are diagnosed with advanced disease," the authors wrote.
Breast Cancer Family History Boosts Risk, Even without BRCA Mutations
Women who test positive for gene mutations associated with breast cancer often take steps to prevent the disease. But little is known about what steps, if any, women should take if they have a family history of breast cancer but lack BRCA1 or BRCA2 gene mutations. Now, a new study suggests that the risk among these women may be high enough that they should discuss prevention options with their physicians, such as screening with breast MRI and chemoprevention with tamoxifen or raloxifene, according to the study's author.
Women with a significant family history of breast cancer have an approximately fourfold increased risk of developing breast cancer compared with the general population, even if they do not carry the high-risk mutations, the study found. A significant family history is defined as two or more close relatives with the disease under age 50, or three close relatives with the disease at any age.
Dr. Steven Narod of the University of Toronto presented the findings last month at the Frontiers in Cancer Prevention Research conference near Washington, D.C. He urged physicians to discuss prevention with the thousands of women who test negative but have a significant family history of breast cancer. "We've been doing this testing since 1995, and there is a huge population of women out there whose risk is very high. And yet they have not been targeted for prevention," he said at a press briefing.
"We don't need to wait for the future," Dr. Narod added. "We should be able to implement this now."
His team followed 1,492 women who tested negative for BRCA1 and BRCA2 mutations from 365 families for at least 5 years. Based on the number of breast cancers in the group versus the number expected in the general population, the researchers calculated that a significant family history was associated with a 30 to 40 percent lifetime risk. By comparison, carriers of BRCA mutations have a lifetime risk of the disease as high as 85 percent.
|
 |
|
|
 |