|
CFSAN/Office of Nutrition, Labeling, and Dietary Supplements
April 2008
Answer: The statement of identity is the name of the food. It must appear on the front label, or PDP as well as any alternate PDP. 21 CFR 101.3
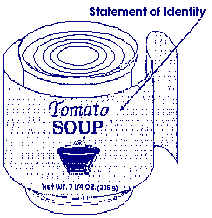 Should the statement of identity stand out?
Should the statement of identity stand out?
Answer: Use prominent print or type for the statement of identity. It shall be in bold type. The type size must be reasonably related to the most prominent printed matter on the front panel and should be one of the most important features on the PDP. Generally, this is considered to be at least 1/2 the size of the largest print on the label. 21 CFR 101.3(d)
Answer: The name established by law or regulation, or in the absence thereof, the common or usual name of the food, if the food has one, should be used as the statement of identity. If there is none, then an appropriate descriptive name, that is not misleading, should be used. 21 CFR 101.3(b)
Answer: Place the statement of identity in lines generally parallel to the base of the package. 21 CFR 101.3(d)
 When are fanciful names permitted as the statement of identity?
When are fanciful names permitted as the statement of identity?
Answer: When the nature of the food is obvious, a fanciful name commonly used and understood by the public may be used. 21 CFR 101.3(b)(3)
Answer: The common or usual name must be used for a food if it has one. It would be considered misleading to label a food that has an established name with a new name. If the food is subject to a standard of identity it must bear the name specified in the standard. 21 CFR 101.3(b)(2)

 Should modified statements of identity be used for sliced and unsliced versions of a food?
Should modified statements of identity be used for sliced and unsliced versions of a food?
Answer: Labels must describe the form of the food in the package if the food is sold in different optional forms such as sliced and unsliced, whole or halves, etc. 21 CFR 101.3(c)

 What food must be labeled as an "imitation"?
What food must be labeled as an "imitation"?
Answer: Generally a new food that resembles a traditional food and is a substitute for the traditional food must be labeled as an imitation if the new food contains less protein or a lesser amount of any essential vitamin or mineral.
Answer: Use the same type size and prominence for the word "imitation" as is used for the name of the product imitated.
 Are there restrictions on label artwork?
Are there restrictions on label artwork?
Answer: Do not use artwork that hides or detracts from the prominence and visibility of required label statements or that misrepresents the food. 21 CFR 1.21(a)(1), 21 CFR 101.3(a), 21 CFR 101.105(h)
 Where should the country of origin be declared on an imported food?
Where should the country of origin be declared on an imported food?
Answer: The law does not specifically require that the country of origin statement be placed on the PDP, but requires that it be conspicuous. If a domestic firm's name and address is declared as the firm responsible for distributing the product, then the country of origin statement must appear in close proximity to the name and address and be at least comparable in size of lettering. (FDA/CBP (Customs and Border Protection) Guidance and Customs regulation 19 CFR 134)
 Are foreign language labels permitted?
Are foreign language labels permitted?
Answer: All required label statements must appear both in English and in the foreign language if any representations appear in a foreign language. 21 CFR 101.15(c)(2)
Answer: Beverages that purport to contain juice (fruit or vegetable juice) must declare the % of juice. Included are beverages that purport to contain juice by way of label statements, by pictures of fruits or vegetables on the label, or by taste and appearance causing the consumer to expect juice in the beverage. This includes non-carbonated and carbonated beverages, full-strength (100%) juices, concentrated juices, diluted juices, and beverages that purport to contain juice but contain no juice. 21 CFR 101.30(a)
Answer: The % juice must be on the information panel (for packages with information panels), near the top. Only the brand name, product name, logo, or universal product code may be placed above it. Use easily legible boldface print or type that distinctly contrasts with the other printed or graphic material. The type size for the % juice declaration must be not less than the largest type on the information panel, except that used for the brand name, product name, logo, universal product code, or the title phrase Nutrition Facts. The percentage juice declaration may be either "contains____% juice" or "____% juice." The name of the fruit or vegetable may also be included (e.g., "100% Apple Juice"). If the package does not contain an information panel, the percent juice must be placed on the PDP in a type size not less than that required for the net contents declaration and placed near the name of the food. 21 CFR 101.30(e); 21 CFR 101.30(g)
Answer: An exception is that beverages containing minor amounts of juice for flavoring are not required to bear a % juice declaration provided that: (a) The product is described using the term "flavor" or "flavored," (b) The term "juice" is not used other than in the ingredient list, and (c) The beverages do not otherwise give the impression they contain juice. 21 CFR 101.30(c)
Answer: For juice expressed directly from fruit or vegetables: Compute on a volume/volume basis.
For juice made by adding water to concentrate: Calculate using values from the Brix table in 21 CFR 101.30(h)(1) as the basis for 100% juice. 21 CFR 101.30(j), 21 CFR 101.30(h)
Answer: Beverages that are 100% juice may be called "juice." However, beverages that are diluted to less than 100% juice must have the word "juice" qualified with a term such as "beverage," "drink," or "cocktail." Alternatively, the product may be labeled with a name using the form "diluted ____ juice," (e.g. "diluted apple juice"). 21 CFR 102.33(g)
Answer: Juices made from concentrate must be labeled with terms such as "from concentrate," or "reconstituted" as part of the name wherever it appears on the label. An exception is that, in the ingredient statement, the juice is declared as "concentrated ____ juice and water" or "water and concentrated ____ juice," as appropriate. 21 CFR 102.33(g)
Answer: When stated, names of juices (except in the ingredient list) must be in descending order of predominance by volume, unless the label indicates that the named juice is used as a flavor. Examples:
If the label represents one or more but not all the juices (except in the ingredient list), then the name must indicate that more juices are present. Examples:
When one or more, but not all, juices are named and the named juice is not the predominant juice, the name of the beverage must either state that the beverage is flavored with the named juice or declare the amount of the named juice in a 5% range. Examples (for a "raspcranberry" beverage that is primarily white grape juice with raspberry and cranberry juices added):
Answer: The term "from concentrate" or "reconstituted" must be no smaller than one-half the height of the letters in the name of the juice. The 5% range information generally should be not less than one-half the height of the largest type appearing in the common or usual name (may not be less than 1/16th inch in height on packages with 5 sq. in. or less area on the PDP, and not less than 1/8 inch in height on packages with a PDP greater than 5 sq. in.
Answer: Under 21 CFR 101.30(a), a beverage purports to contain fruit or vegetable juice if the product's advertising, label, or labeling, bears the name of, or makes any other direct or indirect representation with respect to any fruit or vegetable juice, or the label or labeling bears any vignette (i.e., depiction of a fruit or vegetable) or another pictorial representation of any fruit or vegetable, or product contains color and flavor that gives the appearance and taste of a fruit or vegetable juice. The beverages may be carbonated or noncarbonated, full strength, diluted, or contain no juice.
Answer: Bar mixes are subject to the same requirements as other beverage products. Thus, a percent juice declaration would be required on labels of bar mixes that meet the definition set out in 21 CFR 101.30(a).
Answer: No. A percent juice declaration would not be required on the whiskey sour mix if the only reference to the lemon juice is in the ingredient statement and no pictures of fruits/fruit juice appear on the label or in its labeling.
Answer: A strawberry daiquiri mix would purport to contain strawberries or strawberry juice because the term "strawberry" appears in the identity statement. Also, there is no indication that the strawberry is present only as a flavor or flavoring. If its label or labeling also includes pictures of the juice dripping from strawberries or if the product looks and tastes like it contains strawberry juice or strawberry pulp, the product would have to bear a declaration of the percent of juice or the absence of such juice on the information panel of the label. However, if the product were labeled "Strawberry flavored daiquiri mix " and did not otherwise purport to contain strawberry juice, it would not need a percent juice declaration.
Answer: Bloody mary mix, by appearance and taste, purports to contain tomato juice and thus would be required to bear a statement as to the percentage of juice contained in the product.
Answer: The declaration is required if the product purports to contain juice. However, because FDA has not established specific procedures for calculating the percentage of juice when beverages are prepared by rehydrating juice solids, it will evaluate labels of products made by this process on a case by case basis. Brix values, where provided in 21 CFR 101.30(h), may be used as guidelines in calculating the level of total juice solids necessary to prepare full strength juices, provided the beverage does not contain other non-juice ingredients.
Answer: Yes. The percentage juice declaration would be based on the anhydrous citric acid content of the lemon juice or lime juice, listed in 21 CFR 101.30(h)(1).
Answer: Apple cider is juice that is expressed from apples and must bear a declaration of the percent of juice.
Answer: No. Apple cider vinegar does not purport to be a beverage and thus is not required to bear a percent juice declaration. Although the product is made from apple juice, it is not considered to be a juice beverage.
Answer: Concentrated juice products must bear a percentage juice declaration and that declaration may not be greater than 100 percent. The label may explain that when the product is diluted according to label directions, the product yields a "___percent juice from concentrate," with the blank being filled in with the correct percentage based on the Brix values set out in 21 CFR 101.30(h)(1), as applicable.
Answer: No, there is no specific exemption from the requirement that the percent juice declaration be on the information panel of individual juice packages packed in a multi-unit shrink wrap pack.
Answer: The entire common or usual name must be in one place. If some or all of the juices listed in the name are from concentrate, the term "from concentrate" must follow the names and may be in a smaller type size, but not less than one half the height of the letters in the other part of the common or usual name.
Answer: FDA has not established specific requirements for vignettes on labels of juice beverages. FDA urges manufacturers to use vignettes that accurately depict each fruit or vegetable contained in the multiple juice products. However, a vignette depicting only some of the fruits or vegetables may not be considered misleading, if the name of the food adequately and appropriately describes the contribution of the pictured juice. For example, a 100 percent juice consisting of apple, grape and raspberry juices, in which raspberry juice provides the characterizing flavor, and bears a vignette that only depicts raspberries, would not necessarily be misleading if the identity statement were "raspberry juice blended with apple and grape juices." Alternatively, the statement of identity may be "raspberry flavored fruit juice blend" or "raspberry juice in a blend of two other juices, 3 to 8 percent raspberry juice" (58 FR 2897 at 2921).
Answer: Yes. The soluble solids content for tomato juice must be determined before addition of any spices. The soluble solids for tomato juice, determined by refractometer, should be corrected for salt content as prescribed in 21 CFR 156.3(b) and (c).
Answer: If ascorbic acid is added at levels consistent with fortification of the juice, its declaration in the name of the 100 percent juice would constitute a nutrition claim, triggering compliance with the "more" claim for vitamin C and the comparison statements. However, it is not necessary to specifically list the added ingredient by name in the 100 percent juice statement (i.e., the statement of identity could be "100 percent ___juice with preservative"). In this case the ascorbic acid would be added as a chemical preservative and listed by name in the ingredient statement in accordance with 21 CFR 101.22(j).
Answer: No. Section 102.33(g) states that if one or more of the juices in a juice beverage is made from concentrate, then the name of the juice must include the term "from concentrate" or "reconstituted." Because the names "fruit punch" and "lemonade" do not include the name of a specific juice, these names do not have to contain the term "from concentrate" or reconstituted."
Answer: Yes, before adding sugar.
Answer: No. There are no exemptions from the requirement for label declaration of the percentage of juice on food service containers of juices.
Answer: The regulation is applicable to both.
Answer: There are several alternatives. In the first case, the common or usual name may be "a blend of 3 citrus juices from concentrate with ______ and ____juices", the blanks filled in with names of the expressed juices. In the second case, the citrus juice that is not from concentrate should be listed as in the example given above in order of predominance, i.e., a blend of 2 citrus juices from concentrate with _____, ______, and ______juices, with the third citrus juice listed in one of the blanks, along with the other expressed juices. Alternatively, a name such as "citrus punch" or "citrus flavored punch" may be used as the statement of identity without further identification of the component juices.
Answer: Yes, sometimes. If the juices are specifically named in the statement of identity, and the juices are from concentrate, their names must be followed by the term "from concentrate" in accordance with 21 CFR 102.33(g). If no reference is made to specific juices in the name of a punch that is made from concentrated juices, the statement of identity does not have to include the term "from concentrate." However, each of the concentrated juices used in the punch must be declared in order of predominance in the ingredient statement of the label.
Answer: No. FDA does not have a specific definition or standard of identity for punch, or any other requirement that a punch contain fruit juice. A punch may be an artificially flavored beverage, with or without natural flavorings, or it may be made from tea and other ingredients, exclusive of fruit juice. Such products must be clearly distinguished from products which are made from fruit juices or fruit concentrates or purees. Products containing artificial or natural flavors must be labeled in accordance with 21 CFR 101.22.
Answer: Yes.