
Questions Decision Trees Table Key Words/Related Topics
Citation:
900.12(e)(9)(i),(ii),(iii),(iv),(v): Surveys.
(i) At least once a year, each facility shall undergo a survey by a medical physicist or by an individual under the direct supervision of a medical physicist. At a minimum, this survey shall include the performance of tests to ensure that the facility meets the quality assurance requirements of the annual tests described in paragraphs (e)(5) and (e)(6) of this section and the weekly phantom image quality test described in paragraph (e)(2) of this section.
(ii) The results of all rests conducted by the facility in accordance with paragraphs (e)(1) through (e)(7) of this section, as well as written documentation of any corrective actions taken and their results, shall be evaluated for adequacy by the medical physicist performing the survey.
(iii) The medical physicist shall prepare a survey report that includes a summary of this review and recommendations for necessary improvements.
(iv) The survey report shall be sent to the facility within 30 days of the date of the survey.
(v) The survey report shall be dated and signed by the medical physicist performing or supervising the survey. If the survey was performed entirely or in part by another individual under the direct supervision of the medical physicist, that individual and the part of the survey that individual performed shall also be identified in the survey report.
Approved Alternative Standards:
System Artifact Testing of Target Filter Combinations
Discussion:

Yes. The report of the medical physicist’s survey must contain the name of the individual who performed the survey. This is essential since the individual who performed the survey is the one whose qualifications must be evaluated to determine if he/she meets the MQSA requirements. If another individual was also involved in conducting the survey, that individual’s name must be included in the report as well.
The inspector will include the following comment in the printable remarks section of the report: "The latest available physicist’s survey report was reviewed during the previous annual inspection. It is less than 14 months old and therefore does not constitute a non-compliance."
The inspector will discuss with the facility the date by which they must have a new physicist’s survey performed in order to remain in compliance.
Direct supervision means that the supervisor (if the supervision is done after 4/28/99, the supervising medical physicist must have qualified under the Master’s or higher pathway) is present to observe and correct, as needed, the performance of the supervisee. This requires that the supervisor be in the room during the performance of the individual equipment tests to assure that any mistakes made by the supervisee are corrected before the test is completed. The supervisor must review any calculations made from, and any conclusions drawn from the test results, before those results are provided to the facility.
Furthermore, when conducting a physics survey, the supervisor and supervisee must jointly review the QC program records. The supervisor does not have to be present when the supervisee initially reviews the QC program records. However, the supervisor must review, discuss, confirm, and if necessary, correct the findings made by the supervisee prior to either the initial or final survey report being issued.
The goal of direct supervision is to provide reasonable assurance that any mistakes made by the supervisee are corrected before the QC program review or tests are completed.
Once a year, each facility must be surveyed by a medical physicist or by someone in training under his or her direct supervision. The survey will include:
(1) performance of the annual tests described in the regulations (Table 2 above and the QC tests for other modalities, if applicable) and the weekly phantom image quality test;
(2) review of the results of the QC tests conducted by the facility (Table 1 above), as well as of written documentation of any corrective actions taken and their results; and
(3) a report, which must be provided to the facility within 30 days of the date of the survey, that includes a summary of the areas tested and reviewed and recommendations for necessary improvements.
The annual tests must be performed using technique factors and test conditions as stated in the regulations whenever those factors are specified. Otherwise, technique factors that are clinically used in the facility for which the annual survey is conducted should be used whenever possible. For test procedures that determine dose to the average breast, the same kVp value (within +/- 1 kVp of that used clinically for the average breast) must be used for measuring both the entrance skin exposure and the HVL.
The report must be dated and signed by (or contain the identification of) the medical physicist performing or supervising the survey and any other individual who performed or assisted in the survey. If the survey is done over a period of time, the dates of completion of the individual parts must be indicated in the report.
For a unit with multiple target/filter combinations, the following tests must be performed for each clinically used target/filter combination:
Focal spot condition (for different target materials (tracks) only) (21 C.F.R. 900.12(e)(5)(iii)(A)(3)
X-ray field/light field/image receptor/compression paddle alignment (for different target materials (tracks) only) (21 C.F.R. 900.12(e)(5)(vii))
Beam quality and half-value layer (21 C.F.R. 900.12(e)(5)(iv) and (vi))
System artifacts (21 C.F.R. 900.12(e)(5)(ix)) Also see Approved Alternative Standards
It depends. For the majority of the QC tests, the type of screen-film combination used in the test is irrelevant to the test outcome. However, for the following QC tests, the regulations spell out specific requirements:
1. System Resolution - must be measured for each screen-film combination used at the facility with its corresponding unit(s).
2. Phantom Image and Dose – each of these must be conducted for each screen-film combination clinically used for the standard breast.
Note that the phantom image test applies to both the weekly QC and the annual test conducted by the medical physicist as part of the survey report. If only one combination is routinely used for the standard breast and the other combination is used for non-routine examinations of the standard breast, FDA recommends that the dose and phantom image QC tests also be conducted for the other combination, because the outcome of both tests is heavily influenced by the film-screen combination used.
Note that testing for the uniformity of screen speed must be conducted for all screens and cassettes respectively. Hence, by default, it includes all types of screens used, but this does not preclude performing this test with only one type of film. System artifacts must be performed for each cassette size.
Yes. While the medical physics survey must be performed annually, FDA realizes that surveys cannot usually be scheduled exactly on the anniversary date of the previous survey. Therefore an occasional period of up to 14 months between surveys is acceptable. Facilities may choose, however, to have the physics surveys performed at higher frequencies (shorter intervals) during any annual cycle.
It depends. If your medical physicist recommended that you fix the part(s) of your equipment or practices that failed to meet FDA’s requirements, then you will be cited for not implementing those recommendations. You will not be cited for failure to implement recommendations regarding items that are not required in the regulations. If your medical physicist’s recommendations concerned problems that he/she thought you may encounter if you did not take action, and if you chose not to implement the recommendations, you should at least document that you considered such recommendations and the reasons you did not implement them.
Yes. All testing conducted to satisfy the annual physicist survey requirements of 900.12(e)(9) must be performed by, or under the direct supervision of, an MQSA qualified medical physicist.
|
Facility Survey |
All tests as described in 900.12(e)(2), (e)(5), and, if applicable, (e)(6). Evaluate adequacy of the results of all tests conducted by the facility in accordance with 900.12(e)(1) through (e)(7)
|
|
Survey of a mammography unit |
All tests as described in 900.12(e)(2), (e)(5) {except e(5)(viii)}, and if applicable (e)(6)
|
|
Equipment evaluation of a unit or processor that has been installed or disassembled and reassembled
|
All applicable tests and equipment requirements described in 900.12(b) and (e) |
|
Equipment evaluation of a unit or processor that has undergone a major repair |
Only those tests and equipment requirements described in 900.12(b) and (e) that are applicable to the repaired component of the unit or processor. The decision as to what constitutes applicable tests and equipment requirements for the repaired component should be made by the medical physicist.
|
For more information, check additional guidance about the specific item being installed, reassembled or repaired.
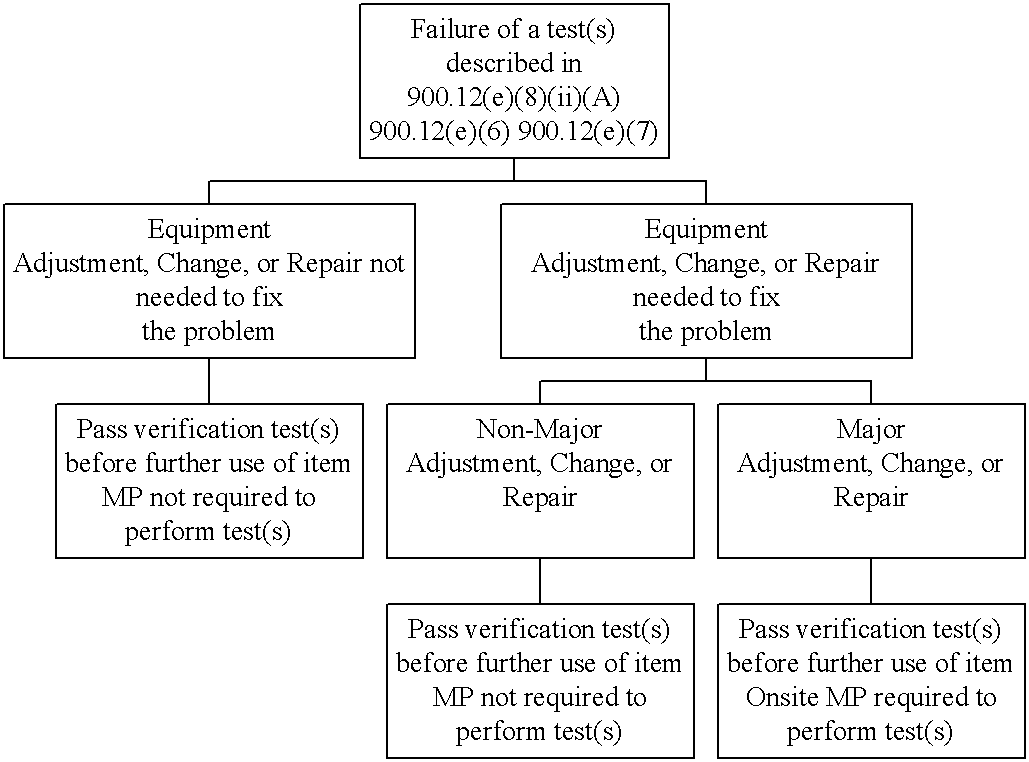
After the adjustment, change, or repair has been completed, the test(s) have to be repeated and must show that the unit is within the appropriate action limit (verification test). For failures of the tests described in 900.12(e)(8)(ii)(A), 900.12(e)(6), 900.12(e)(7) and/or after adjustments, changes, or repairs that are classified as major, the verification test must be passed prior to using the item clinically. Note: verification testing performed after a major repair must be performed on-site by the medical physicist as required by 900.12(e)(10).
For failures of the tests described in 900.12(e)(8)(ii)(B), the facility has a maximum of 30 days from the date of the initial test failure to correct the problem and pass the verification test. Facilities should have the verification test(s) performed (either by the medical physicist or by a qualified individual) as soon as possible after the adjustment, change, or repair is done, so as to allow time to repeat the adjustment, change, or repair if the equipment does not pass the verification test. Note: if the verification test is not passed within the 30 day time period, the item cannot be used clinically until such time as it is truly repaired and passes the test.
For non-major equipment adjustments, changes, or repairs that are performed for any reason other than for failure of a required test, the facility must correct any problems found and perform verification tests to ensure that the equipment remains in compliance with the regulations. This should be accomplished as soon as reasonably possible.
If the adjustment, change, or repair constitutes a "major repair (see Medical Physicist Involvement in Equipment Adjustments, Changes, or Repairs table)," the medical physicist must repeat the test(s) as part of the required follow-up equipment evaluation. If the adjustment, change, or repair does not constitute a "major repair," the test(s) can be performed by any qualified person (e.g. radiologic technologist or service representative with appropriate training/experience). However, in this circumstance, FDA recommends that the medical physicist be consulted during this process.
The following decision trees may be used to help in determining the timing and extent of physicist and facility involvement in response to test failures, equipment adjustments/changes/repairs and equipment evaluations.
Decision Tree #1. Failure of a test(s) described in 900.12(e)(8)(ii)(A): Use of test results, 900.12(e)(6): Quality control tests -- other modalities, 900.12(e)(7): Mobile units.
Decision Tree #2. Failure of a test(s) described in 900.12(e)(8)(ii)(B): Use of test results.
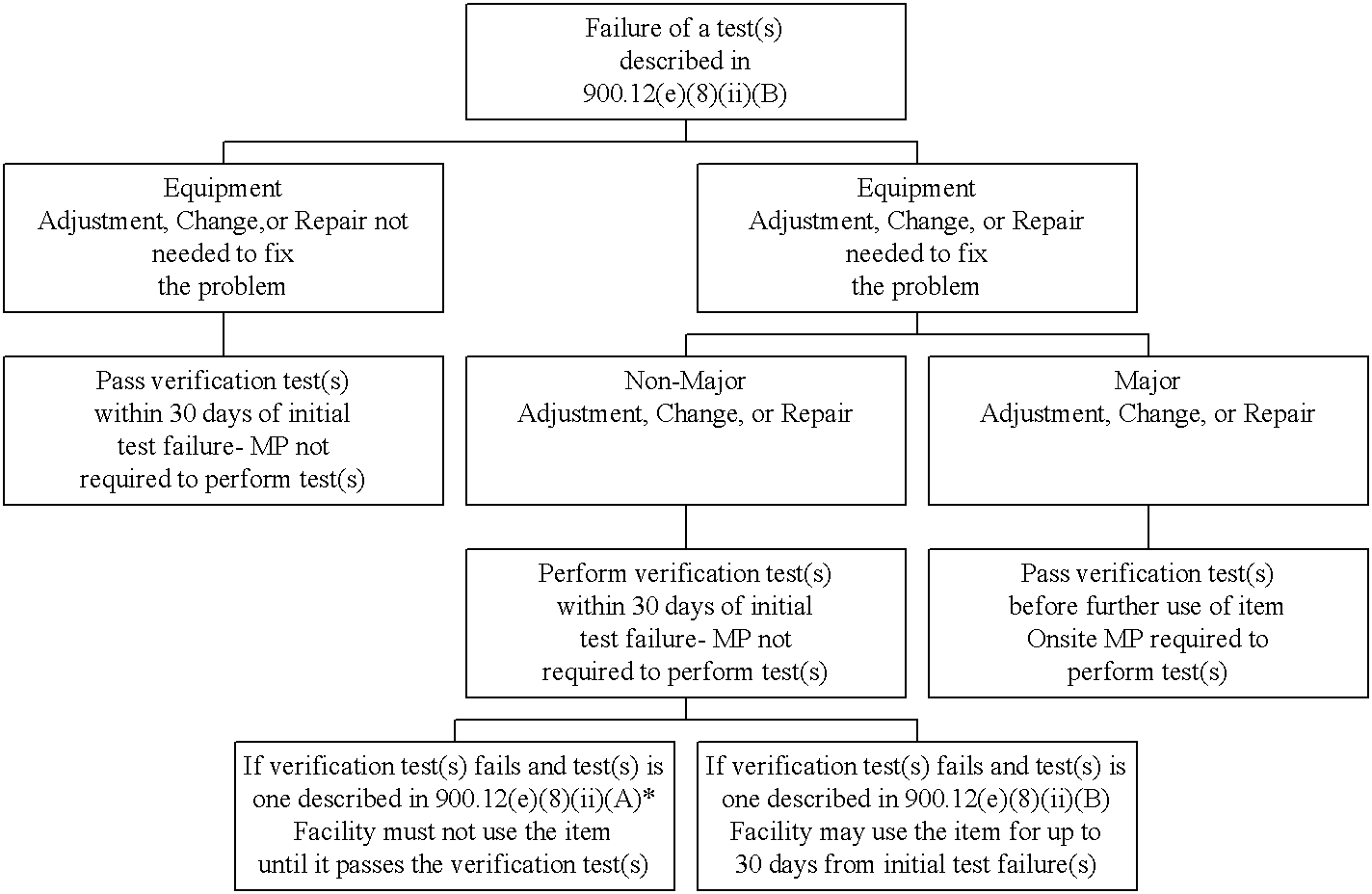
*Note: In some cases, failures of the test(s) described in 900.12(e)(8)(ii)(B) and non-major adjustments, changes, or repairs may necessitate a verification test(s) described in 900.12(e)(8)(ii)(A). In those cases where an item fails its 900.12(e)(8)(ii)(A) verification test(s), the item cannot be used until such time as the verification test is passed.
Decision Tree #3. Non-major equipment adjustment, change, or repair for any reason other than failure of a required test.
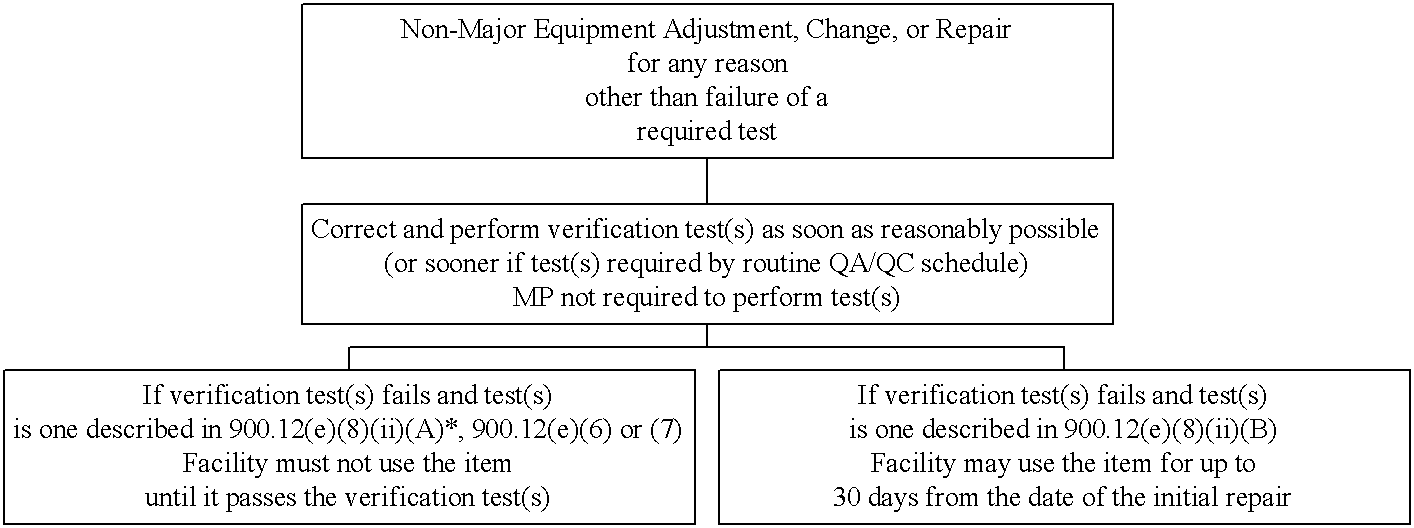
*Note: In some cases, failures of the test(s) described in 900.12(e)(8)(ii)(B) and non-major adjustments, changes, or repairs may necessitate a verification test(s) described in 900.12(e)(8)(ii)(A). In those cases where an item fails its 900.12(e)(8)(ii)(A) verification test(s), the item cannot be used until such time as the verification test is passed.
Decision Tree #4. Equipment evaluations (new equipment, reassembly, or major adjustments, changes, or repairs).
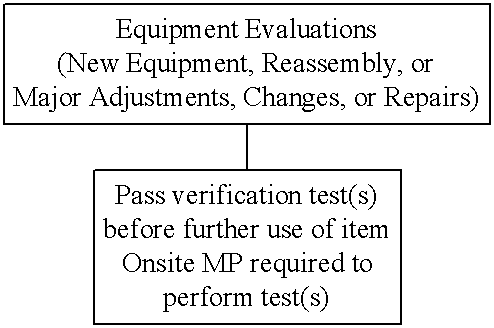
At a minimum, the medical physicist must be on-site to perform or to provide direct supervision for the performance of:
1. The annual survey;
2. Mammography equipment evaluations. A mammography equipment evaluation must be conducted whenever a new unit or processor is installed, a unit or processor is disassembled and reassembled at the same or a new location, or major components of a mammography unit or processor are changed or repaired.
Determining when a medical physicist must be on-site in connection with adjustments, changes, or repair of equipment requires further discussion. Adjustments, changes, or repairs of equipment can occur as corrective actions for problems that caused the equipment to fail a quality control test, as the result of an unexpected equipment failure, or as a measure intended to prevent possible future inadequate equipment performance. All adjustments, changes, or repairs must include some form of verification testing to demonstrate that the affected equipment meets the applicable standards.
As noted, in the case of major adjustments, changes, or repairs, the medical physicist is required to conduct a mammography equipment evaluation on-site to confirm that the applicable standards in 21 CFR 900.12(b) and (e) are met. FDA also recommends that the medical physicist have a role in some other changes or repairs through the provision of "medical physicist (MP) oversight." By "MP oversight," we mean that the medical physicist should be consulted as to whether an on-site visit is required or if other personnel can verify that the standards are met, with direction by telephone or printed material from the medical physicist if needed. Finally, FDA recognizes that there are adjustments, changes, or repairs for which verification (that the adjusted, changed, or repaired equipment meets standards) can be performed by other qualified personnel (e.g. radiologic technologist or service representative with appropriate training/experience) without involving the medical physicist. However, the facility can consult their medical physicist in these situations if they wish.
The first column of the table, "Medical Physicist Involvement in Equipment Repairs," lists the adjustments, changes, or repairs in which the regulations require a mammography equipment evaluation. It also includes a number of adjustments, changes, or repairs that do not require a mammography equipment evaluation. The second column indicates whether the item is considered by FDA to be a major adjustment, change, or repair. If so, it is stated in the third column that the "MP conducts evaluation in person." For other adjustments, changes, or repairs, the third column indicates that FDA recommends either "MP oversight" or "MP involvement optional." In cases where the recommendation is "MP oversight" or "MP involvement optional", the verification may be accomplished by a qualified individual (as described in the previous paragraph) other than the medical physicist.
It is important to remember that in all cases, even those where FDA is recommending "MP oversight" or verification by a non-medical physicist, that some form of verification testing must be included as part of the adjustment, change, or repair.
For any adjustment, change, or repair not listed in the table below, or if the facility is unsure as to the full extent of the adjustment, change, or repair, the facility should consult their medical physicist to determine the proper extent of his or her involvement in evaluating the item.
|
Item |
Major Repair |
Medical Physicist Involvement |
|
Automatic Exposure Control |
|
|
|
AEC replacement |
Y |
MP conducts evaluation in person |
|
Thickness compensation internal* adjustment |
N |
MP oversight |
|
AEC sensor replacement |
Y |
MP conducts evaluation in person |
|
AEC circuit board replacement |
Y |
MP conducts evaluation in person |
|
Density control – internal* adjustment
|
N |
MP oversight |
|
Bucky (New to Facility) Replacement |
|
|
|
AEC sensor also replaced |
Y |
MP conducts evaluation in person |
|
AEC sensor not replaced
|
N |
MP oversight |
|
FFDM detector also replaced
|
Y |
MP conducts evaluation in person |
|
FFDM detector not replaced
|
N |
MP oversight |
|
Cassette Replacement |
|
|
|
Same screen speed |
N |
MP involvement optional |
|
Faster screen speed
|
N |
MP oversight |
|
Slower screen speed where the dose increase may exceed 3.0 mGy for the standard breast
|
Y |
MP conducts evaluation in person |
|
Collimator |
|
|
|
Replacement |
Y |
MP conducts evaluation in person |
|
Reassembly with blade replacement |
Y |
MP conducts evaluation in person |
|
Adjustment
|
N |
MP oversight |
|
Compression Device |
|
|
|
Pressure adjustment |
N |
MP involvement optional |
|
Thickness scale accuracy adjustment but only if it affects AEC performance |
N |
MP oversight |
|
Repair of auto decompression
|
N |
MP involvement optional |
|
Compression Paddle |
|
|
|
Paddle (new to facility) replacement |
N |
MP oversight |
|
Deflection adjustment |
N |
MP oversight |
|
Adjustment due to extension beyond allowable limit, or visibility on images
|
N |
MP oversight |
|
Darkroom |
|
|
|
Repair of light leaks |
N |
MP involvement optional |
|
Safe light change
|
N |
MP involvement optional |
|
Film Type/Speed Change
|
N |
MP oversight |
|
Processor |
|
|
|
Chemistry type change |
N |
MP involvement optional |
|
Fixer/Developer replacement |
N |
MP involvement optional |
|
Installation |
Y |
MP conducts evaluation in person |
|
Reassembly |
Y |
MP conducts evaluation in person |
|
Replenishment rate adjustment |
N |
MP involvement optional |
|
Roller replacement
|
N |
MP involvement optional |
|
X-ray Unit |
|
|
|
kVp, mA or time internal* adjustments |
N |
MP oversight |
|
High voltage generator replacement |
Y |
MP conducts evaluation in person |
|
X-ray tube replacement |
Y |
MP conducts evaluation in person |
|
Filter replacement |
Y |
MP conducts evaluation in person |
|
Installation |
Y |
MP conducts evaluation in person |
|
Reassembly |
Y |
MP conducts evaluation in person |
|
Manufacturer's software modifications (see approved alternative standard) |
Y |
MP conducts evaluation in person |
|
FFDM detector replacement or repair |
Y |
MP conducts evaluation in person |
|
FFDM Display (monitor)/Printer Replacement |
Check FFDM manufacturer’s QC manual |
Follow FFDM manufacturer’s QC manual |
* Internal adjustments refer to equipment adjustments that typically cannot be made by the operator.
For failures of the tests described in 900.12(e)(8)(ii)(A), 900.12(e)(6), 900.12(e)(7) and/or after adjustments, changes, or repairs that are classified as major, the verification test must be passed prior to using the item clinically. Note: verification testing performed after a major adjustment, change, or repair must be performed on-site by the medical physicist.
For failures of the tests described in 900.12(e)(8)(ii)(B), the facility has a maximum of 30 days from the date of the initial test failure to correct the problem and pass the verification test. Facilities should have the verification test(s) performed (either by the medical physicist or by a qualified individual) as soon as possible after the adjustment, change, or repair is done, so as to allow time to repeat the adjustment, change, or repair if the equipment does not pass the verification test. Note: if the verification test is not passed within the 30 day time period, the item cannot be used clinically until such time as it is truly repaired and passes the test.
For non-major equipment adjustments, changes, or repairs that are performed for any reason other than for failure of a required test, the facility must correct any problems found and perform verification tests to ensure that the equipment remains in compliance with the regulations. This should be accomplished as soon as reasonably possible.
The decision trees included in the Question 6 answer may be used to help in determining the timing and extent of physicist and facility involvement in response to test failures, equipment adjustments/changes/repairs and equipment evaluations.
Related Topics:
Annual Equipment Quality Control Tests
Medical Physicist Responsibilities