|
|
||||||||||||||||
|
|
|
|
|
|||||||||||||
|
|
Control and Prevention Division of Cancer Prevention and Control 4770 Buford Hwy, NE MS K-64 Atlanta, GA 30341-3717 Call: 1 (800) CDC-INFO TTY: 1 (888) 232-6348 FAX: (770) 488-4760 E-mail: cdcinfo@cdc.gov Submit a Question Online |
|
|
|
Screening Capacity Assessment
In 2000, CDC began a national assessment of the capacity to perform colorectal cancer screening tests and follow-up for the U.S. population aged 50 and older. This national assessment concluded in 2004. With CDC technical support, state-level capacity assessments were initiated in 15 states beginning in 2002. State-level capacity assessments comprise:
Both the national- and state-level capacity assessments provide baseline data for the planning of widespread colorectal cancer screening at the national, state, and local levels. For the purposes of the SECAP study, screening capacity is defined as the availability of endoscopists to perform screening by flexible sigmoidoscopy and/or colonoscopy. Study ObjectivesEach of these objectives applies to the national- and state-level studies.
State-Level Survey of Endoscopic Capacity (SECAP) Sites 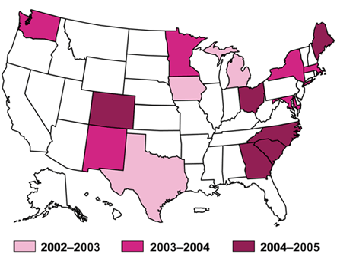
Year 1 states (2002–2003): Iowa, Michigan, and Texas Year 2 states (2002–2003): Maryland, Massachusetts, Minnesota, New Mexico, New York, and Washington Year 3 states (2002–2003): Colorado, Georgia, Maine, North Carolina, Ohio, and South Carolina MethodsThe colorectal cancer capacity study began with the development of the study protocol and survey instruments, the design of the sampling frame, and the design of a forecasting model. The capacity assessment includes two separate but related research efforts. Survey of Endoscopic Capacity (SECAP) This survey was conducted among U.S. physician practices to determine the current volume of sigmoidoscopies and colonoscopies performed. Survey instruments were designed in draft and reviewed by external practicing endoscopists of all specialties and by recognized colorectal cancer screening experts. The survey instruments were tested and approved by the CDC Institutional Review Board for protection of human subjects and the U.S. Office of Management and Budget under the Paperwork Reduction Act. The sampling frame for SECAP included all U.S. medical facilities known to have purchased or leased lower endoscopic (sigmoidoscopy and colonoscopy) equipment between January 1, 1996 and December 31, 2000. For the state studies, the sampling frame was extended to include purchases and leases through 2002. This sampling frame is protected by legal agreements as proprietary and sensitive business information. After physician practices were selected from the sampling frame, a telephone screening questionnaire was administered to confirm study eligibility and to identify the most appropriate person at the practice to receive the mail survey. A self-administered questionnaire was then sent to the identified respondent. Nationally, 74% of surveys were returned, and 82% of the respondents were physicians. Forecasting Model To determine the number of people currently unscreened for colorectal cancer, we first estimated the total population 50 years and older and then identified and removed persons at high risk for colorectal cancer. This left an estimate of the population 50 years and older at average risk for colorectal cancer. We determined the number of people who had been screened based on National Health Interview Survey (NHIS) data, and subtracted that number from the overall population to determine the number of people not yet screened for colorectal cancer. To determine the number of tests needed to screen the unscreened population, several screening programs were proposed under which screening might occur, and the number of screening tests needed for each screening program was determined. The first program assumed that screening would occur with a combination of screening tests in proportions consistent with current tests used based on the 2000 NHIS. The other four programs were selected because they represent screening options recommended by national guidelines. These programs assumed that the unscreened population would be screened with a) annual fecal occult blood test (FOBT) with diagnostic colonoscopy for positive tests, or b) FOBT plus sigmoidoscopy, or c) sigmoidoscopy with diagnostic colonoscopy for positive tests, or d) colonoscopy. While survey data were being collected, a forecasting model was designed using publicly available data to determine the number of average-risk people aged 50 years or older at the national and state level who have not been screened for colorectal cancer. The forecasting model was used to estimate (1) the number of people in the United States requiring colorectal cancer screening and follow-up examinations and (2) the number of tests needed to screen the unscreened people in a variety of hypothetical screening scenarios. The capacity from SECAP (the potential maximum number of procedures minus the current number of procedures) was compared to the number of tests needed in a variety of hypothetical screening scenarios. This helps to assess the capacity to offer screening to the unscreened population. National- and State-Level Study Differences The national- and state-level studies differed in the SECAP methods, the survey instruments and the forecasting model used.
Results and PublicationsResults. The following key results are available from the SECAP capacity assessments. More detailed information is provided in the published articles listed below.
Additional publications will describe the following key data:
Publications.
Next StepsThe current capacity assessments focus on the capacity to get the entire unscreened population screened at least once. The next proposed step will assess the capacity to sustain colorectal cancer screening over a 10- to 20-year period by taking into account the target population growth rate, the need for repeat testing and follow-up testing and compliance with screening recommendations, along with the current volume of procedures and test needs.
Page last reviewed: August 15, 2008
Page last updated: August 15, 2008 Content source: Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion |
|
|
|
|
||||||||||||
|