|
Strategies for Outreach and Education
Understanding Clinical Trial Barriers
General Strategies for Educating Diverse Populations
Specific Strategies for Educating Ethnically Diverse Populations
Information on Other Underserved Groups
References
It is important to understand the reasons why so few adults with
cancer participate in clinical trials. A few are listed here.
Many people with cancer, or those at high risk for developing
cancer, are:
Unaware of the option of participating in clinical
trials. Research has consistently shown that most people are not
aware that clinical trials could be an option for cancer treatment
or prevention. Unwilling to go against physician's advice or direction.
Research has shown if a person's doctor does not recommend a trial
as an option for cancer treatment or prevention, he or she is very
unlikely to participate in one. Fearful, distrusting, or suspicious of research. For
many people, the idea of being "randomized" to one treatment makes
them feel they have less control over their care. Many are also
fearful of being "experimented upon" and not receiving treatment
for their cancer. Many people distrust those in the medical
community, based on past negative experiences or the historical
abuses of research participants. Unaware of clinical trials. The
reality or the perception that there are no trials in their local
community is a barrier for many people. Concerned about potential costs of trials. Some people who are insured
fear that their insurance company won't cover participation in a clinical trial.
Those who do not have insurance may worry about costs that are not covered by the
trial's sponsor. Facing personal or practical obstacles. There are many
costs, financial and otherwise, to participating in a clinical
trial. Time and travel that are required to seek care at a distant
trial site may be a concern for many people. The indirect costs of
being away from work and family may also be a concern. Finally,
some people may not wish to temporarily leave the care of their
physician to participate in a trial.
Many doctors are:
Unaware of clinical trials. Physicians are not
always aware of available clinical trials. Some may not be aware of
the local resources or may assume that none would be appropriate
for the people they treat. Unwilling to "lose control" of patient's care. Most
doctors feel that relationships with the people they care for are
very important. They want what is best for each person. Some
doctors fear that if a person must be referred elsewhere to
participate in a trial, they may lose control of the person's
care. They may not understand that every effort is made to
maintain the physician relationship, even when a person is on a
trial. In addition, many doctors may fear the loss of income if a
person is referred elsewhere for his or her cancer care. Under the impression that standard therapy is best. Many
physicians may not adequately understand how clinical trials are
conducted or the importance of clinical research. Some physicians
believe that the treatment in clinical trials is not as good as
the standard treatment they might provide to people. They also may
be uncomfortable admitting that there is uncertainty about which
treatment is best in a phase 3 clinical trial.
Additional Clinical Trial Barriers for Ethnically Diverse Populations
There is long-standing fear, apprehension, and skepticism in minority populations about medical research due to real abuses that have happened in the past (e.g., the legacy of the Tuskegee syphilis study). Among these populations, there is often widespread fear and distrust of the medical care system as a result of discrimination, indifference, and disrespect. Some may feel that they do not want to give up their rights by participating in a trial, or lose their power by being "experimented upon." Others may be skeptical about the quality of care that would be provided in a clinical trial. Some may find that trial recruitment strategies are not sensitive to their needs.
Doctors may not mention clinical trials as an option for cancer care.
As noted above, many physicians do not refer their patients to clinical trials. However, some physicians may avoid suggesting a clinical trial to their minority patients, out of concern that patients would see him/her as insensitive. Moreover, some physicians may unwittingly discriminate against older patients, or those who are from certain ethnic or cultural backgrounds.
Many people may face additional problems accessing clinical trials. Depending on where they live or their access to transportation, people may have difficulty getting back and forth from a clinical trial site. Those with low income may find it difficult to take time off work or find appropriate childcare. Other barriers, such as a lack of health insurance or lack of general health care, clearly present difficulties in accessing trials.
Cultural or ethnic backgrounds may include values and beliefs that are very different from Western Medicine. Many people have cultural beliefs that Western medicine cannot address their health concerns. Different ethnic and cultural views of health and disease (e.g., fatalism, family decisions about treatment, use of traditional healers, prayer, herbal medicines, or use of complementary/alternative health practices) may make clinical trials a less attractive treatment option. For prevention trials, many may feel that the risk of a potential disease and its consequences may be less important than meeting daily needs.
Language and/or literacy barriers may make it difficult for some people to understand and consider participating in clinical trials. The complexity of forms, including informed consent documents, may also be a barrier to those considering participation in a clinical trial. Translation can also be difficult if the person translating information has not had specialized training.
One of the biggest hurdles for clinical trial education is
overcoming suspicion of medical research.
It is important to note that strategies for clinical trial
outreach and education will vary, based on the type of trial and its
requirements for participation. Although some of the following
strategies were designed for cancer prevention trials, many may also
be used for other types of clinical trials.
Strategy 1
Educators should be familiar (and preferably a part of) the
communities they are trying to reach. People who are known, trusted,
and accountable will be more effective and more believable when
discussing clinical trials with community members.
Suggested Steps
Use easy to understand language. In some cases
this may mean using a community's first language. Involve people from the community, especially community
leaders. Find ways to develop collaboration and encourage
ownership in the outreach program.
|
Tip
If you are not from the population(s) you seek to work
with, it is critical that you or your organization develop
meaningful collaborative partnerships with organizations
within those communities.
|
Strategy 2
Address important concerns and perceptions, benefits, and risks
about clinical trials through one-on-one contact. One-on-one contact
is one of the best ways to educate others.
Suggested Steps
Make sure you do not judge someone's values if
they are different from your own. Find ways to present information
that complement the values someone holds. Address risks and costs in a frank, open, and honest
way. Stress the importance of enrollment in trials to the
family and to future generations. Stress the importance of equal access to the highest
quality care, including clinical trials. Each person has the right
to know and understand every option available with regard to his
or her health care. Promote the balance of spirituality, faith, medicine,
and science. See the next section for suggested messages for specific
ethnic/ racial groups.
Strategy 3
Discuss potential benefits of participating in a clinical trial;
but do not overlook the risks.
Suggested Step
Discuss the fact that because people are monitored closely under
clinical trial protocols, they often receive a higher quality of
medical care and follow up than do those who are not enrolled in
clinical trials.
Strategy 4
Avoid disrupting home and work schedules when conducting education
or outreach activities.
Suggested Steps
|
Key Points for Outreach and Education
Clinical trial outreach strategies must
incorporate an understanding of a potential participant's
decision-making process, his or her culture, family and work
life, and economic concerns. Those conducting education and
outreach must find ways to present clinical trial
information that complement the values people in the
community hold. Education and outreach strategies should stress
the importance of equal access to the highest quality care,
including clinical trials. Each person has the right to know
and understand every health care option available. Clinical trials must be explained in a way that is
respectful and easy to understand, addresses someone's fears
and concerns, and addresses risks and benefits. The research team must ensure that the informed
consent process truly reflects a participant's understanding
of the risks and benefits of the clinical trial. Involving
family, members from the participant's community, and
culturally competent staff are some ways to help verify that
the participant has received the information in a way that
he or she can understand and made the decision to
participate voluntarily.
|
Research has shown that there are many differences in who gets
cancer among people of different races, ethnicities, and
socioeconomic backgrounds. Certain racial and ethnic groups, as noted
on the pages that follow, are also more likely to die of cancer than
other groups. These differences may be due to a variety of reasons,
such as late stage of disease at diagnosis, barriers to health care
access, history of other diseases, biologic and genetic differences
in tumors, health behaviors, and the presence of other risk factors
for cancer. In addition, some cancers that have a high rate of
developing in one ethnic group are rare and may not be listed among
the top 10 cancers in the U.S. population as a whole.* Because rare
cancers may not receive as much
attention as those in the "top 10," it is even more important for
people of particular ethnic and cultural groups to be aware of
research so that they can work to find
ways to decrease the burden of these unusual cancers in their
populations.
Differences in cancer screening and treatment have also been
documented for people of different ages, as well as those from
different socioeconomic, educational, and racial/ethnic
backgrounds.
*Based on the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program. SEER is
the most authoritative source of information on cancer incidence and
survival in the United States.
Information on data sources used in this section:
Cancer incidence data come from the NCI SEER program, covering 14 percent of the U.S. population.
Cancer mortality data come from the National Center for Health Statistics (NCHS) and
covers the entire U.S. population. Cancer incidence and death rates for some racial and ethnic
populations may be limited by problems in ascertaining race and by the misreporting of race and ethnicity on forms used to collect information on cancer incidence, deaths, and the populations at risk. For instance, while reporting race for African-American and White populations is generally considered reliable, biases are more serious for smaller populations, particularly American Indian/Alaskan Natives, as well as for groups living in smaller geographical areas. Additionally, it is important to note that Hispanics can be of any race and are not mutually exclusive from White, African Americans, Asian/Pacific Islanders, and American Indian/Alaskan Natives. These biases can affect trends and comparisons among groups.
Ethnically Diverse Populations-Some Definitions
Diverse populations include minority, ethnic, and racial groups
designated by the U.S. Government, including:
American Indian or Alaska Native Asian American Black or African American Hispanic or Latin American Native Hawaiian or other Pacific Islander
Ethnically diverse populations are growing rapidly, and according
to the 2000 Census, about 25 percent of the U.S. population reported
their race as something other than White.
About 17 percent of the U.S. population over age 5 (more than 44 million people)
do not speak English at home. Of these,
NCI's working definition of diverse populations also includes
medically-underserved populations, such as rural, low-income, and
low-literacy level individuals of any racial or ethnic group.
Medically underserved populations are those that lack easy access to,
or do not make use of, high-quality cancer prevention, screening and
early detection, treatment, or rehabilitation services. In general,
these groups experience higher cancer death rates than the U.S.
population as a whole.
Outreach Strategies
The strategies listed below are not meant to be a complete
overview of barriers and strategies; nor should the information be
generalized to all people in these groups. Attitudes within various
populations vary greatly, depending on a person's age, socioeconomic
status, community, and other factors. The broad outline here provides
some background, context, and potential strategies for potential
education and advocacy efforts.
|
Participation of Different Groups in
NCI Treatment Clinical Trials2
The percentage of White patients enrolled in NCI clinical trials parallels that of the overall U.S. population. However, different patterns are seen for Black, Asian American, and Hispanic cancer patients.
Black children and young adults have accrual to clinical trials comparable to their White peers, as do Black women.
Asian American and Hispanic children and young adults have accrual to clinical trials comparable to their White peers.
The percentage of Black men who have cancer and are 30 to 59 years old who participate in clinical trials is markedly lower than the percentage of White men with cancer in this age group.
The percentage of Asian Americans and Hispanics aged 30 to 80 that accrue to clinical trials is less than that of Whites.
In terms of age, those 80 years of age or older are least likely to be enrolled, followed by those aged 30 to 39 years.
|
Patient accrual for NCI-sponsored cancer treatment trials by sex and race/ethnicity
2
| Race/Ethnic Group |
Males (%) |
Females (%) |
Percentage of U.S. Population |
| White |
36 |
46 |
72 |
| Black |
4 |
5 |
12 |
| Hispanic |
4 |
3 |
12 |
| Asian |
1 |
1 |
4 |
| American Indian |
0.5 |
0.5 |
1 |
| Hawaiian/Pacific Islander |
0.5 |
0.5 |
0.5 |
| Total |
44 |
56 |
100 |
Percentage of estimated United States female population represented in NCI-sponsored
cancer treatment clinical trials: log scale (by age and race/ethnic group)2
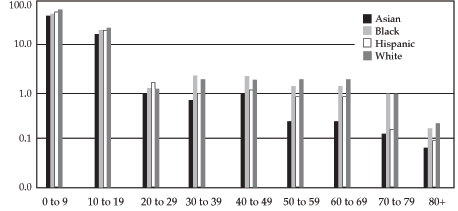
Percentage of estimated United States male population represented in NCI-sponsored
cancer treatment clinical trials: log scale (by age and race/ethnic group)2

African Americans and Clinical Trials
Background
A person who is Black or African American has origins in any of
the Black racial groups of Africa. This definition includes:
Cancer in African Americans
(Unless otherwise noted, all data is age-adjusted incidence or mortality rates between 1992 and 1999.)3
Overall, African Americans had the highest risk of any U.S.
racial/ethnic group of not only getting cancer, but also for dying from cancer.
African American Men:
Of all ethnic and gender groups, African American men have the highest overall rate of having cancer.
Of all ethnic and gender groups, African American men have the highest overall rate of dying from cancer.
Of all men, African Americans have the highest incidence and death rates from many cancers, including lung and bronchus, prostate, and colon and rectum cancer. In addition, in 2001, African American men were at least 50 percent more likely to get prostate cancer than were men of any other ethnic group.4
Top five cancers: Prostate, lung and bronchus, colon and rectum, oral cavity and pharynx, and stomach
African American Women:
Of all women, African Americans have the highest incidence rates for colon and rectal cancer, lung and bronchial cancer, and pancreatic cancer.
Of all women, African Americans have the highest death rates from many cancer, including breast, colon and rectal, pancreatic, uterine, and cervical cancers.
While they have the second highest rates of all women of getting breast cancer, they have the highest rate of dying from the disease.
Top five cancers: Breast, colon and rectal, lung and bronchial, uterine, and pancreatic
Challenges
The legacy of the Tuskegee Syphilis Study (in which researchers
studied but did not treat African American men with syphilis) has
contributed to long-standing mistrust in African American communities
concerning clinical research. Widespread skepticism about the medical
care system exists as a result of a long history of discrimination,
indifference, and disrespect. The oral history contributing to this
mistrust is particularly important to recognize.
Some African Americans may believe that if they agree to
participate in a trial, they will not be appropriately cared for, nor
honestly informed of the risks or the benefits. They may fear
that:5
Placebos would be substituted for lifesaving
interventions Treatments that work would be deliberately withheld They would not receive a full course of treatment,
especially if funding sources for the clinical trial were no
longer available
Other cultural beliefs and attitudes that affect research
participation include hopelessness, fatalism, and doubt about the
usefulness of cancer prevention and control. Faith, folk
remedies, and the role of the family are other important influences
for African Americans.6
Clinical trials may be a lower priority to African Americans and
others who have a low income, less access to transportation and
health care, less information about clinical trials, and low levels
of literacy. Concerns about family and work responsibilities may also
be a significant barrier.7 African American men have
noted concerns about researchers not giving back to the community,
being uncomfortable talking about prostate cancer, and past negative
experiences with the medical care system.8
Potential Solutions
Cultural strategies:
Find people who are already active in
organizations to help spread the word about clinical trials.
People who are known, trusted, and accountable in the community will be better
messengers than will outsiders. Explore partnerships with African American churches,
particularly for health issues central to the mission of the
church. Faith is a very important part of many African American
cultures and the most successful outreach efforts usually involve
churches that have two or more paid clergy, and medium or large
memberships. Ensure that the informed consent process truly reflects
the participant's understanding of the risks and benefits of the
clinical trial. Involving family, members from the participant's
community, and culturally competent staff are some ways to help
verify that the participant has received the information in a way
that he or she can understand and made the decision to participate
voluntarily.
Program strategies:
Talk about trials using one-on-one
contact-preferably with another African American person-through
churches, schools, civic organizations, and African American
sororities and fraternities. Word of mouth can be an effective
way to reach others. Conduct in person outreach to complement other education
efforts using videos, brochures, or advertisements. Present real-life situations that exemplify statistics
or written messages. Effective dialogue can take place through a
church-sponsored forum or an educational session that allows for
open discussion and questioning. Involve local celebrities, including DJs at African
American radio stations, by asking them to share messages about clinical
trials. Ask newspapers and local media to join in education
efforts. Provide personal, "real world" discussion of clinical trials and
follow up in any education program.
Key Messages
Distrust of medical research is a critical issue, but it is
important that African Americans be presented with an opportunity to
find answers to research questions. The prospect of learning
information that will help all people access treatment and better
manage disease can be a powerful motivator.
Some African Americans may be more interested in clinical trials
if they understand that participation means they:
Contribute to their community and their families Join a group of people like themselves nationally, and
in their local community
Clinical trial educational messages need to include information on
the following topics:
Severity of the cancer problem nationwide Underlying myth that African Americans don't need to be
concerned about cancer Toll that cancer (especially breast and prostate) is
taking on African American women and men-and that we don't know
why Prevention and treatment options for high-risk African
Americans-and the need for more research Importance of clinical trials-and what it means for all
people if all groups are not represented in a trial Laws on participant protection and rights What risk means for the individual and others in his or
her community
Asian Americans and Clinical Trials
Background
A person who is Asian has origins in any of the original peoples
of the Far East, Southeast Asia, and Indian subcontinent. The term
"Asian" refers to persons from the following and other Asian,
Southeast Asian, and South Asian backgrounds:
- Chinese
- Vietnamese
- Pakistani
- Filipino
- Cambodian
- Thailandian
- Japanese
- Hmong
- Indian
|
- Korean
- Laotian
- Bangladeshi
- Indonesian
- Sri Lankan
- Nepalese
- Bhutanese
- Sikh
- Burmese
|
|
"Asian Americans" and "Pacific Islanders" are two discernibly distinctive groups, comprised of numerous heterogeneous ethnic subpopulations. These broad categories fail to show mortality rates that, in some instances, strongly differ among ethnic groups. Whenever possible, this publication separates out these groups.
|
U.S. residents who reported they were Asian* make up 4.2 percent of the total population. Chinese is the leading Asian group (2.7 million) followed by Filipino (2.4 million) and East Indian (1.9 million).1
*Self report, alone or in combination with one or more other races
The Asian population includes many groups who differ in language, culture, and length of residence in the United States.
Some of the Asian groups, such as the Chinese and Japanese, have been in the United States for several generations and often have
literacy, education, and socioeconomic characteristics that are above the national average. On the other hand, groups such as the Hmong, Vietnamese, Laotians, and Cambodians are comparatively recent immigrants, and tend to have limited acculturation and poverty rates below the national average. It is important to note that 88 percent of Asian Americans and Pacific Islanders (AAPI) are either foreign-born themselves or have at least one foreign-born parent. Of those who speak Asian and Pacific Island languages at home, more than 22 percent say they speak English "not well" or "not at all."1
Cancer in Asians
(Unless otherwise noted, all data is age-adjusted incidence or mortality rates between 1992 and 1999).)3
Some Asians are much more devoted to traditional medical practices
than to Western medicine. For example, a study of breast and cervical
cancer screening in Chinese women found that more than 2/3 had gone
to traditional providers for preventive health care, went to temples
to pray for their health, and looked to fortune-tellers for
guidance.9 Another study of cervical cancer screening in
Cambodian women indicated beliefs that fate cannot be changed by
detection, cancer is incurable, and cancer will not develop if
traditional practices are used.10
Like the Hispanic/Latino populations, Asian/Pacific Islanders experience lower incidence and mortality rates overall compared with other minority groups. However, they do experience higher incidence and mortality rates for certain cancers. Based on three-year averages, more than 18 percent of Asian and Pacific Islanders lack health insurance.
Asian/Asian Pacific Islander men:
Of all men, AAPIs have the highest incidence rates of liver and stomach cancer.
Of all men, AAPIs have the highest death rates from liver cancer.
Asian/Asian Pacific Islander women:
Seventy percent of Asian Americans come from countries with the world's lowest overall rates of breast cancer, yet after living in the U.S. for as little as 10 years, Asian women have an 80 percent higher risk of getting the disease than recent immigrants.
In addition, third and fourth generation Asian American women have rates of developing breast cancer that are similar to their neighboring Caucasian women.11
Asian American women in general have the lowest rates of Pap test, mammogram, and breast exam screening of any ethnic group.6,11
Of all women, Asians have the highest rates of liver and stomach cancer.
Of all women, Asians have the highest death rates for liver and stomach cancer.
|
Cancer in Specific Asian Groups
The NCI SEER cancer incidence rates noted in this publication are available for different levels of racial/ethnic detail in each of the time periods (1988-1992 and 1992-1999).
Although the SEER program routinely collects detailed racial/ethnic information on the cancer patients in its coverage areas, the lack of comparable detail in the racial/ethnic county-level population estimates from the U.S. Census Bureau means that incidence rates for certain racial/ethnic groups can be calculated only for time periods centered on the decennial census. For example, county-level census information for Asian and Pacific Islanders by subgroups (Chinese, Filipinos, Hawaiians, etc.) is still only available from the 1990 Census.
|
From 1988 to 1992, Chinese, Japanese, Korean, and Vietnamese* groups all had higher rates of getting liver and intrahepatic bile duct (IHB) and stomach cancers than Whites, and Chinese and Japanese had higher mortality than Whites from these two cancers, as well.12 In this same time period, Korean and Vietnamese women had higher incidence rates of cervical cancer than White women.12
*Liver and IHB not calculated for Vietnamese women
From 1988 through 1992, the top five cancers for many Asian groups
are as follows:12
Women:

Men:

Challenges
Values in many Asian cultures may be different than the
Euro-American system, with decisions reached by consensus, group
welfare being of primary value, and individual life not as sacred.6
In some Asian cultures, the family is responsible for treatment
decisions and the patient is not told of his or her diagnosis.
Many in the Asian immigrant communities need a lot of support as
they learn about clinical trials, and they need to feel safe asking
questions. "Saving face" in public is important. In many cases, Asian immigrants
may feel it is disrespectful to ask questions of doctors or health
professionals.
Recent immigrants also may be dealing with a combination of
educational, social, and health problems, along with emotional
difficulties related to separation and isolation. Many are in
low-wage jobs and need to get permission to take time off work to
take care of health care needs.
The language barrier also is difficult to overcome. Many Asians do
not speak English, and this may not be readily apparent. There are so
many Asian languages that deciding on the language(s) in which
information should be printed is difficult. This barrier is
particularly important in issues surrounding informed consent. The
informed consent process is intimidating for all people and is
especially so for those with limited English skills.
Potential Solutions
Cultural strategies:
Assess how long the group you are trying to reach
has been in this country as well as the countries of origin represented. It is
important to fit the educational outreach to the culture and to
use people from the communities to reach community members. Involve family members in learning about the risks and
benefits of clinical trials, but resist using them as translators of medical information. In particular, avoid having children-who may be more proficient in English than their parents-serve as translators. It is preferable to enlist someone who is trained for this work. Ensure that the informed consent process truly reflects
the participant's understanding of the risks and benefits of the
clinical trial. Involving family, members from the participant's
community, and culturally competent staff are some ways to help
verify that the participant has received the information in a way
that he or she can understand and made the decision to participate
voluntarily.
Language strategies:
Find respectful ways to make sure that information
is being understood. Someone who does not understand English may
say "yes" or nod, even if they do not understand what is being
said. To ensure that the person you are talking with understands what you have said, staff may ask, "Many people have a hard time understanding information like this. I want to make sure that I explain it clearly. Could you please tell me, in your words, what I have just said." When translating materials, at least two bilingual, bicultural translators should be used. In addition, materials should be pilot-tested with the target audience.
Program strategies:
Use health fairs, which have been successful when
organized by community members, and involve the leadership of
existing Asian groups in clinical trial outreach. If possible, translate clinical trial information to be
used in ethnic newspapers. Many immigrant groups get their health
information from press that is printed in their own languages. Invite a professional to deliver information about
clinical trials. Preferably, a doctor or nurse from that community
should be invited, who can encourage others to ask questions.
Often, laypeople do not have much credibility as an information
source. Keep workshops or programs short (30 to 40 minutes) because many new immigrants
have more than one job, in addition to other responsibilities. While recent immigrants may not have been concerned with breast cancer in their countries of origin, it is important to educate these women about their increased risk for this disease.
Key Messages
A family's receptivity to
cancer treatments and trials will depend on the experience that
relatives have had with the medical system. For example, if a relative did not
survive cancer, it may be taboo to talk about him or her. Remember
to consider feminine modesty and traditional gender role values.
Native Hawaiians and Other Pacific Islanders and Clinical
Trials13
|
Native Hawaiians and Pacific Islanders are often
aggregated nationally into the "Other" category or currently
the "Asian American/Pacific Islander or AAPI" category.
It is important to note that these are two discernibly distinctive groups, comprised of numerous
heterogeneous ethnic subpopulations.
These groupings have obscured disparate mortality rates
that are prevalent in one group and not the other. Whenever possible, this publication separates out these groups.
|
Background
Native Hawaiians and other Pacific Islanders of Polynesian,
Micronesian, and Melanesian ancestry* made up 0.3
percent of the total U.S. population.1
This group comprises
more than 25 diverse groups with variations in historical
backgrounds, languages, and cultural traditions.
*Alone or in combination with one or more races.
Among Pacific Islanders in the United States, Native Hawaiians are
the largest group, 58 percent (211,014). Three-fourths of Pacific
Islanders live in the states of California and Hawaii and they are a
relatively young population, with a median age of 25 years and an
average family size of 4.1.
The term "other Pacific Islanders," refers to the peoples of
Polynesia, Micronesia, and Melanesia, and includes:
- Chamorros
- Samoans
- Fijians
- Tongans
- Tahitians
- Marshallese
|
- Chuukese
- Kosraen
- Yapese
- Pohnpeian
- Palauan
- Other Pacific Islanders
|
Within this group are six U.S.-associated Pacific Island
jurisdictions--the Federated States of Micronesia, the Republic of
Palau, the Republic of the Marshall Islands, Guam, American Samoa,
and the Commonwealth of the Northern Marianas-that have various
political relationships with the U.S.
The population of the U.S.-associated Pacific Island jurisdictions
is approximately 427,000. The health status varies within and among
the jurisdictions, but is generally worse than for Americans. The
jurisdictions must contend with health conditions found in both
developing countries (e.g., malnutrition, dengue fever, cholera, and
tuberculosis) and developed countries (e.g., diabetes, heart disease,
and cancer).
Cancer in Native Hawaiians
Hawaiian Men:
(For the years 1988-1992)12
Have the second highest cancer mortality,
behind only African Americans
Of all men and of all ethnic groups,
Top five cancers: Lung and bronchus, prostate, colon and rectum, stomach, and non-Hodgkin's lymphoma
Hawaiian Women:
(For the years 1988-1992)12
Top five cancers: Breast, lung and bronchus, colon and rectum, uterine, and stomach
Cancer in Pacific Islanders
Cancer surveillance and databases are rudimentary or non-existent
in most of these jurisdictions, rendering cancer rates unknown. What
is known is that cancer is among the top three causes of death. The
most commonly reported cancers for males were cancers of the lung and
prostate, and for females, cancers of the breast, cervix, and
lung.
Challenges
A history of oppression, higher prevalence of
behavioral risk factors, ineffective cancer prevention and control
efforts, and poor access to state-of-the-art services for cancer
prevention, early detection, and treatment (including low
representation in clinical trials) contribute to increased cancer
risk and mortality among Native Hawaiians and other Pacific
Islanders. Many Native Hawaiians and Pacific Islanders are
socio-economically disadvantaged and underserved in terms of
access to health and social services. In the U.S., many Pacific Islanders do not speak English
at home. There is a general distrust of research among island
communities. This distrust can also have negative consequences for
those participating in a trial, such as poor compliance or
avoidance. Geographic barriers are a problem for many Pacific
Islanders; clinical trials are unavailable for most Western
Pacific and Samoan communities and rural Hawaiian communities. The conduct of many clinical trials lacks cultural sensitivity
and does not address language needs; it also does not interpret cultural
behaviors and preferences.
Potential Solutions
Cultural strategies:
Tailor the educational outreach to the culture and
use "cultural brokers" (members of the community) to reach other community
members. Include the family unit, which for many Native Hawaiians
and Pacific Islanders includes extended family members and
friends, when educating about the risks and benefits of clinical
trials. The role of the woman is central to the family in many
Pacific Island cultures. Ensure that the informed consent process truly
represents the participant's understanding of the risks and
benefits of the clinical trial. Involving family, members from the
participant's community, and culturally competent staff are some
ways to help verify that the participant has received the
information in a way that he or she can understand and made the
decision to participate voluntarily.
Program strategies:
Use personal contacts through a family member or a
friend to do education and outreach. Explain the benefits of research to the community at
large. Address issues of medical care that are not covered by
clinical trials, as these are an important concern for
participants who may not have insurance or are underinsured.
Native Americans and Clinical Trials14,15
Background
A person who is considered Native American has origins in any of
the original peoples of North or South America (including Central
America) and maintains tribal affiliation or community
attachment.
The term "Native American" refers to:
American Indians Alaska Natives:
Native Americans are made up of culturally distinct and diverse
communities. The U.S. contains 511 federally recognized tribes, with
Native American people living in every State. The largest tribes are
Cherokee and Navajo. More than 9 percent of the U.S. population
reported American Indian or Alaska Native status in the 2000
Census.
Indian Health Service
There are more than 300 hospitals and health clinics, located on
or near Indian reservations, run by the Indian Health Service (IHS).
In recent years, many tribes have assumed management of some of these
health care facilities.
The Indian clinics and hospitals are unable to provide the
high-tech medical care needed to diagnose and treat cancer. For this
reason, the IHS Contract Health Services program pays for Indian health
care provided by non-IHS providers. However, this program is
chronically short of funds. Depending on the region/tribe, its local
priorities, and funding remaining in the service contract, certain
treatments may not be available. At present the Contract Health
Services does not reimburse for many treatments that are deemed
"experimental," which keeps many Indian people out of clinical
trials.
Although 54 percent of Native Americans live in urban areas, less
than 2 percent of the IHS budget is spent in urban
clinics.6 These clinics are severely underfunded and must
rely on other sources of support, including Medicaid revenue. Many
urban Indians choose to return to their home reservations for
care.
Based on 3-year averages, American Indians and Alaska Natives were the least likely of the major racial groups to have health insurance.1A
Cancer in Native Americans
(Unless otherwise noted, all data is age-adjusted incidence or mortality rates between 1992 and 1999.)3
While American Indian/Alaska Natives experience some of the lowest cancer rates among all ethnic groups, they do experience higher incidence and mortality for certain cancers. Cancer is the second leading cause of death among Native Americans
and is the leading cause of death among Alaska Native
women.16,17
American Indian/Alaska Native Men:
Top five cancers: Prostate, lung and bronchial, colon and rectal, kidney and renal pelvis, and stomach
American Indian/Alaska Native Women:
Top five cancers: Breast, colon and rectum, lung and
bronchial, uterine, and ovarian
|
Alaska Natives
(For the years 1988-1992)12
Alaska Native men had the highest rates of getting colon and rectal cancer among all ethnic groups.
In this same time period, Alaska Native women had the highest rates for getting and dying from colon, rectal, and lung cancer among ethnic groups, and their rates of getting cervical cancer were twice as high as those for White women.
The top cancers:
Men: Lung and bronchus, colon and rectum, prostate, and stomach
Women: Breast, colon and rectum, lung and bronchus, and cervical
American Indians (Living in New Mexico)
American Indian men had the highest rates of all ethnic groups of getting kidney cancer. In this same period, American Indian women had higher rates of cervical and ovarian cancers than the U.S. White female population.
Top cancers in American Indians:
Men: Prostate, colon and rectal, kidney and renal pelvis, and lung and bronchus
Women: Breast, ovarian, colon and rectal, gallbladder, and uterine
|
Challenges
A history of disrespect, racism, and poverty has
contributed to a distrust of science and research by Native
American populations. To protect the interests of Native American people, many
tribes have their own Institutional Review Board (IRB), in
addition to those required by Indian Health Service (IHS) or
tribal facility. Successful clinical trial outreach requires that
the investigator work closely with the tribal IRB in addition to
those of the IHS. Native Americans are a culturally distinct and diverse
community and their beliefs about cancer, and experiences with
diabetes, alcoholism, poverty, and traditional roles can
significantly affect the success of clinical trial educational
programs. On average, Native Americans are younger, have a lower
rate of high school completion, and have higher rates of poverty
and unemployment than Whites.6 Many Native people do not have access to quality health
care. Many tribes do not have a word for cancer in their
languages and historically, the disease was thought of as
something that affected only the "White man." Some Native
Americans may hold a fatalistic attitude toward cancer, and fear
that if they talk about cancer or even think about it, they might
catch it. It is unlikely that Native Americans would participate
in a cancer trial without hearing messages from other Native
Americans, yet there are few cancer survivors to serve as role
models. Informed consent forms and procedures may serve as a
barrier to recruitment because the language used in such forms may
not be well understood. Because of other pressing health issues, such as
diabetes, obesity, and substance abuse, in addition to extreme
poverty, cancer screening and treatment may not be as important to
some Native people. There may be a tribal taboo on the loss of body parts
that needs to be discussed in relation to clinical trials. Transportation is an important barrier for Native
Americans who live in rural areas. Differences in communication styles are important to consider.
Many Native Americans are reserved, reluctant to ask questions, or
don't discuss their health problems. Body language also is
important, with respect for personal space and friendly gestures
such as smiling and eye contact being key. Traditional roles are such
that women are usually caretakers and often place their needs
last. In addition, Native American women value modesty and
privacy, and many traditional Native American couples find a male
health care provider for the woman unacceptable.
Potential Solutions
Cultural strategies:
Use group activities such as sharing and caring
for others because they are universal concepts among Native
people, and should be a part of any clinical trial education
program. Incorporate the use of traditional healing ceremonies as
well as spiritual connections, which can be very important for
people in these communities. Family plays a central role in American Indian life. The
needs of the family may take precedence over the needs of the
individual. When appropriate, the patient's family should be
involved in the decision-making process. Ensure that the informed consent process truly
represents the participant's understanding of the risks and
benefits of the clinical trial. Involving family, members from the
participant's community, and culturally competent staff are some
ways to help verify that the participant has received the
information in a way that he or she can understand and made the
decision to participate voluntarily.
Language strategies:
Use easy-to-understand language and a gentle
approach to education and outreach. Try to include materials that
portray Native Americans. Make sure that patient consent forms are understood.
Reading out loud or encouraging consultation with others may be
important.
Program strategies:
Emphasize that participation in a trial can help
improve cancer care for the next generation. Use stories and visual tools that focus on the
family. Use one-on-one or small group education and outreach
techniques to respect privacy. Work with community or tribal elders such as community
health representatives and public health nurses, to find out the
best ways to conduct outreach and education efforts. Find out if transportation is needed to get to the site. Use public service announcements on Native American
radio.
Key Messages
Messages should be culturally relevant and discuss
issues related to family and community. Tribal beliefs are very diverse and programs should be
designed on a site-specific basis with the help of tribal
advisors.
Hispanics and Clinical Trials
Background
The terms "Hispanic" and "Latino" refer to people born in North,
Central, and South America, and in the Caribbean whose language is
Spanish. Someone who is Hispanic or Latino is a person of Mexican,
Puerto Rican, Cuban, Central or South American, or other Spanish
culture or origin, regardless of race.
In the mainland United States today
the largest groups within the Hispanic community are:
Mexican (58.5%) Puerto Rican (9.6%) Central American (4.8%) (Salvadorian, Guatemalan,
Honduran, Nicaraguan, Panamanian, and other people from countries
in Central America) South American (3.8%) (Colombian, Ecuadorian, Peruvian,
Argentinean, Venezuelan, Chilean, and other people from countries
in South America) Cuban (3.5%)
The Hispanic population is the fastest growing ethnic group in the
U.S. In the 2000 Census, Hispanics eclipsed African Americans to
become the second largest ethnic group, with 12.5 percent of the
population reporting Hispanic or Latino status. Hispanics as a group
comprise many different races and ethnicities. Within these
subpopulations, other differences exist according to culture,
beliefs, lifestyles, and experiences, but Hispanics agree that
certain commonalities go beyond specific nationalities. In general,
the U.S. Hispanic population is younger, with more people per
household, and has lower rates of employment, less education, and
lower economic status than do Whites.6
There are 28 million U.S. residents aged 5 and older who speak Spanish at home-about 10 percent of the U.S. population. These percentages vary greatly throughout the United States; for example, states like California, Texas, and New Mexico, have approximately 30 percent of residents who speak languages other than English.1 Among all those who speak Spanish at home, almost half speak English "less than very well." It is important to note that in 2000, 68 percent of the U.S. population is foreign-born or had at least one parent who was foreign-born.1
Cancer in Hispanics*
(Unless otherwise noted, all data is age-adjusted incidence or mortality rates between 1992 and 1999.)3
*"Hispanic" is not mutually exclusive from Whites, African American, Asian/Pacific Islanders, and Native Americans.
Although Hispanics had the largest reduction in cancer mortality
rates of any U.S. ethnic group (-1.6 percent) they also had the greatest number of uninsured
people. Based on three-year averages, more than 33 percent of all Hispanics lack health insurance coverage.1a A recent study showed that uninsured
Hispanic women are more than two times more likely to be diagnosed
with breast cancer at a later stage than other women, and uninsured
Hispanic men are almost four times more likely to be diagnosed with a
later stage of prostate cancer than non-Hispanics.19
While Hispanics/Latinos have lower incidence and death rates overall compared with those of African Americans and Whites, they do experience higher rates for certain cancers.
Latino/Hispanic men:
Top five cancers: Prostate, lung and bronchus, colon and rectal, non-Hodgkin's lymphoma, and stomach
Latina/Hispanic women:
Top five cancers: Breast, colon and rectal, lung and bronchus, cervical, and uterine
Challenges20
Many Hispanics have strong religious and cultural
beliefs. Some may believe strongly in "fatalismo" (fatalism) and
"resignación" (resignation)-that diseases or illness cannot
be controlled because they are inherited. Many may use folk remedies [such as "uña de
gato"(cat's nail)] to treat cancer or wait until they are in
serious pain to see a doctor. In one study, barriers that were identified by Latinas
considering a cervical cancer trial included transportation, fear
of getting a placebo, care of children and family, and care from a
male provider.7 Although Spanish is one language, regional dialects need
to be considered when translating materials. The language barrier is particularly important in issues
surrounding informed consent. The informed consent process is
intimidating for all people and is especially so for those with
limited English skills. Some Hispanics think that cancer treatment will only
prolong life but that no effective cure exists for the disease.
Even if people are treated, it is felt that the type of treatment
depends on the person's ability to pay. Hispanics may not obtain health care until they are very
sick and cannot perform normal functions. This adds to the number
and severity of health problems that need to be evaluated.
However, women are more likely than men to seek medical care. Many in the Hispanic community feel that doctors do not
communicate well with them and they do not feel well informed
about trials. Many hold the perception that doctors may have
financial interests in a trial, and there is a lack of trust
around participating in scientific research. A sense of fatalism and resignation is usually strongest
in women and older men. This may make people feel that treatment
is useless. Machismo is a barrier because men feel they are the
family protectors and should not show weakness. Hispanics' biggest barriers to accessing health care are
money, time, and language. Other common problems include lack of
insurance, problems with transportation or childcare, and getting
off work to see a doctor. Although face-to-face interaction is important,
Hispanics would not welcome unknown health educators or volunteers
into their homes. Men lack trust in a Federal Government source,
while most women tend to trust it.
Potential Solutions
Cultural strategies:
Collaborate with people who are from the
communities and speak Spanish. Community members can identify with
people who have a direct tie to their situation. Personal
interaction is very important. Testimonials from local pastors,
Hispanic celebrities, or doctors who have experienced cancer
themselves are beneficial. Involve family members in learning about the risks and
benefits of clinical trials. Sometimes children have learned to
speak English more quickly than their parents, so they can be
helpful in translating forms and brochures. It is important to
note, however, that using children as translators has both pros
and cons. It is preferable to use someone who is trained for this
work. Ensure that the informed consent process truly reflects
the participant's understanding of the risks and benefits of the
clinical trial. Involving family, members from the participant's
community and culturally competent staff are some ways to help
verify that the participant has received the information in a way
that he or she can understand and made the decision to participate
voluntarily.
Language strategies:
Find respectful ways to make sure that information
is understood. When people do not understand English, they may say
"yes" or nod, even if they do not understand what is being said.
To ensure that the person you are talking with understands what you have said, staff may ask, "Many people have a hard time understanding information like this. I want to make sure that I explain it clearly. Could you please tell me, in your own words, what I have just said?" When translating materials, at least two bilingual, bicultural translators should be used. In addition, materials should be pilot-tested with the target audience.
Program strategies:
Take the outreach program out to community and
neighborhood centers as well as to other sites that are already
familiar to Hispanics. Consider partnering with existing groups. Use family-oriented, positive messages as much as
possible to offer hope. Use radio and newspapers for outreach. Spanish-language
media-especially television and radio talk shows-are popular sources
of health-related information for many in these communities.
Printed materials with many pictures/illustrations and minimal text are preferred.
Older Adults
When considering older people and clinical trials, it is important
to note that approximately 60 percent of all cancers occur in people
aged 65 and older, and the number of people over age 65 is expected
to double by the year 2033. The elderly are an important group to
participate in clinical trials.
Because of mobility problems, transportation (including escort
assistance) is one of the most important challenges specific to older
people. Literacy issues (see below) are also a challenge that must be
addressed when educating older adults.
Fostering positive doctor-patient interaction is another
difficulty because often the older population is reluctant to
question or challenge doctors and may be afraid to offend by changing
doctors. This is compounded when doctors do not refer older adults to
trials because of the assumption that they are too old or sick for a
trial.
Older persons are more likely to be living on fixed incomes, so
the financial aspects of clinical trial participation may be
heightened.
The family, or other social support, is another important
consideration because it is often involved in the older person's
treatment and decision-making process.
It is important to inform older adults that Medicare reimburses
for all routine care costs for its beneficiaries participating in
clinical trials.
People with Low Literacy Skills19
"Many Americans face the serious problem of not being able to read
or understand information. According to the 1992 National Adult
Literacy Survey (NALS), some 40 to 44 million of the 191 million
adults in the United States are functionally illiterate. Another 50
million are only marginally literate. Functional literacy represents
more than just the ability to read. It involves reading comprehension
as well as the ability to compute, communicate, write, and solve
problems. These skills are especially important for patients in
acquiring general information and applying it to their specific
circumstances."
"When applied to the health system, low functional literacy
translates into low health literacy. Health literacy is defined as
the ability to obtain, interpret, and understand basic health
information and services, as well as competence and motivation to use
such information and services in ways that enhance one's health. Most
health-related educational materials use simplified printed materials
to convey information, assuming that people can read. Most adults do
read, but many have difficulty understanding what they read and
applying generalized information to their own specific
situation."
"One common assumption is that certain populations have low levels
of functional literacy. For example, traditionally "underserved"
populations such as those with low incomes are labeled as having low
levels of functional literacy simply because they are, on average,
less educated. However, low functional literacy is not defined by
race, class, or even educational attainment."
Ways to Help People with Low Health Literacy Skills
"One-on-one assistance is the most effective technique for
educating this group. In addition to helping people gain a better
understanding of the clinical trial and their health needs,
one-on-one assistance fosters trust between patients and the
counselors or health care professionals who help them. Comprehension
should be ascertained, but not by asking, "Do you understand?" Often
the "teach back" method works well."
"Group assistance offers an arena in which people can
obtain information from educators and through the questions asked by
others in the group. This technique often supplements one-on-one
counseling."
"Visual tools are designed to simplify concepts such as
instructions for care that are too complicated to understand in
written form or through verbal communication. Visual tools are
particularly useful to those who cannot read at all. Videotapes may
be useful tools, but follow up discussion is necessary in order to
ascertain comprehension."
Lesbian, Gay, and Bisexual Individuals
The lesbian, gay, and bisexual (LGB) community is diverse in terms
of cultural background, ethnic or racial identity, age, education,
income, rejection or acceptance of societal stereotypes, and
prejudice. As with other minority groups, discrimination and bias can
play a role in inadequate medical assessment, treatment, and
prevention of LGB health problems. In addition, lesbians may be at a
greater risk of cancer because of issues associated with health care
access, delayed or lack of childbearing, screening, and
insurance.
Little information is available about specific clinical trial
barriers for the LGB population. Significant barriers that must be
addressed include:
Previous negative health care experiences Fear of sexual orientation disclosure Perceived or actual exclusion from health promotion
campaigns Misinformation about risks and screening Exclusion of significant others
1. U.S. Census Bureau. U.S. Department of Commerce, Census 2000. Available at http://www.census.gov
1a. U.S. Census Bureau. U.S. Department of Commerce Health Insurance Coverage: 2001 Issued September 2002. Available at http://www.census.gov
2. Sateren, W. B., Trimble, E. L., Abrams, J., Brawley, O., Breen, N., Ford, L.,
McCabe, M., Kaplan, R., Smith, M., Ungerleider, R., Christian, M. C. (2002).
How sociodemographics, presence of oncology specialists, and hospital
cancer programs affect accrual to cancer treatment trials. Journal of Clinical
Oncology Apr 15;20(8):2109-17.
3. Edwards, B.K., Howe, H.L., Ries, L.A.G., Thun, M.J., Rosenberg, H.M., Yanick, R., Wingo, P.A., Jemal, A., Feigal, E.G. (2002). Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on the U.S. cancer burden. Cancer May 15;94(10):2766-2792.
http://seer.cancer.gov
4. American Cancer Society. (2002). Cancer facts and figures
2002.
5. Roberson, N. L. (1994). Clinical trial participation:
Viewpoints from racial/ethnic groups. CANCER Supplement, 74(9),
2687-2691.
6. Guilano, A. R., Mokuau, N., Hughes, C., Tortolero-Luna, G.,
Risendal, B., Ho, R. C. S., Prewitt, T. E., & McCaskill-Stevens,
W. J. (2000). Participation of minorities in cancer research: The
influences of structural, cultural, and linguistic factors.
Annals of Epidemiology, 10(8), S22-S34.
7. Brown, D.R., Fouad, M. N., Basen-Enguist, K., &
Tortolero-Luna, G. (2000). Recruitment and retention of minority
women in cancer screening, prevention, and treatment trials.
Annals of Epidemiology, 10(8), S13-S21.
8. Royal, C. D., Baffoe-Bonnie, A., Kittles, R., Powell, I.,
Bennett, J., Hoke, G., Pettaway, C., Weinrich, S., Vijayakumar, S.,
Ahaghotu, C., Mason, T., Johnson, E., Obeikwe, M., Simpson, C.,
Mejia, R., Boykin, W., Roberson, P., Frost, J., Faison-Smith, L.,
Meegan, C., Foster, N., Furbert-Harris, P., Carpten, J.,
Bailey-Wilson, J., Trent, J., Berg, K., Dunston, G., & Collins,
F. (2000). Recruitment experiences in the first phase of the
African American hereditary prostate cancer (AAHPC) study. Annals
of Epidemiology, 10(8), S68-S77.
9. Lee, M. (1999). Breast and cervical cancer early detection
in Chinese American women. Asian-American Pacific Islander
Journal of Health, 6, 358-367.
10. Taylor, V., Jackson, J., Schwartz, S., Yasui, Y., Tu, S.,
& Thompson, B. (1999). Cervical cancer control in a Cambodian
American population. Asian-American Pacific Islander Journal of
Health, 6, 368-377.
11. Shinagawa, S. M., Kagawa-Singer, M., Chen, M. S., Tsark, J.
U., Palafox, N. A., & Mackura, G. (1999). Cancer registries
and data for Asian Americans and Native Hawaiians and Pacific
Islanders: What registrars need to know. Journal of Registry
Management, 26(4), 128-141.
12. Miller, B.A., Kolonel, L.N., Bernstein, L., Young, Jr., J.L., Swanson, G.M., West, D., Key, C.R., Liff, J.M., Glover, C.S., Alexander, G.A., et al. (Eds). (1996). Racial/ethnic patterns of cancer in the United States 1988-1992 (NIH Publication No. 96-4104). Bethesda, MD: National Cancer Institute.
13. Papa Ola Lokahi. (May 1, 2001). Personal communication.
Honolulu, HI and Tsark, J.U. (1998). Cancer in Native Hawaiians.
Pacific Health Dialog, 5, 315=327.
14. Hodge, F. S., Weinmann, S., & Roubideaux, Y. (2000).
Recruitment of American Indians and Alaska Natives into clinical
trials. Annals of Epidemiology, 10(8), S41-S48.
15. Burhansstipanov, L. (Director, Native American Cancer Research
Corporation). (April 2001). Interview. Pine, CO.
16. U.S. Department of Health and Human Services. (1997). Regional differences in Indian health. Rockville, MD: Indian Health Service.
17. Lanier, A. P., Holck, P., Kelly, J., Smith, B., & McEvoy,
T. (1999). Alaska Native cancer survival report. Anchorage,
AK: Alaska Native Health Board.
18. U.S. Census Bureau. US Department of Commerce, Health Insurance Coverage, by Selected Characteristics: Issued October 1999. Available at http://www.census.gov
19. American College of Physicians-American Society of Internal
Medicine. (2000). No health insurance? It's enough to make you
sick. Latino community at great risk. Philadelphia: Author.
Available: American College of Physicians-American Society of
Internal Medicine, 190 N. Independence Mall West, Philadelphia, PA,
19106.
20. National Cancer Institute. (1996). Communicating with
Hispanic cancer patients: A focus group study. Washington, DC:
Author.
21. Center for Medicare Education. (2000). Considering health literacy. (Issue brief, volume 1, no. 6. [Online], Available: http://www.MedicareEd.org
Other sources utlized for this section include:
Alexander, G. A., Chu, K. C., & Ho., R. C. S. (2000).
Representation of Asian Americans in clinical cancer trials.
Annals of Epidemiology, 10(8), S61-S67.
Atkinson, J., & Hartmuller, V. (1994). Strategies for
minority recruitment. In PCPT minority recruitment manual.
Bethesda, MD: National Cancer Institute.
Brant, J. (1996). Breast cancer challenges in American Indian
women. In K. H. Dow (Ed.), Contemporary issues in breast cancer
(pp. 243-252). Sudbury, MA: Jones and Bartlett.
Brant, J., Fallsdown, D., & Iverson, M. (1999). The
evolution of a breast health program for Plains Indian women.
Oncology Nursing Forum, 26(4), 731-739.
Bunn, P., & Krebs, L. (1997). Colorado blueprint: Women and
minorities in cancer care trials. Denver, CO: University of
Colorado Comprehensive Cancer Center.
Center for Lesbian, Gay, Bisexual, and Transgender Health. (2000).
Lesbian, gay, bisexual, and transgender health: Findings and
concerns. New York: Columbia University's Joseph L. Mailman
School of Public Health.
Chen, A. M. (1996). Demographic characteristics of Asian and
Pacific Islander Americans: Health implications. Asian-American
and Pacific Islander Journal of Health, 4, 40-49.
Chu, K. C. (1998). Cancer data for Asian Americans and Pacific
Islanders. Asian-American and Pacific Islander Journal of Health,
6(2), 130-139.
Haynes, M. A., & Smedley, B. D. Committee on Cancer Research
Among Minorities and the Medically Underserved. (1999). The
unequal burden of cancer: An assessment of NIH research and programs
for ethnic minorities and the medically underserved. Health
Sciences Policy Program, Health Sciences Section, Institute of
Medicine. Washington, D.C.: National Academy Press.
Hughes, C. K, Tsark, J. T., Kenui, C. K., & Alexander, G. A.
(2000). Cancer research studies in Native Hawaiians and Pacific
Islanders. Annals of Epidemiology, 10(8), S49-S60.
Kagawa-Singer, M., Millon-Underwood, S., Burhansstipanov, L.,
& Munet-Vilaro, F. (1994). Nursing research and underserved
populations. In Proceedings of the Third National Conference on
Cancer Nursing Research. Atlanta, GA: American Cancer Society.
Lin-Fu, J. S. (1994). Ethnocultural barriers to health
care: A major problem for Asian and Pacific Islander Americans.
Asian-American and Pacific Islander Journal of Health, 2,
290-298.
McCabe, M. S., Varricchio, C. G., & Padberg, R. M. (1994).
Efforts to recruit the economically disadvantaged to national
clinical trials. Seminars in Oncology Nursing, 10(2),
123-129.
Millon-Underwood, S. (1994). Barriers to minority participation
in clinical trials. In PCPT minority recruitment manual.
Bethesda, MD: National Cancer Institute.
Millon-Underwood, S., Sanders, E., & Davis, M. (1993).
Determinants of participation in state-of-the-art cancer
prevention, early detection/screening, and treatment trials among
African-Americans. Cancer Nursing, 16(1), 24-33.
National Cancer Institute. (1997). Talking to patients and the
public about cancer clinical trials: Findings from NCI's "Science
Awareness Research." Bethesda, MD: Author.
National Clearinghouse for Alcohol and Drug Information. (2000).
[Online], Available:
http://www.health.org/
Paskett, E. D., DeGraffinreid, C., Tatum, C. M., & Margitic,
S. E. (1996). The recruitment of African-Americans to cancer
prevention and control studies. Preventive Medicine, 25,
547-553.
Shinagawa, S. M., Kagawa-Singer, M., Chen, M., Tsark, J., Palafox,
N., & Mackura, G. (1999). Cancer registries and data for
"Asian Americans" and "Native Hawaiians and Pacific Islanders": What
registrars need to know. Journal of Registry Management, 26(4),
128-141.
Susan G. Komen Breast Cancer Foundation. (1999). Suggestions to
enhance the participation of women of color in breast cancer
prevention trials. In Clinical Trial Community Outreach Efforts.
Dallas, TX: Author.
The Mautner Project for Lesbians with Cancer. (2001).
[Online], Available: http://www.mautnerproject.org.
Thomas, S. B., Quinn, S. C., Billingsley, A., & Caldwell, C.
(1994). The characteristics of Northern Black churches with
community health outreach programs. American Journal of Public
Health, 84(4), 575-579.
Underwood, S. (2000). Minorities, women and clinical cancer
research: The charge, promise, and challenge. Annals of
Epidemiology, 10(8), S3-S12.
Back to Top
< Previous Section | Next Section > |