|
| Appropriate
Vaccine and Diluent Storage Conditions |
Live
Vaccines
| Live vaccines are sensitive to heat. |
Live
vaccines are sensitive to heat. MMRV, varicella, and zoster vaccines
must be stored in a continuously frozen state in a freezer at 5°F
(-15°C) or colder until administration. MMRV, varicella, andzoster vaccines deteriorate rapidly after they are removed from the freezer.
Measles, mumps, and rubella vaccine (MMR)
is routinely stored in the refrigerator, but it also can be stored in
the freezer. The National Center for Immunization and Respiratory Diseases
recommends keeping MMR in the freezer along with MMRV, if adequate space
is available. This may reduce the risk of inadvertent storage of MMRV
in the refrigerator. LAIV and rotavirus vaccines are also live virus vaccines, but they should be stored in the refrigerator. Do NOT store these vaccines in the freezer.
Inactivated
Vaccines
| Inactivated vaccines are sensitive to both excessive heat and freezing. |
Inactivated
vaccines are sensitive to both excessive heat and freezing. They should
be stored in a refrigerator at 35° to 46°F (2° to 8°C),
with a desired average temperature of 40°F (5°C). Exposure
to temperatures
outside this range results in decreased vaccine potency and increased
risk of vaccine-preventable diseases. Inactivated vaccines may tolerate
limited exposure to elevated temperatures, but they are cold sensitive
and are damaged rapidly by freezing temperatures.
Vaccine
Light Sensitivity
| HPV, MMR, MMRV, rotavirus, varicella, and zoster vaccines are sensitive to light, which causes loss of potency. |
HPV, MMR, MMRV, rotavirus, varicella, and zoster vaccines are sensitive to light, which causes loss of potency. These vaccines must be protected from light at all times. Therefore, store these vaccines at the appropriate temperatures in their boxes with the tops on until they are needed.
Lyophilized
(Freeze-Dried) Vaccines and Diluents
 |
Diluents packaged separately from their corresponding vaccines can be stored at room temperature or in the refrigerator. |
 |
Diluents packaged with their vaccines should be stored in the refrigerator next to their vaccines. |
MMR,
MMRV, varicella, and zoster diluent is packaged separately from the
corresponding lyophilized (freeze-dried) vaccine and can be stored
at room temperature or in the refrigerator. To conserve space, diluents
packaged separately from their vaccines may also be stored in the
door of the refrigerator. Diluents
packaged with their vaccines (such as ActHIB® and Menomune®)
should be stored in the refrigerator next to their vaccines.
| Vaccine
Storage Locations and Positioning |
Freezers
In the freezer,
vaccine should be stored in the middle of the compartment, away from the
walls, coils, and peripheral areas. Vaccines should not be stored in the
freezer door. The temperature in the door is not stable and differs from
that in the main compartment. MMRV, varicella, and zoster vaccines may
be stored in either a manual defrost or a frost-free freezer at 5°
F (-15° C) or colder.
| |
|
Note
Vaccines should not be stored in freezer door |
|
In the freezer, vaccine should be stored in the middle of the compartment,
away from the walls, coils, and peripheral areas. |
Refrigerators
In the refrigerator,
vaccine should be stored in the middle of the compartment, away from the
coils, walls, floor, and cold air vent. The temperature near the floor
of the refrigerator is not stable and differs from that in the middle
of the compartment. For this reason, vaccine should never be stored in
the vegetable bins. Vaccines should not be stored in the refrigerator
door. The temperature in the door is not stable and differs from that
in the main compartment. In a combination refrigerator-freezer unit, the
top shelf of the refrigerator may be colder than the recommended temperature
range because of cold air venting on it from the freezer. Refrigerated
vaccines should always be stored far enough away from the air venting
from the freezer compartment to avoid freezing the vaccines. Ideally,
vaccine should be stored on the middle shelf, away from the cold air vent.
However, if vaccine can be situated away from the cold air vent, and the
temperature in this area is within the recommended range, vaccine may
also be stored on the upper shelf. If the upper shelf must be used for
vaccine storage, it would be best to place MMR on this shelf because MMR
is not sensitive to freezing temperatures like the other refrigerated
vaccines.
Vaccine
Spacing
Vaccine should be placed with space between the vaccine and the compartment wall, and with space between each large box, block, or tray of vaccine to allow for cold air circulation around the vaccine. Adequate cold air circulation helps each vaccine to reach a consistent temperature throughout its mass and is necessary for the storage unit to maintain a consistent temperature inside the compartment. Packing any vaccine storage unit too tightly will affect the temperature.
Vaccine
Packaging
Vaccine products that have similar packaging should be stored in different locations to avoid confusion and medication errors. For example, if you have pediatric and adult versions of the same vaccine, storing them in different locations lessens the chance that someone will inadvertently choose the wrong vaccine. Likewise, vaccines that
|
|
Note
Diluents may be stored in refrigerator door.
Vaccines
should not be
stored in
refrigerator door. |
In the refrigerator, vaccine should be stored in the middle of the compartment,
away from the walls and coils and off the floor. |
have
similar sounding names should be stored in different locations. For example,
DTaP and Tdap vaccines might be easily confused, as could Hib and hepatitis
B vaccines.
The location of each specific vaccine inside the storage unit should be clearly labeled. This can be accomplished by attaching labels directly to the shelves on which the vaccines are sitting or by labeling containers in which boxes of the same vaccine type are placed. Storing each vaccine in its own specifically labeled section of the refrigerator or freezer helps decrease the chance that someone will mistakenly administer the wrong type of vaccine.
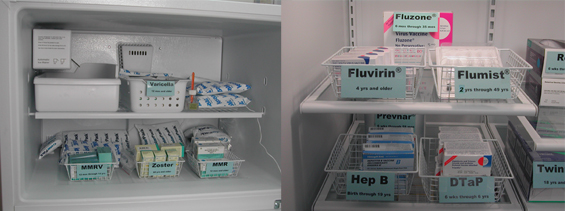
Attach labels directly to the shelves on which the vaccines are sitting or
label trays or containers according to the vaccines they contain. |
In addition to labeling the location of vaccines, mark each opened multidose vial with the date it was first opened. Mark reconstituted vaccine with the date and time it was reconstituted. Dating these vials is important for two reasons. First, some vaccines expire within a certain time after opening or after reconstitution. This may not correspond to the expiration date printed on the vial by the manufacturer. For example, multidose vials of meningococcal vaccine should be discarded if not used within 35 days after reconstitution, even if the expiration date printed on the vial by the manufacturer has not passed. Second, dating opened or reconstituted vials helps manage vaccine inventory by identifying vials that should be used first.
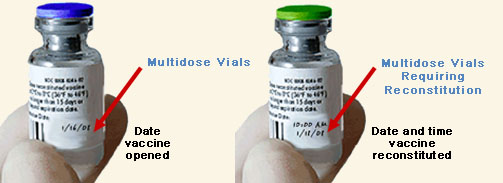
Mark each opened multidose vial with the date it was first opened.
Mark each reconstituted vaccine with the date and time it was reconstituted. |
Whenever possible, use all the vaccine in one multidose vial before opening another vial. Similarly, use all the reconstituted vaccine in one vial before reconstituting another vial. This policy helps to reduce vaccine waste.
Diluents should be clearly labeled, whether they are stored at room temperature or in the refrigerator. Label the boxes of corresponding vaccines and diluents from the same manufacturer so that they will be used together. This avoids confusion and helps to ensure that you use only the specific diluent provided by the manufacturer for each type of lyophilized (freeze-dried) vaccine. This is particularly important if you store two or more lyophilized vaccines using different diluents.

Diluents should be clearly labeled, whether they are stored at room temperature or in the refrigerator. |
Vaccine
Boxes
| Store
all opened and unopened vials of vaccine in their boxes inside the
appropriate storage unit. |
To avoid
confusion, vaccine boxes should be stored together by type and arranged
in rows. Boxes should be stacked
according to expiration dates. Vaccines with the shortest expiration dates
should be closer to the front of the storage unit compartment for easy
access. Store all opened and unopened vials of vaccine in their boxes
inside the appropriate storage unit so that their contents and expiration
dates are easily identifiable. HPV, MMR, MMRV, rotavirus, varicella,
and zoster vaccines should always be stored in their boxes with the
lids on to protect them from light. Storing loose vaccine vials outside
of their boxes is not recommended. This practice makes inventory management more difficult, makes tracking expiration dates more difficult, predisposes to administration errors when vials are confused, and exposes the vaccines to light.
Trays
and Containers
Trays and containers may be used to organize vaccine boxes. Each tray or container should only store vaccine of the same type. Other medications and biologic products, if they must be stored in the vaccine storage unit, must not be stored on the trays or in the containers to avoid medication errors. Clearly label the tray or container with the name of the vaccine and place vaccine boxes of that type on the tray or in the container inside the refrigerator or freezer. Trays and containers must not be stacked or placed so closely together that air circulation inside the vaccine storage unit compartment is impeded.
| Storage
of Non-Vaccine Products |
Food
and Beverages
Never store food or beverages inside the vaccine refrigerator or freezer. This practice results in frequent opening of the storage unit door and greater chance for temperature instability and excessive exposure to light. It may also result in spills and contamination inside the compartment.

Never store food or beverages inside the vaccine refrigerator or freezer. |
Medications
and Other Biologic Products
If possible,
other medications and other biologic products should not be stored inside
the vaccine storage unit. If there is no other choice, these products
must be stored below the vaccines on a different shelf. This prevents
contamination of the vaccines should the other products spill, and reduces
the likelihood of medication errors.
|
