Chapter 2
Cells 101: Business Basics
By Alison Davis
Performing as key actors in all living things, cells play an essential set of roles in your body. They roam around in your blood, come together to make organs and tissues, help you adjust to changes in your environment, and do any number of other important jobs. Far from being static structures, cells are constantly working, changing, sending and responding to chemical cues, even correcting their mistakes when possible—all to keep your body healthy and running smoothly.
This frenzied activity takes place with an intricacy and accuracy that nearly defies imagination. In this chapter, we'll focus on several of the basic functions that cells have in common: creating fuel, manufacturing proteins, transporting materials, and disposing of wastes.
Got Energy?

Click for larger image
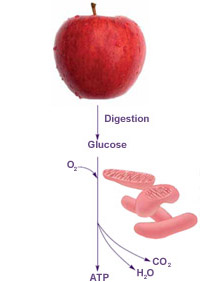
When you think about food, protein, and energy, what may come to mind is the quick meal you squeeze in before racing off to your next activity. But while you move on, your cells are transforming the food into fuel (ATP in this case) for energy and growth.
As your digestive system works on an apple or a turkey sandwich, it breaks the food down into different parts, including molecules of a sugar called glucose. Through a series of chemical reactions, mitochondria transfer energy in conveniently sized packets from glucose into ATP. All that's left are carbon dioxide and water, which are discarded as wastes.
This process is extremely efficient. Cells convert nearly 50 percent of the energy stored in glucose into ATP. The remaining energy is released and used to keep our bodies warm. In contrast, a typical car converts no more than 20 percent of its fuel energy into useful work.
Your body uses ATP by breaking it apart. ATP stores energy in its chemical bonds. When one of these bonds is broken, loosing a chemical group called a phosphate, energy is released.
ATP is plentifully produced and used in virtually every type of cell. A typical cell contains about 1 billion molecules of ATP at any given time. In many cells, all of this ATP is used up and replaced every 1 to 2 minutes!
Priority: Proteins
IMAGE COURTESY OF DAVID S. GOODSELL
Along with the fuel you need to keep moving, eating, thinking, and even sleeping, cells make other important products, including proteins. Scientists estimate that each of your cells contains about 10 billion protein molecules of approximately 10,000 different varieties.
The workhorse molecules of your cells, proteins are responsible for a wide range of tasks, including carrying oxygen in your blood (a protein called hemoglobin), digesting your food (enzymes like amylase, pepsin, and lactase), defending your body from invading microorganisms (antibodies), and speeding up chemical reactions inside your body (enzymes again—they're not all for digesting food). Specially designed proteins even give elasticity to your skin (elastin) and strength to your hair and fingernails (keratin).
Protein production starts in the cell's command center, the nucleus. Your genes, which are made of DNA, contain the instructions for making proteins in your body, although many other factors—such as your diet, activity level, and environment—also can affect when and how your body will use these genes.
Code Reading
Click for larger image
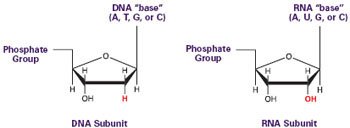
The first step in building proteins is reading the genetic code contained in your DNA. This process is called transcription. Inside the cell nucleus, where your DNA is safely packaged in chromosomes, are miniature protein machines called RNA polymerases. Composed of a dozen different small proteins, these molecular machines first pull apart the two strands of stringy DNA, then transcribe the DNA into a closely related molecule called RNA.
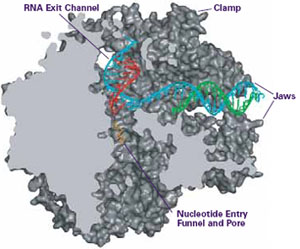
IMAGE COURTESY OF ROGER KORNBERG
Researchers have used a technique called X-ray crystallography to help unravel just how transcription occurs. As one example, Roger Kornberg of the Stanford University School of Medicine in California, used this tool to obtain a detailed, three-dimensional image of RNA polymerase. The image suggests that the RNA polymerase enzyme uses a pair of jaws to grip DNA, a clamp to hold it in place, a pore through which RNA components enter, and grooves for the completed RNA strand to thread out of the enzyme.
Helper molecules may then cut and fuse together pieces of RNA and make a few chemical modifications to yield the finished products—correctly sized and processed strands of messenger RNA (mRNA). Completed mRNA molecules carry genetic messages to the cytoplasm, where they are used as instructions to make proteins.
Specialized proteins and small RNA molecules escort the mRNA out of the nucleus through pores in the nuclear envelope. A sequence of chemical reactions that burn ATP drives this export process.
Translation, Please

Click for larger image
Once in the cell's cytoplasm, each mRNA molecule serves as a template to make a single type of protein. A single mRNA message can be used over and over again to create thousands of identical proteins.
This process, called translation, is carried out by ribosomes, which move along the mRNA and follow its instructions. The mRNA instructions are a string of units that, in groups of three, code for specific protein building blocks called amino acids. Ribosomes read the mRNA units in sequence and string together the corresponding amino acids in the proper order.
Where do ribosomes get the amino acids? From matchmaker molecules called transfer RNAs (tRNAs) that bring amino acids from the cytosol to the ribosome. One end of the L-shaped tRNA matches up with a three-unit mRNA sequence while the other end carries the appropriate amino acid.
One at a time, the tRNAs clip onto the mRNA in a cavern deep within the ribosome, allowing the ribosome to stitch together the amino acids in the right order. A finished amino acid chain can range in length from a few dozen to several thousand amino acids. Some proteins are made up of only one amino acid chain. Others, especially large proteins, contain two or more chains.
Translation consumes lots of energy, but it happens very fast. In bacteria, for example, ribosomes can stitch together 20 amino acids in 1 second.
Some three-unit sequences in the mRNA message can immediately halt protein production. Reading one of these mRNA stop signs indicates to the ribosome that the new protein has all the amino acids it needs, and translation ends.
At this point, most proteins made by free-floating ribosomes are essentially complete. They will remain in the cytosol, where they conduct business—such as passing chemical messages in the cell.
A Sweet Finish

The story is different for proteins made by ribosomes on the rough ER. Inside the rough ER, enzymes add specialized chains of sugar molecules (carbohydrates) to proteins in a process called glycosylation. Next, the proteins traverse the Golgi, where the sugar groups may be trimmed or modified in other ways to create the final protein. Unlike genes and proteins, carbohydrates are not based on a genetic template. As a result, they are more difficult to study because researchers cannot easily determine the sequence or arrangement of their components. Scientists are only just beginning to learn about the critical roles carbohydrates play in many life processes.
For example, without the carbohydrates on its outer surface, a fertilized egg would never implant into a woman's uterus, meaning it would never develop into a baby. Also, without sticky sugar molecules to slow down your immune cells, they would fly right by the cut on your hand without stopping to help fight infection. Sugars attached to lipids on the surface of red blood cells define a person's blood type (A, B, AB, or O). Carbohydrates even help proteins fold up into their proper shape and dictate where proteins go and which other molecules they can interact with.
In extremely rare cases, children are born without the ability to properly glycosylate their proteins, a disorder called carbohydrate deficiency glycoprotein syndrome. As you might imagine, this disease affects virtually every part of the body, causing symptoms like mental retardation, neurological defects, and digestive problems.
Glycosylation, then, does more than just add a sugar coating. It's an essential process that gets proteins ready for action.

ALISA Z. MACHALEK
RNA—it's not just for making proteins anymore. In the last few years, scientists have unearthed several previously unknown functions for the molecule that was regarded mostly as the molecular go-between in the synthesis of proteins from genes. It's not that RNA has suddenly developed any new talents. All of these tasks have probably been going on for millions of years, but researchers are just now discovering them.
In particular, certain types of small RNAs seem to be critical for carrying out important work inside cells. In addition to helping make proteins, small RNA molecules help cells grow and divide, guide developing organ and tissue formation, edit the "spellings" of genes, and control gene activity. This last ability, more generally referred to as gene expression, is key to how cells mature into so many different cell types throughout the body.
One of the most intriguing discoveries is RNA interference (RNAi), a mechanism that organisms use to silence genes when their protein products are no longer needed. The silencing happens when short RNA molecules bind to stretches of mRNA, preventing translation of the mRNA (see main text).
Scientists have found RNAi at work in almost every living thing examined, from worms to people. Researchers are learning that RNAi gone wrong may even contribute to certain diseases. Using experimental fruit flies, Gregory Hannon of Cold Spring Harbor Laboratory on Long Island, New York, has uncovered a link between RNAi and a disorder called Fragile X syndrome, which is one of the most common inherited forms of mental retardation.
Researchers also believe RNAi holds promise for future molecule-based therapies. For example, in lab tests, scientists have recently succeeded in killing HIV, the virus that causes AIDS, by wielding an RNAi-based molecular weapon. If the technique works equally well in people, it could lead to an entirely new class of anti-AIDS drugs.
 Proteins
come in virtually every imaginable shape, each containing a sophisticated
array of bends and folds that allow them to do their jobs. Further proving
that a protein's proper three-dimensional shape is critical to its function,
scientists have linked misfolded proteins to several diseases, including
Alzheimer's, Huntington's, Parkinson's, amyotrophic lateral sclerosis
(Lou Gehrig's disease), and cystic fibrosis.
Proteins
come in virtually every imaginable shape, each containing a sophisticated
array of bends and folds that allow them to do their jobs. Further proving
that a protein's proper three-dimensional shape is critical to its function,
scientists have linked misfolded proteins to several diseases, including
Alzheimer's, Huntington's, Parkinson's, amyotrophic lateral sclerosis
(Lou Gehrig's disease), and cystic fibrosis.
But proteins don't always accomplish their acrobatic folding feats by themselves. Other molecules often help them along. These molecules, which are also proteins, are aptly named chaperones. Like their human namesakes, chaperone proteins work around the clock to prevent inappropriate interactions (molecular ones, in this case) and to foster appropriate bonding. Chaperones are so important in protein folding that some researchers believe that supplying them to cells may someday help treat devastating health problems caused by misfolded proteins.
Of course, it would help if scientists also could understand just how protein folding takes place. But it can happen so fast—small proteins can fold in a few millionths of a second—that researchers have had a difficult time understanding the process in detail.
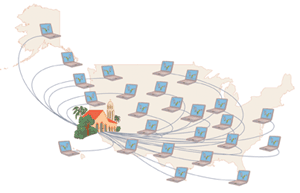
Enter Stanford University scientist Vijay Pande, who decided to couple the power of computers with the help of the public. Computers are adept at simulating biological processes, but it would take a single personal computer a century to simulate the entire folding pathway of a single protein. Pande initiated a project called Folding@Home, a so-called distributed computing project in which anyone who wants to can download a screensaver that performs protein-folding calculations when a computer is not in use. Folding@Home is modeled on a similar project called SETI@Home, which is used to search for extraterrestrial intelligence.
Pande recruited tens of thousands of personal-computer owners who have Internet connectivity. Each idle computer was assigned a different job to help simulate the folding process of a test protein at several different temperatures. With so many computers employed, the simulation was complete in a matter of days. The scientists used data gathered from the screensavers to come up with a folding-time prediction, which was confirmed by lab tests to be correct. You can learn more about this project at http://folding.stanford.edu.
Cellular Rush Hour
To reach its destination, a newly created protein must toil through the cytosol, moving past obstacles, such as organelles, cytoskeletal fibers, and countless molecules. Luckily, the cell has well-organized systems to shepherd proteins to the places where they are needed.
Vesicle Taxis
Perhaps the most challenging obstacle is membranes. It's essentially an oil-and-water problem. The cell's cytosol, the insides of organelles, and many proteins are water-soluble, but the insides of membranes are fat-soluble (oily). As you know, oil and water don't mix. So how do water-loving proteins headed for lysosomes, the ER, or the Golgi cross the fatty membranes surrounding those organelles to get inside them? The cell chauffeurs them around in vesicles, membrane bubbles that essentially solve the problem by eliminating it. Proteins carried in these protective bubbles never really have to "cross" any membranes.
Take, for example, the journey of proteins from the ER to the Golgi. A small portion of the ER membrane pinches off, enveloping exiting proteins in a vesicle that has a special molecular coat. This vesicle then travels to the Golgi. Strategically located docking sites on the Golgi permit vesicles to latch onto and fuse with its outer membrane to release their contents inside. The same process takes proteins in vesicles from the Golgi to lysosomes or to the cell's surface.
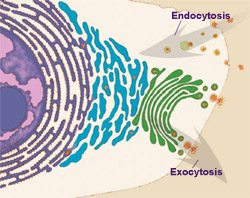
Cells also use vesicles to carry nutrients and other materials into the cell in a process called endocytosis. White blood cells use endocytosis to fight infection. They swallow bacteria whole, engulfing them in large vesicles. The vesicles then fuse with lysosomes, which break down the bacteria into molecular bits and pieces the cell can use.
Endocytosis occurs continuously, and cells essentially eat their entire skin every 30 minutes. So why don't cells continually shrink? Because there is a mirror-image process, called exocytosis, that counterbalances endocytosis. Cells use this process to dump wastes out of the cell and to replace membrane lost at the cell surface through endocytosis.
Molecular Motors
Vesicles don't just wander around aimlessly. Like many other materials inside the cell, including some organelles, they often are carried by small molecular motors along tracks formed by the cytoskeleton. Your body uses motors to get all sorts of things done—copying DNA (and fixing it when a "typo" slips in), making ATP and proteins, and putting molecules in the correct places during development to make sure the body is assembled correctly.
In recent years, scientists have discovered that the workings of every motor they examined hinge on the same two ingredients: an energy source (usually ATP) and chemical reactions. Ronald Vale of the University of California, San Francisco, has found that molecular motors function sort of like a falling row of dominoes. Chemical reactions driven by ATP cause small shape changes in parts of the motor proteins, which then alter the shape of other parts of the proteins, eventually causing a forward (or sometimes backward) movement of the motor along its track.
Tiny Tunnels
While vesicles are ideal for handling large molecules and bulky material, cells have a different way to transport smaller molecules, like water and charged particles (ions), across membranes. These molecules travel through hollow or gated proteins that form channels through membranes.

Channel proteins are just one family of proteins that function within the cell's surface membrane. They transport ions like sodium and potassium that are critical to many biological processes, such as the beating of the heart, nerve impulses, digestion, and insulin release. Unfortunately, channel proteins are tough to study because they cannot easily be isolated from the membrane in either their natural or active states.
Yet with new and improved laboratory techniques and good old-fashioned tenacity, researchers are learning fascinating new things about membrane proteins. One example is work by Roderick MacKinnon of Rockefeller University in New York City, that showed what potassium channel proteins look like at the atomic level. This revealed how these channels precisely control which ions they transmit, why they sometimes conduct ions only in one direction, and how they open and close under different conditions. Just 5 years later, in 2003, MacKinnon received science's highest honor, the Nobel Prize.
The discovery of specialized vesicles called secretory vesicles earned two cell biologists a prestigious prize, the 2002 Albert Lasker Award for Basic Medical Research, an award often known as "America's Nobel Prize." James Rothman of Memorial Sloan-Kettering Cancer Center in New York City and Randy Schekman of the University of California, Berkeley, shared the prize for figuring out that cells use secretory vesicles to organize their activities and communicate with their environment.
How these two scientists made their discovery is an interesting story itself. Despite skepticism from their peers, Rothman and Schekman pursued an unproven research method: using genetically altered yeast cells to study cell secretion. Working independently, the two discovered, in great detail, how cells use vesicles to direct proteins and other molecules to their proper destinations.
The fundamental research of Rothman and Schekman taught scientists that vesicles are vital to the livelihood of cells. Vesicle transport underlies countless processes, such as the secretion of insulin to control blood sugar, nerve cell communication, and the proper development of organs. The work also helped scientists learn to use yeast cells as protein factories. As a result, genetically altered yeast cells now pump out many important products, including approximately one-quarter of the world's insulin supply and a key ingredient in hepatitis B vaccines.
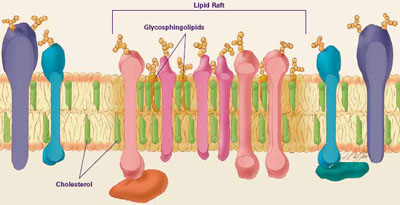
Cellular membranes are sort of like a layer of liquidy Jell-O® studded with fruit. The Jell-O® portion is made up of lipids, and the pieces of fruit are proteins that float around within it. Of course, cell membranes are much more complex than that. Depending on which organelle a membrane encases and where in the body it is located, its proteins (and to a lesser extent, its lipids) can vary widely in type and amount. This allows different processes to be performed in each membrane.
Until recently, scientists thought that individual lipids and proteins floated around independently. New data indicate that certain proteins tend to group together, as if, in the Jell-O® analogy, all the peaches and pears clustered together while the pineapple floated around by itself.
Researchers have learned much of what they know about membranes by constructing artificial membranes in the laboratory. In artificial membranes, different lipids separate from each other based on their physical properties, forming small islands called lipid rafts. These rafts have a higher concentration of certain specialized lipids, called glycosphingolipids, and cholesterol than do non-raft parts of the membrane. Rafts are also distinguished by a different assortment of proteins. Certain types of proteins cluster together in rafts, while others remain mostly outside of rafts. The big question is, to what extent do these rafts, seen readily in artificial membranes, actually exist in living cells?
Using advanced laboratory methods and imaging techniques, some researchers found evidence that rafts, indeed, do form in living cellular membranes, but these rafts may be small and transitory. Although the existence of lipid rafts in cellular membranes remains controversial, many scientists believe they serve as communication hubs by recruiting proteins that need to come together in order to transmit a signal. Researchers are beginning to link lipid rafts with a variety of diseases, including AIDS, Alzheimer's, anthrax, and atherosclerosis. —A.Z.M.