Publications:
Chapter 5: Human Papillomavirus
Manual for the Surveillance of Vaccine-Preventable Diseases (4th Edition, 2008)
On This Page:
![]() Print-friendly version of this page/chapter
Print-friendly version of this page/chapter ![]() (366 KB)
(366 KB)
S. Deblina Datta, MD; Eileen Dunne, MD, MPH; Mona Saraiya, MD, MPH; Elizabeth Unger, MD, PhD; Lauri Markowitz, MD
Background
Genital human papillomavirus (HPV) is the most common sexually transmitted infection in the United States, with an estimated 6.2 million persons becoming newly infected every year.1 More than 100 HPV types have been identified, over 40 of which can infect the genital area.2 Types are classified by their association with cancer. Low-risk, or non-oncogenic types, such as HPV 6 or 11, can cause
- benign or low-grade abnormalities of cervical cells,
- anogenital warts, and
- recurrent respiratory papillomatosis (RRP), a disease of the respiratory tract.
High-risk, or oncogenic types, including types 16 and 18, can cause
- low-grade cervical cell abnormalities,
- high-grade cervical cell abnormalities that are precursors to cancer, and
- anogenital cancers such as cervical, vulvar, vaginal and anal cancers as well as some oropharyngeal cancers.3–5
Among the cancer-related outcomes of HPV infection, cervical cancer causes the largest global burden of disease (over 300,000 deaths due to cervical cancer in 2002).4 High-risk HPV (HR-HPV) types are detected in 99% of cervical cancers;6 approximately 70% of cervical cancers worldwide are due to types 16 and 18.7 While persistent infection with high-risk types is considered necessary for the development of cervical cancer, it is not sufficient because the vast majority of women with high-risk HPV infection do not develop cancer.8-11
In addition to its association with cervical cancer, high-risk HPV infection is associated with cancer of the vulva, vagina, penis and anus (Table 1).4 Each of these is less common than cervical cancer and, unlike cervical cancer, not all cases of these less common anogenital cancers are related to HPV infection.4, 12–16 Oncogenic types of HPV may play a role in the development of some oropharyngeal cancers.17
| Table 1. Cancers attributable to high-risk human papillomavirus infection — United States, 2003 | ||
Anatomic site |
Total cancers* |
% estimated HPV attributable fraction† |
|---|---|---|
| Cervix | 11,820 | 100 |
| Anus | 4,187 | 85 |
| Vulva/vagina | 4,577 | 40 |
| Penis | 1,059 | 40 |
| Oral/pharyngeal | 29,627 | 15 |
* CDC. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR 2007;56(No. RR-2):1–24. † Parkin M. Presented at the International Papillomavirus Conference, Vancouver, Canada, 2005 |
||
Genital HPV infection is primarily transmitted by genital contact, usually (but not necessarily) through sexual intercourse.2, 18 Most HPV infections are transient and asymptomatic, causing no clinical manifestations. More than 90% of new HPV infections, including those with HR-HPV types, clear within 2 years, and clearance usually occurs in the first 6 months after infection.8, 10, 11, 19 Persistent infection with high-risk HPV, typically over several decades, is the most important risk factor for cervical cancer precursors and invasive cervical cancer.10, 19-22
Non–cancer-related outcomes of HPV infection include anogenital warts and RRP. Anogenital warts are due to infection with low-risk (LR) HPV types. Approximately 90% of anogenital warts are associated with types 6 and 11.23 The prevalence of genital warts has been examined using health-care claims data.24 An estimated 1% of sexually active adolescents and adults in the United States have clinically apparent genital warts.25 Rarely, infection with LR-HPV results in RRP, a disease characterized by recurrent warts or papillomas in the upper respiratory tract, particularly the larynx. There are juvenile onset and adult onset forms. The juvenile onset (JORRP) form is believed to result from HPV infection acquired perinatally from a mother with genital warts during delivery. Estimates of the incidence of JORRP are relatively imprecise but in two cities in the United States have ranged from 0.12 to 2.1 cases per 100,000 children younger than 18 years.26 Less is known about the adult form of RRP.
Disease Description
Most instances of HPV infection are asymptomatic (no clinical manifestations). However, even if asymptomatic, cervical infection can result in cervical changes which can be detected by Pap testing or cervical biopsy. Cervical cytology testing, or Pap testing, can detect changes in cervical epithelial cells (cells found on the surface of the cervix which can be either squamous or glandular). Most abnormal Pap tests results are categorized (by increasing grade of abnormality in squamous cells) as atypical squamous cells of unknown significance (ASC-US); atypical squamous cells—cannot rule out high-grade squamous intraepithelial lesion (ASC-H); low-grade intraepithelial lesions (LSIL); high-grade intraepithelial lesions (HSIL); and squamous cell carcinoma (SCC). Glandular cell abnormalities are either atypical cells of glandular origin (AGC) or adenocarcinoma in situ (AIS). HPV types 16 and 18 are more commonly found in higher grade lesions than lower grade lesions. In one study, the prevalence of HPV 16 was 12.9% among women found to have ASC-US, 23.6% among those with LSIL, and 51.8% among those with HSIL Pap tests.27 Each year, approximately 50 million women undergo Pap testing; approximately 3.5–5 million of these Pap tests will require some further evaluation including 2–3 million ASC-US, 1.25 million LSIL and 300,000 HSIL Pap tests.28–30
Abnormal Pap test results (typically LSIL, and HSIL) require further evaluation by colposcopic examination of the cervix. If a biopsy specimen is obtained during colposcopy, the cervical tissue is classified as normal, invasive cervical cancer (either squamous cell carcinoma or adenocarcinoma), or precancerous lesions. Precancerous lesions include cervical intraepithelial neoplasias (CIN) grades 1, 2, or 3; carcinoma in situ (CIS [based on increasing degree of abnormality in the cervical squamous epithelial cells]); or adenocarcinoma in situ (AIS). Cervical cancer incidence rates have decreased approximately 75%, and mortality rates approximately 70% since the 1950s, largely because of Pap testing.28, 29 In 2003, cervical cancer incidence in the United States was 8.1 per 100,000 women, with approximately 11,820 new cases reported.31 The median age of diagnosis for cervical cancer was 48 years.32
Anogenital warts develop approximately 2–3 months after HPV infection (typically types 6 and 11). However, not all persons infected with HPV types 6 and 11 develop genital warts. Anogenital warts can be treated, although 20%–30% regress spontaneously. Recurrence of anogenital warts is common (approximately 30%), whether clearance occurs spontaneously or following treatment.33
JORRP, believed to result from vertical transmission of HPV from mother to infant during delivery, is diagnosed at a median age of 4 years. A multicenter registry of JORRP in the United States, using data collected during 1999–2003, demonstrated that the clinical course of JORRP was associated with extensive morbidity, requiring a median of 13 lifetime surgeries to remove warts and maintain an open airway.34
Treatment of HPV-Associated Diseases
HPV infections are not treated; instead treatment is directed at the HPV-associated conditions. Current treatment options for anogenital warts and cervical, vaginal and vulvar cancer precursor lesions (e.g., CIN) include topical agents (which can be patient-applied), cryotherapy, electrocautery, laser therapy, and surgical excision.
Cervical Cancer and Precancer
Persistent HPV infection can result in precancerous cervical lesions as well as invasive cervical cancer. Treatment decisions are based on cervical biopsy results (e.g., obtained with colposcopy) not on the Pap test result.
For mild precancerous cervical biopsy lesions (mild dysplasia, i.e., CIN 1), the recommended management is follow-up with further evaluation.35 For severe precancerous cervical lesions such as CIN 2 or CIN 3, treatment options include removal of the area of abnormality (laser, loop electrosurgical excisional procedure [LEEP], cold knife conization) or destruction of the area of abnormality (cryotherapy, laser vaporization). Each method has its indications, advantages and disadvantages, but cure rates are comparable.
For invasive cervical cancer, treatment options include surgery, radiation therapy and chemotherapy, alone or in combination, depending on stage of disease. Depending on the stage of disease at diagnosis, a woman may be able to keep her ovaries. The survival rate 5 years after diagnosis of cervical cancer varies depending upon the stage of cervical cancer. The risk increases with higher stages of disease.
Anogenital warts
The primary goal of treating visible anogenital warts is wart removal. In the majority of patients, treatment can induce wart-free periods. If left untreated, visible anogenital warts might resolve on their own, remain unchanged, or increase in size or number. It is unknown if treatment of anogenital warts affects genital transmission of HPV. No single treatment is ideal for all patients. Most patients require a course of therapy rather than a single treatment.
Treatment regimens are classified into topical medications applied by the patient and provider-applied modalities, such as cryotherapy, podophyllin resin 10%–25%, trichloroacetic acid or bichloroacetic acid, or surgery. Other regimens include intralesional interferon or laser surgery.36
Importance of Surveillance
HPV cannot be detected through culture methods; detection requires molecular testing. HPV testing has a clinical role in identifying individuals with an increased risk of an HPV-associated cervical precancer or cancer. The FDA-approved clinical test, (HPV Hybrid Capture®2 [HC2] High Risk Test, Digene, Gaithersburg, MD) uses exfoliated cervical cells (or cervical biopsy) and detects the presence of one or more of 13 high-risk types. It does not determine the specific type or types present, but indicates the presence of high-risk HPV. The HC2 High Risk test is approved
- for use in women with equivocal cervical cytology results (i.e., ASC-US) to help determine if referral to colposcopy is needed, and
- as an adjunct to cervical cancer screening with cytology in women older than 30 years.
HPV infection of epithelial cells is associated with characteristic morphologic changes, and the presence of HPV may be suggested on the basis of pathologic findings. However, definitive detection of HPV requires polymerase chain reaction (PCR) testing; most PCR testing involves research procedures. HPV testing is not used for screening of HPV-associated lesions in anatomic sites other than the cervix, and it is not useful in diagnosis or clinical management of cancer, cancer precursors, or warts.
For epidemiologic and research questions using HPV as an endpoint, type-specific HPV tests have many advantages. There are many different formats, and results are dependent on the nature of the assay and the type of sample. The most common approach is to use a PCR that amplifies all mucosal HPV types (consensus PCR) with type(s) being determined by subsequent hybridization and/or sequencing of the products.
Research tests such as serologic testing for HPV antibodies may be useful to monitor population exposure to HPV. Because HPV infection is confined to the epithelium and infected cells are shed before cell death, natural HPV infection results in minimal host immune response, and not all those infected have detectable antibodies. However, laboratory reagents are not standardized and serologic assays are currently available only in research settings.
HPV Vaccine
A quadrivalent HPV vaccine (GARDASIL™ produced by Merck and Co., Inc.) was licensed by the Food and Drug Administration in 2006.37 The L1 protein found on the HPV capsid of HPV is the antigen used for HPV immunization. The vaccine protects against infection with HPV types 6, and 11, which are associated with anogenital warts, and types 16 and 18, associated with precancerous lesions and anogenital cancers. The vaccine is licensed for use in females only. Study of vaccine efficacy in males and need to vaccinate males is ongoing.
Clinical trials have demonstrated high levels of efficacy in preventing cervical precancers caused by the targeted HPV types, and vulvar and vaginal precancers and genital warts caused by the targeted HPV types among women who have not been infected with that HPV type. Among women in the clinical trials with no evidence of prior infection with HR-HPV, efficacy against these endpoints was almost 100%. In immunogenicity and safety studies conducted among females 9–15 years of age, over 99% of study participants developed antibodies after vaccination; titers were higher for young girls than for older females participating in the efficacy trials.
The vaccine is prophylactic has no therapeutic effect on HPV-related disease. If a girl or woman is already infected with one of the HPV types in the vaccine, the vaccine will not prevent disease associated with that type.
Quadrivalent HPV vaccine is administered intramuscularly as three separate 0.5-ml doses. The second dose should be administered 2 months after the first dose, and the third dose 6 months after the first dose.
Table 2 summarizes The Advisory Committee on Immunization Practices recommended schedules for routine and catch-up HPV vaccination.31
| Table 2. Recommended age groups, schedule, dosages and route of administration for Gardasil™ quadrivalent HPV vaccine | ||||
| Age group | Schedule | Dosage/route | Comment | |
|---|---|---|---|---|
| Routine vaccination | Females 11–12 yrs | 0, 2, 6 mos | 0.5 ml/intramuscular injection | Provider may initiate series as early as age 9 yrs. |
| Catch-up vaccination | Females 13–26 yrs | 0, 2, 6 mos | 0.5 ml/intramuscular injection | |
A second prophylactic bivalent vaccine against HPV types 16 and 18 is currently under development and has not been reviewed by FDA at this time. This vaccine may be licensed as early as 2008.
Ideally, vaccine should be administered before potential exposure to HPV through sexual contact. Sexually active females who have not been infected with any of the HPV vaccine types would receive full benefit from vaccination. However, the great majority of females who may have already been exposed to one or more of the HPV vaccine types can benefit from vaccination, even though benefit would be less. Pap testing and screening for HPV DNA or HPV antibody are not needed prior to vaccination at any age.
Cervical cancer screening among vaccinated females
At present, cervical cancer screening recommendations have not changed for females who receive HPV vaccine. Healthcare providers administering quadrivalent HPV vaccine should educate women about the importance of cervical cancer screening as recommended by national organizations.
Laboratory Testing
Identification of every instance of HPV infection is not necessary. This is because
- most sexually active individuals will acquire HPV infection at some point in their lives,
- most infections will not have any associated clinical disease, and
- the commercially available test for HPV infection requires laboratory testing of cervical specimens.
However it is important to monitor rates of cervical cancer since this is the primary goal of HPV vaccination. Cervical cancer surveillance data (as well as data on other HPV-associated anogenital cancers) are measured by population-based cancer registries participating in CDC’s National Program of Cancer Registries (NPCR) and/or the Surveillance Epidemiology and End Results (SEER) program of the National Cancer Institute, which cover over 96% of the U.S. population.
This surveillance activity is especially important because the vaccine protects against only four types of HPV, and over 40 types can infect the anogenital area. Data from cancer surveillance will be invaluable in measuring the success of HPV vaccination, but useful data are not expected until several decades after widespread adoption of the vaccine. The types of data currently available through cancer registries will be limited in answering these and other important surveillance-related questions:
- How can surveillance systems be used to evaluate vaccine effectiveness and identify possible vaccine failures?
- What type of surveillance systems can be used to provide data on more proximal endpoints (e.g., genital warts, cervical precancers) of HPV infection?
- What is the impact of vaccination on the distribution of HPV type-specific infection? Specifically, does vaccination against types 16 and 18 result in increased prevalence of other oncogenic types (“replacement lesions”)?
- How can type-specific data on HPV-associated anogenital cancers be collected (in order to compare vaccine type–associated cancers with non-vaccine type–associated cancers)?
- What is the impact of vaccination on medical costs related to procedures such as follow-up Pap tests (after an abnormal screening Pap test), colposcopy, cervical biopsy, and treatment of cervical lesions?
- What will be the impact of vaccination with the quadrivalent (against types 6, 11, 16, 18) versus the bivalent (types 16, 18 only) vaccine?
- How can surveillance systems measure disease rates among populations not adequately covered by vaccination and inform vaccination programs?
- How can surveillance systems inform cervical cancer screening programs in the HPV vaccine era?
CDC is currently exploring the feasibility and usefulness of surveillance activities to answer these questions. See section on Enhancing Surveillance.
Disease Reduction Goals
Because the quadrivalent HPV vaccine was licensed in 2006, the Healthy People 2010 Midcourse Review does not state a goal for vaccination coverage at this time.38 However, it does include a goal to “Reduce the proportion of females with human papillomavirus (HPV) infection,” although no target proportion is identified. It also states as a goal to “Reduce the death rate from cancer of the uterine cervix below a target of 2 deaths/100,000 females (from a baseline of 3 deaths/100,000 in 1998).” Another stated goal is to “increase the proportion of women receiving a Pap test,” but no quantitative goal regarding the proportion has been suggested. No goals are currently stated for reduction of anogenital warts, recurrent respiratory papillomatosis or non-cervical anogenital cancers.
Another Healthy People 2010 objective addresses surveillance to “increase the number of states that have a statewide population-based cancer registry that captures case information on at least 95 percent of the expected number of reportable cancers.”
Case Definition
There are currently no case definitions approved by the Council of State and Territorial Epidemiologists (CSTE) for the National Notifiable Diseases Surveillance System for any HPV-associated conditions, including HPV infection, anogenital warts, RRP, precancerous anogenital lesions, or anogenital cancers. The following descriptions of diagnosis and classification of HPV-associated conditions are included as aids to understanding possibilities for surveillance:
HPV infection: Tests for LR-HPV infection are not used for clinical purposes and are primarily research tools. Testing for HR-HPV infection status is important for its adjunctive role in cervical screening. Routine testing for HR-HPV infection is not recommended, but testing is clinically indicated in two specific clinical situations:
- in order to triage women with ASCUS Pap tests for further evaluation, and
- as an adjunct to Pap testing for women age 30 years and older.
Abnormal Pap tests and precancerous anogenital lesions: Abnormal Pap test categories are listed by increasing grade of severity: atypical squamous cells of unknown significance (ASCUS); atypical squamous cells—cannot rule out high-grade squamous intraepithelial lesion (ASC-H); low-grade intraepithelial lesions (LSIL); and high-grade intraepithelial lesions (HSIL). Precancerous lesions diagnosed by pathologists on specimens from cervical biopsy lesions provide a much more specific diagnosis of potential cancer than abnormal Pap tests. These precancerous lesions are grouped into cervical intraepthelial neoplasia (CIN) 1, CIN 2, and CIN 3; carcinoma in situ (CIS); or adenocarcinoma in situ (AIS). Precancerous lesions are also defined for vaginal intraepithelial neoplasias (VAIN), vulvar intraepithelial neoplasias (VIN), and anal intraepithelial neoplasia (AIN). These are defined and used for clinical diagnostics and management.
Anogenital cancers: The primary sites and pathologic diagnoses of the cancers are coded using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3). In the United States, disease burden from cervical, vaginal, vulvar and anal cancers is measured by population-based cancer registries participating in NPCR and/or SEER.
Anogenital warts: A diagnosis of anogenital warts is made based on visual inspection of the lesion(s). There are no case definitions for anogenital warts used for surveillance purposes.
Recurrent respiratory papillomatosis: RRP is diagnosed by a specialist based upon clinical evaluation. No case definitions for RRP are currently in use for surveillance purposes.
Reporting
HPV infection and HPV-associated clinical conditions are not nationally reportable diseases and notification is currently not required by CDC. Persons reporting should contact the state health department for state-specific reporting requirements.
Figure 1. United States cervical cancer incidence rates*, by state, 2003† 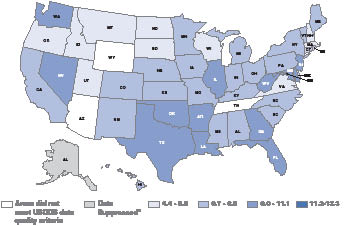
* Rates are per 100,000 and are age-adjusted to the 2000 U.S. standard population. † Source: U.S. Cancer Statistics Working Group. United States Cancer Statistics: 2003 Incidence and Mortality (preliminary data). Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2006. |
Current established national systems that can monitor HPV and its associated conditions include the following:
- The National Health and Nutrition Examination Survey (NHANES) conducts annual surveys of HPV infection.
- CDC’s National Program of Cancer Registries (NPCR) and the National Cancer Institute’s (NCI) Surveillance Epidemiology and End Results (SEER) program continuously monitor incident anogenital cancer cases as well as anogenital cancer–related deaths. These data are collected at the state (central cancer registries) as well national (NPCR/SEER) levels (Figure 1).
Other less-established systems are discussed in the following section.
Enhancing Surveillance
The goal of HPV vaccination is to prevent clinical conditions associated with infection with vaccine HPV types (6, 11, 16, and 18), with the primary goal being prevention of cervical cancers. However, because infection with HPV is relatively commonplace and a high proportion of infections are asymptomatic and the consequences are not seen for many years, monitoring the impact of a vaccination program poses many challenges.
The proximal measures of vaccine impact include outcomes such as HPV infection cervical cancer precursors, and anogenital warts. Currently, the only national surveillance program in the United States for proximal measures is NHANES, which measures HPV infection. The distal measures of vaccine impact include anogenital cancers, which are monitored through an excellent system of state-based cancer registries that cover approximately 96% of the U.S. population. However, these distal measures may take as long as 20 or 30 years before any impact can be accurately detected.
A potentially important limitation to the collection of surveillance data on anogenital warts, Pap tests or cervical cancer precursors is the lack of any HPV-associated nationally notifiable conditions. Despite this limitation, CDC is currently considering surveillance approaches to answer other questions related to HPV vaccination impact. (See section on Importance of Surveillance.) Approaches recently initiated include
- a pilot study exploring enhanced surveillance by select central cancer registries to include population-based statewide data on cervical intraepithelial neoplasia and cervical carcinoma in situ,
- creating a network of investigator-led sentinel surveillance sites in catchment areas (typically, a county) within four states to establish an enhanced system for population-based assessment of CIN 2/3 and, importantly, linkage of cases with HPV vaccination status and HPV type,
- monitoring HPV types and cervical precancers among patients in managed care organizations, and
- monitoring volume of patient visits for anogenital warts through a sentinel network of sexually transmitted disease clinics.
Other approaches being considered include using family planning clinic data to monitor abnormal Pap tests and referral patterns for colposcopy/treatment. CDC is also currently exploring the feasibility of using administrative datasets such as health insurer claims databases (Marketscan Medstat Dataset, Ingenix Dataset) and vaccine safety datasets (Vaccine Safety Datalink) to monitor HPV-associated outcomes. Specifically, administrative data consisting of ICD-9-CM codes in conjunction with Current Procedure Terminology (CPT) codes are being examined for usefulness in identifying specific Pap test diagnoses, cervical precancer diagnoses, and anogenital warts.
Currently, there are no recommendations for collection of routine surveillance data on HPV-associated conditions at the national level, other than the cancer-related data already being collected through cancer registries. However, several states have initiated various case reporting and other surveillance activities to measure HPV-related disease burden in their areas. To address the questions of usefulness of national reporting requirements, selection of appropriate disease endpoints for surveillance, and feasibility of data collection, CDC has initiated the activities described above. In preparation for future surveillance activities, CDC encourages state and local health programs to investigate the feasibility of making certain HPV-associated clinical conditions reportable, especially cervical precancers such as CIN 2/3 or CIS. CDC currently recommends that state and local health programs
- educate providers and the public about the link between HPV vaccination and cervical cancer prevention, and
- increase awareness of the availability of the newly licensed quadrivalent HPV vaccine and the importance of vaccinating 11- and 12-year-old girls, and
- continue to emphasize the importance of ongoing cervical screening with the Pap test, even for vaccinated women.
References
- Weinstock H, Berman S, Cates W, Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004;36:6–10.
- Holmes KK, Sparling P, Mardh P, et al. (eds). Sexually Transmitted Diseases. Third ed. New York: McGraw-Hill; 1999.
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518–27.
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118:3030–44.
- Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi F, Carcinogenicity of human papillomaviruses. Lancet Oncology 2005;6:204.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9.
- Bosch FX, de Sanjosé SS. Human papillomavirus and cervical cancer—burden and assessment of causality. J Natl Cancer Inst Monogr 2003;31:3–13.
- Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Désy M, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis 1999;180:1415–23.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423–8.
- Molano M, van den Brule A, Plummer M, Weiderpass E, Posso H, Arslan A, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol 2003;158:486–94.
- Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998;132:277–84.
- Daling JR, Weiss NS, Klopfenstein LL, Cochran LE, Chow WH, Daifuku R. Correlates of homosexual behavior and the incidence of anal cancer. JAMA 1982;247:1988–90.
- Daling JR, Weiss NS, Hislop TG, Maden C, Coates RJ, Sherman KJ, et al. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med 1987;317:973–7.
- Holly EA, Whittemore AS, Aston DA, Ahn DK, Nickoloff BJ, Kristiansen JJ. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J Natl Cancer Inst 1989;81:1726–31.
- Koblin BA, Hessol NA, Zauber AG, Taylor PE, Buchbinder SP, Katz MH, et al. Increased incidence of cancer among homosexual men, New York City and San Francisco, 1978–1990. Am J Epidemiol 1996;144:916–23.
- Ries L, Kosery C, Hankey B, MillerB, Clegg L, Edwards B. SEER Cancer Statistics Review 1973–1996. Bethesda, MD: National Cancer Institute, 1999.
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467–75.
- Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218–26.
- Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst 1995;87:1365–71.
- Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Chang T, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis 1994;169:235–40.
- Schiffman M, Kjaer SK. Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr 2003;31:14–9.
- Schlecht NF, Platt RW, Duarte-Franco E, Costa MC, Sobrinho JP, Prado JCM, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst 2003;95:1336–43.
- Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol 1995;33:2058–63.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003;36:1397–403.
- Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med 1997;102(5A):3–8.
- Armstrong LR, Preston EJ, Reichert M, Phillips DL, Nisenbaum R, Todd NW, et al. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis 2000;1:107–9.
- Datta SD, Koutsky L, Ratelle S, et al. Type-specific high-risk human papillomavirus prevalence in the US, HPV Sentinel Surveillance Project, 2003–2005. Presented at the 44th Annual Meeting of the Infectious Disease Society of America, Toronto, October 2006. Abstract 1084.
- Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002;52:342–62.
- Schiffman M, Brinton L, Devesa S, Fraumeni J. Cervical cancer. In: Schottenfeld D, Fraumeni J, eds. Cancer epidemiology and prevention. Second edition. New York Oxford: Oxford University Press; 1996: 1090–128.
- Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study. Gynecol Oncol 2000;78:97–105.
- CDC. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007;56(No. RR-2):1–24.
- National Cancer Institute. SEER—Surveillance Epidemiology and End Results. National Institutes of Health. Available at http://seer.cancer.gov/statfacts/html/cervix.html?statfacts_page=cervix.html&x=14&y=18.
- Chuang TY, Perry HO, Kurland LT, Ilstrup DM. Condyloma acuminatum in Rochester, Minn., 1950–1978. I. Epidemiology and clinical features. Arch Dermatol 1984l;120:469–75.
- Reeves WC, Ruparelia SS, Swanson KI, Derkay CS, Marcus A, Unger ER. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg 2003;129:976–82.
- Wright TC, Jr., Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120–9.
- CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR 2006;55 (No. RR-11):1–94.
- Food and Drug Administration. Product Approval Information. Human papillomavirus (types 6, 11, 16, 18) recombinant vaccine. US Department of Health and Human Services, Food and Drug Administration. Available at http://www.fda.gov/cber/products/hpvmer060806.htm
- U.S. Department of Health and Human Services. Healthy People 2010. 2nd. ed. With understanding and improving health and objectives for improving health (2 vols.). Washington DC: US Department of Health and Human Services, 2000. Available at http://www.healthypeople.gov
* Due to the large size of this file, please be patient while the file is being downloaded. If the file does not download after a few minutes, close all open applications and reboot your computer before trying again.
Non-CDC Link Disclaimer: Links to non-Federal organizations found at this site are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. The CDC is not responsible for the content of the individual organization web pages found at these links.
.pdf files: To view and print the .pdf files on this site, you will need Adobe Acrobat Reader. Use this link to obtain a free copy of Adobe Acrobat Reader (exit). We highly recommend that you upgrade to the latest version if haven't already.
Content last reviewed on August 20, 2008
Content Source: National Center for Immunization and Respiratory Diseases