 |
 |
FDA
Home Page | Search FDA Site |
FDA A-Z Index | Contact FDA
![]()
FDA faces a critical issue today. Because of a convergence of challenges outlined in previous sections, the Agency has been unable to fully meet its explicit statutory obligations; nor has it been able to completely guarantee the more implicit health and safety responsibilities the statute requires and the public demands. Figure 2 illustrates that a sizable gap still exists between statutory requirements of "on-time review" for several product areas, and what FDA currently is able to deliver. Figure 3 shows a similar gap between mandated and actual inspectional coverage for FDA-regulated industries.
The Agency has listened carefully to its stakeholders over the past several months and has combined their views with its own emerging strategies to develop a plan for narrowing the gap. The following section provides a summary of stakeholder views.
FDA's assessment of the challenges it faces in fulfilling its mission and the identification of the disparity between expectations and what is achievable given the current climate set the stage for consultations with its external stakeholders. This consultation is necessary to determine the most effective ways of narrowing the gap. FDA depends on the views of its stakeholders for two crucial reasons:
In the sections that follow, the process of stakeholder consultation is discussed, and a summary of their views is provided.
Section 406(b) of FDAMA prescribes that the plan for statutory compliance be developed:
"after consultation with appropriate scientific and academic experts, health care professionals, representatives of patient and advocacy groups, and the regulated industry."
The experts and representatives referenced in Section 406(b) comprise the constituency of the FDA. The Agency informally consults with these constituents on a regular basis. Section 406(b) codifies this process and provides a mechanism for formal input from and feedback to its constituency.
In response to this requirement, the Agency designed a process that provided multiple avenues for input, including the following:
FDA adopted a uniform approach in framing the stakeholder discussions and comments. Agency officials first outlined the stakeholder consultation process. The leadership then provided a framework outlining the emerging technological and public health challenges faced by FDA. Finally, to focus stakeholder comments and discussion, questions (Appendix B) were developed that related to each of the six objectives addressed by the 406(b) plan and were available to stakeholders prior to the meetings.
The process of engaging the Agency's stakeholders and receiving useful feedback is an ongoing one. This initial round of stakeholder views will continue to be analyzed and interpreted during Fall 1998. Results of the analysis will be shared with FDA's external as well as internal audiences. The next round of formal stakeholder meetings is being scheduled for Spring 1999, and regular contacts will continue to be maintained. Although longer term assessment is forthcoming, a preliminary evaluation of stakeholder views has been conducted. An overview of these views is provided in the next section. Stakeholder comments are assessed in greater detail in Part Two of the Plan and are related to Agency strategies.
FDA's stakeholders commented on many aspects of the Agency's operations. The recommendations made by stakeholders regarding the Agency's priorities and the strategies FDA should use in carrying out its responsibilities reflect a wide range of concerns and perspectives. The full context of stakeholder views expressed at public meetings and in written comments are captured in transcripts and dockets that are available on FDA's Internet Web page. Appendix B-4 also provides a compendium of stakeholder recommendations, classified both by 406(b) objectives and by the strategic directions that are identified in the next section of the Plan. Major themes that emerged from the stakeholder comments are summarized below.
Most stakeholders agree on several broad issues. Many agreed that FDA priorities should be risk-based, scientifically rational, and focused on protecting public health. In addition, the Agency should view meeting its statutory obligations as a high priority. A number of organizations cautioned that the Agency should limit its participation in new activities, especially those that go beyond the scope of its core statutory requirements. Although stakeholders varied in their interpretations of core responsibilities, some stakeholders highlighted the importance of preserving FDA's regulatory role and encouraged the Agency to develop more creative strategies to exercise its regulatory responsibilities. Many stakeholders acknowledged the difficulties inherent in making trade-offs among program activities when resources are constrained.
Making new safe and effective treatments available to patients in a timely manner is also a high priority for FDA. To optimize the performance of the premarket review and approval system, stakeholders recommended that FDA continue to re-engineer its systems and strive for internal efficiencies; communicate earlier in the premarket review process, more frequently, and more openly with industry and other stakeholders; and make FDA policies and procedures more consistent and more transparent to industry and the public. Several groups would like FDA to adopt a more uniform and consistent approach to addressing risks of public health significance. Consistency of FDA policies and procedures seemed to be a greater concern than their transparency.
Requests for improved communication emphasized two-way communication--not only from the FDA to its stakeholders, but also from stakeholders to FDA beyond adverse event reporting. Stakeholders value FDA developing a strong scientific and analytic base for its regulatory decisions. They believe that FDA should use the expertise of other organizations to help meet its goals. For example, delegating or collaborating on certain functions (such as research, standard-setting, and some aspects of product review) to third parties was offered as a means of leveraging limited resources.
Several stakeholder groups want to be more involved in FDA advisory committees. These views are consistent with FDA's transition to a more open and collaborative relationship with its regulatory counterparts and industry. Continued FDA leadership and participation in the international arena was encouraged to ensure that international standards and guidelines are consistent with U.S. requirements. Even though it was recognized that FDA had limited resources to meet all of its statutory obligations and to meet public expectations, industry representatives opposed the collection of user fees for medical devices and the blood banking industry, as well as for veterinary products, as a means of funding premarket review activities. Similarly, the concept of an "FDA seal", viewed as a form of user fees, was not supported.
Although the first order of concern of all stakeholders is consumer health protection and availability of medical products, there is no consensus on the role FDA should play nor what approach should be taken in this daunting task. Key differences among stakeholders include the following:
FDA's role in education
Stakeholders differed sharply in their opinions on the legitimacy and primacy of FDA's role in consumer education. While some stakeholder groups believe that industry and health professionals should be responsible for consumer education, others assert that FDA should play an essential role in providing objective information about regulated products to consumers and in facilitating patient participation in ongoing clinical trials of promising new therapies. One consumer advocacy group, the National Council on Patient Information and Education, requested FDA's support in developing a collaborative, national consumer medicine safety and education program.
FDA's enforcement activities
Some stakeholders called for expanded FDA authority and additional resource appropriations to allow the Agency to carry out its responsibilities, for example, in the areas of drug safety monitoring and monitoring the sale of unapproved veterinary products. Other stakeholders acknowledged that FDA would need to share enforcement responsibilities with others. For example, one group supported a division of tasks in the inspection arena, with FDA covering the imports, and states being responsible for domestic inspections.
Use of Third Parties
There were mixed views in this area as well. Many consumers preferred that FDA regulate the industry more directly, while several industry representatives advocated for greater use of third parties, as long as the arrangement was carefully monitored by the Agency.
Advisory Committees
Views regarding the composition of FDA advisory committees diverged greatly. Some pressed for broader representation of interested persons while others advocated that FDA place greater emphasis on the depth of knowledge of advisory committee members. The Agency was urged to recruit renowned experts to serve on advisory committees. Some advisory committees were criticized for favoring nonscientific issues over science when they make recommendations.
Perhaps the issue that remains most problematic is the overall question of balance among FDA's functions. The appropriate mix of premarket review, post-market inspection, and surveillance activity is an ongoing topic of debate among the Agency's stakeholders. One stakeholder summed up the issue:
"How should FDA balance the need for strong and timely premarket review programs with the need for effective postmarket inspection, surveillance, and enforcement programs? That is like asking the American people to find a balance between building safe aircraft and providing adequate maintenance over the course of a plane's life." (Patient Group)
Although stakeholders expressed their views regarding the emphasis FDA should place on various issues, these comments frequently focused on a single FDA Center or two competing issues. FDA does not have sufficient information at this time about the priority Agency stakeholders wish to assign to a particular issue relative to other issues competing for resources within an FDA Center or within the Agency as a whole. In some instances the proposed strategies appear to be contradictory. For example, how should the Agency balance setting risk-based priorities or meeting public expectations when doing so directly competes with meeting its statutory obligations?
The six objectives specified in FDAMA Section 406(b) and outlined on page 1 of this Plan, provide FDA with a broad framework for meeting its statutory requirements and public expectations. The Agency's senior leadership believes the following strategic directions are necessary to focus its efforts in achieving the objectives set forth by Congress. These directions represent an amalgam of approaches that have been emerging for several years, and which have been modified both by new FDA challenges and by the productive suggestions made by external stakeholders. Figure 4 identifies the link between key stakeholder themes and the strategic directions outlined in this section of the plan.
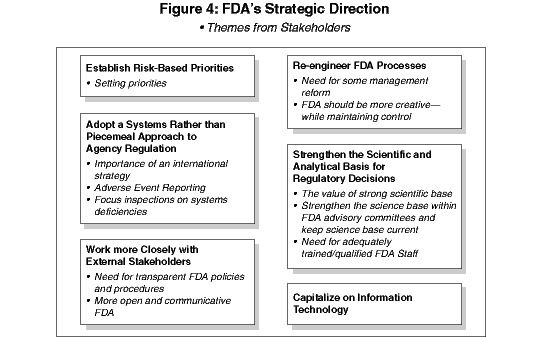
The strategic directions are broad in scope and cross-cut all components of the organization. As such, they provide a context to guide all of the Agency's more specific goals and programs. They also serve as a way to galvanize diverse activities into a set of unified directions for the long-term.
Although the importance of setting risk-based priorities was a concept repeatedly endorsed by many stakeholder groups, there was not consensus regarding what constituted the highest risk areas. FDA must listen to its stakeholder community, but then it must decide, based on continuing consultation with its stakeholders, which health and safety risks most directly threaten the well-being of U.S. consumers, and allocate its resources accordingly. In the harsh light of limited resources, FDA simply cannot meet everyone's demands and cannot address all risks with the same degree of urgency or intensity. For example, the Agency is unable to respond to its highest priority health risks and at the same time fully meet its biennial statutory inspection requirements for drugs, biologics, and medical devices. It may be appropriate to reassess the practicality of mandates that emphasize industry coverage, regardless of risk, when those mandates may divert limited resources away from addressing serious health and safety concerns. The Agency has and will continue to increase the efficiency of "fast track" processes to address the most urgent needs for therapies so that these therapies can enter the marketplace rapidly. Resources will continue to be redirected toward the review of these products. Surveillance and compliance efforts also will continue to be directed toward identifying and taking action to correct the most serious health and safety problems associated with products that are in the marketplace or about to enter the market. The Presidential Food Safety Initiative will continue to focus attention and devote resources to those areas of the food supply that pose the greatest risk of illness and/or death to consumers.
A strong science base continues to underpin each of the Agency's regulatory decisions. Such decisions must be made throughout the lifespan of FDA-regulated products from initial research, development and testing, through production, marketing and consumption. A strong science base consists of the expertise, the risk assessment protocols, the test methods, product guidance and performance standards, and the facilities and equipment necessary for conducting excellent science. The emerging emphasis in this strategic area is to seek means for achieving synergies in science capability through access to and collaborative efforts with sources of scientific expertise beyond FDA. A recent example that the Agency hopes will achieve research synergies through collaboration is the pharmaceutical quality and drug development science initiative that the Agency has begun to pursue under a cooperative research agreement among FDA, professional societies, and industry. The initiative will provide a venue to conduct research on pressing questions about pharmaceutical manufacturing that can inform regulatory decisions regarding needs in such areas as supplement submission requirements or bioequivalence studies after there are manufacturing changes. Such collaborative efforts are reinforced in the objectives identified in FDAMA Section 406(b). The key lies in "ensuring access to the expertise," wherever it is most cost-effective.
FDA will need to multiply the Agency's capability to address complex public health problems by working with stakeholders in planning, implementing, and evaluating solutions to these problems. The solutions don't lie solely in expanding the mass of the Agency. Consumers, the regulated industry, health professionals, and FDA's regulatory counterparts in the U.S. and abroad each represent components of a total network that can potentially improve health outcomes. To help "activate" that network, FDA is engaged in several strategies, some just emerging and others in a more mature phase. These "activation strategies" include: collaboration with stakeholders to create synergies in protecting the public health; ensuring that stakeholders are well informed about the Agency's regulatory processes [the processes should be as transparent as possible] and the products that are affected by these processes; involving stakeholders early in the Agency's processes; and ensuring that all affected stakeholder groups' interests are well represented in product testing and approval decisions.
FDA is striving to create synergies through collaboration with appropriate outside colleagues in product research and testing, development, production, marketing, and consumption/use to ensure safety, quality, and efficacy. The Agency's Joint Institute for Food Safety and Applied Nutrition [JIFSAN](with the University of Maryland) and the Moffett Center in Illinois are illustrative of such synergies working at the level of applied research and development to ensure safe foods.
Industry representatives and health professionals made it clear to FDA during the stakeholder consultation process that they can be more effective colleagues in improving health outcomes in their role as product developers and users if they are 1) well informed about the Agency's regulatory review, surveillance, and compliance processes; and 2) consulted prior to regulatory decisions on both the pre- and post-market side of product commercialization. FDA will continue implementing strategies to engage in preventive problem solving, as well as initiatives that will make the Agency's processes as clear and understandable as possible to participants.
Consumers and patients expressed a need to have prompt, complete, understandable, and unbiased information about products that FDA regulates, particularly new therapies. Well-informed consumers are more effective contributors to the management of their own health risks. FDA has launched several initiatives that are intended to keep the consumer well-informed through such vehicles as publishing the availability of important new drugs on the Internet. FDA is also attempting to ensure that the interests of all affected patients are well represented in such areas as clinical trial designs for new therapies. In addition, FDA will ensure that the interests of the consumer are represented in such deliberative bodies as advisory committees when recommendations on new products are being considered.
FDA has used both an internal and an external focus in redesigning many of its regulatory review processes. From the external perspective, FDA is implementing several protocols that will result in simplified regulatory approaches and, as a result, a reduced burden for the regulated industry. Many of these regulatory reinventions are embodied in provisions in FDAMA. For example, the Agency may start review of a "fast-track" drug application before the application is complete if preliminary clinical data demonstrate that the product may be effective. Fast-track status also is being established for humanitarian medical devices, and new product development protocols will allow medical device sponsors to use recognized study results that have been generated by other sources as part of their own application submission. Other regulatory simplification strategies have been instituted independent of FDAMA. For example, a phased review process for animal drugs has been designed that enables the Agency to provide periodic feedback to product sponsors throughout the drug review process to foster "continuous improvement" in the application.
FDA is also focusing internally to achieve greater efficiencies and effectiveness in its review and tracking processes. For example, implementation of project management techniques allows an opportunity for convergent thinking and action to occur so that multiple disciplines can coordinate their efforts in providing thorough but timely reviews of product sponsors' applications.
Several stakeholders during the public meetings noted that they could be more efficient and effective participants in promoting and protecting public health if they could understand the total context of what the Agency was trying to do and what its future directions were. The establishment of a systems approach within FDA is closely related to the establishment of risk-based priorities. Use of a systems orientation is an effective way to identify what is truly high-priority risk and then to address that risk in a systemic manner. Systems solutions, such as the Food Safety Initiative, the integrated adverse event reporting initiative, and the import monitoring system, are examples of FDA acting in concert with other collaborators to address the highest priority, most pervasive risks facing consumers.
The Agency also has adopted a systems orientation in many of its individual programs. To illustrate, medical device inspectors have embarked on a new approach to determine industry compliance with Good Manufacturing Practices (GMPs). They are pilot-testing a systems-oriented inspectional strategy whereby medical device plants are given guidance on the establishment of a total Device Quality System, so that the control of product safety and quality is owned by the firm, rather than their having to respond to a series of external compliance requirements that must be responded to one at a time. The seafood Hazard Analysis and Critical Control Points (HACCP) initiative provides another example where FDA worked with the seafood industry to implement a systems approach to ensure the safety of seafood consumed by the American public.
FDA has been on a long course of improvement in taking advantage of the opportunities offered by a rapidly evolving information technology environment. Information technology has been used for quite some time by the Agency in order to improve internal efficiencies. For example, a key element in accelerating the review of new drug therapies has been automating major portions of the drug review process. When both product sponsor and Agency reviewer can use electronic communication to establish a common ground of understanding, then all parties benefit. It is a critical element that has become pervasive in all mission-oriented as well as support activities.
More recently, the Agency has turned its attention to using information technology as a way of improving communication with external stakeholders. One of the most powerful examples of how stakeholders are assisted is in the rapid provision of information on new drug therapies via the Internet to consumers and patients. FDA's home page provides an opportunity for all of FDA stakeholders to be aware of recent Agency regulatory decisions, and, just as important, to receive input in the form of suggestions and other opinions from Agency officials. The Agency will expand use of information technology to bring relevant information to bear in the area of product surveillance and adverse event reporting. Well-designed and integrated information systems will dramatically reduce the gap between adverse effects associated with consumption and problem correction.
The strategic directions outlined above provide the context for understanding Part Two of the 406(b) Plan. In Part Two, specific performance targets and associated strategies are outlined for FY 1999. Part Two is organized into sections that correspond to the six objectives outlined in Section 406(b) of FDAMA {Section 903(f) of the FD&C Act as amended}. Thus, specific performance targets can be directly related to achieving the objectives of the Act.
Within each objective, strategies for FY 1999 reflect the Agency- wide strategic directions identified in Part One. Thus, the Agency's targeted planning for FY 1999 is strategically aligned with its intended directions over the next several years.
![]()