|
CFSAN/Office of Nutrition, Labeling, and Dietary Supplements
April 2008
Answer: There are no specific size requirements for the nutrition label. However, the "Nutrition Facts" heading must be in a type size larger than all other print size in the nutrition label (21 CFR 101.9(d)(2)). Minimum type sizes of 6 point and 8 point are required for the other information in the nutrition label (21 CFR 101.9(d)(1)(iii)), and there are minimum spacing requirements between lines of text (21 CFR 101.9(d)(1)(ii)(C)).
Answer: The illustration below indicates an example of the graphics FDA uses to display the Nutrition Facts label. Format requirements are specified in 21 CFR 101.9(d).
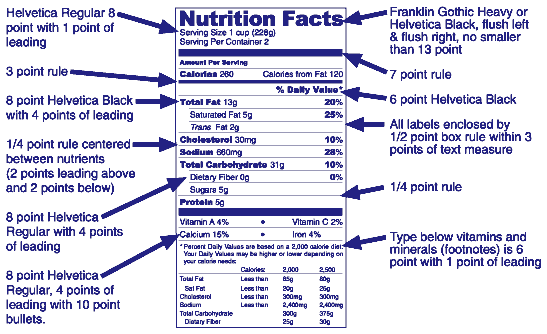
Answer: No. The mandatory type specifications are listed in 21 CFR 101.9(d). Unlike the illustrative example of VII L2 (above):
Answer: The requirement for 6 and 8 point type sizes are minimum requirements. Larger type sizes may be used.
Answer: Under 21 CFR 101.9(j)(13)(ii)(D) the Nutrition Facts may be presented on any label panel when the total surface available for labeling is 40 or less square inches. Packages with more than 40 square inches of available space must place the nutrition information on either the PDP or information panel as defined in 21 CFR 101.2.
Answer: Yes, however, if condensing results in a label that does not meet minimum type size requirements, FDA would consider the label misleading. 21 CFR 101.9(d)(1)(iii).
Answer: On packages with more than 40 square inches available to bear labeling, the "side-by-side" format may be used if the regular Nutrition Facts label does not fit. In this format, the bottom part of the Nutrition Facts label (following the vitamin and mineral information) is placed immediately to the right and separated with a line. If additional vitamins and minerals are listed after iron and the space under iron is inadequate, they may also be listed to the right with a line that sets them apart from the footnotes.
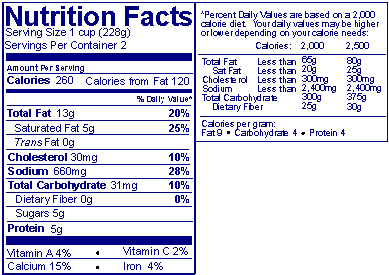
Also, if the package has insufficient continuous vertical space (i.e., about 3 inches) to accommodate the above format, the nutrition label may be presented in a tabular (i.e., horizontal) display.
Answer: If the window is used for any labeling, including promotional stickers, the window is considered to be available labeling space. However, if no labeling is present it is not considered to be available space.
Answer: Yes, required label information must be presented in a manner so that it is not obscured. Firms having difficulties in presenting nutrition information on such packages may wish to request a special allowance pursuant to 21 CFR 101.9(g)(9) by writing to the Office of Nutritional Products, Labeling, and Dietary Supplements, HFS-800, 5100 Paint Branch Pkwy., College Park MD 20740.
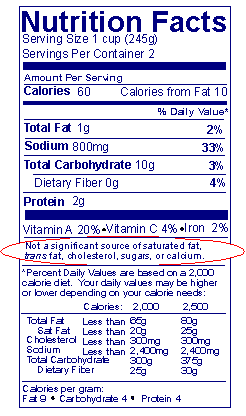
Answer: The nutrients listed below may be omitted from the list of nutrients and included in a single sentence when present at "zero" levels in a food. This is done by putting the label statement ("not a significant source of _________") immediately below the listing of vitamins A and C, calcium, and iron. When the statement "Not a significant source of_____________" is used for more than one nutrient, nutrients must be listed in the order in which they would have been listed in the regular format (e.g., "Not a significant source of calories from fat, saturated fat, trans fat, cholesterol, dietary fiber, sugars, vitamin A, vitamin C, calcium and iron"). The footnote can be used, with any format, to list one or more of the following nutrients: (21 CFR 101.9(c))
| Nutrient | Level per serving | Label statement |
|---|---|---|
| Calories from fat 21 CFR 101.9(c)(1)(ii) |
Less than 0.5 g fat | "Not a significant source of calories from fat" |
| Saturated fat 21 CFR 101.9(c)(2)(i) |
Less than 0.5g of total fat | "Not a significant source of saturated fat" |
| Trans fat 21 CFR 101.9(c)(2)(ii) |
Less than 0.5g of total fat | "Not a significant source of trans fat" |
| Cholesterol 21 CFR 101.9(c)(3) |
Less than 2 mg | "Not a significant source of cholesterol" |
| Dietary fiber 21 CFR 101.9(c)(6)(i) |
Less than 1g | "Not a significant source of dietary fiber" |
| Sugars 21 CFR 101.9(c)(6)(ii) |
Less than 1g | "Not a significant source of sugars" |
| Vitamins A and C, calcium, and iron 21 CFR 101.9(c)(8)(iii) |
Less than 2% of RDI | "Not a significant source of _________" (listing the vitamins or minerals omitted) |
Answer: Part 101.9(d)(1)(i) states that the nutrition information "shall be all black or one color type, printed on a white or other neutral contrasting background whenever practical." This does not prohibit reverse print or use of other colors. However, if reverse type is used, FDA expects that any impairment in readability resulting from such a technique will be compensated for by use of other graphic techniques to improve readability, such as increased type size. Reverse printing is not permitted as a form of highlighting under 21 CFR 101.9(d)(1)(iv) because it would interfere with the consistent look of the label.
Answer: No, the use of that footnote is optional. 21 CFR 101.9(d)(10)
Answer: The listing of percent of the Daily Values needs to be in a column aligned under the heading and can be either centered or right justified.
Answer: The Nutrition Facts label can be placed on the film package provided that the color contrast of the print and the indentations made by the product do not prevent consumers from being able to read the information at the point of purchase.
Answer: No. However, if a continuous print label includes one uncut Nutrition Facts label it would be acceptable.
Answer: Yes, as long as the sticker adheres to the product under the intended storage conditions. Some companies use generic cartons or bags and affix product specific labeling.
Answer: When nutrition labeling must be presented in a second language, the nutrition information may be presented in separate nutrition labels for each language or in one label with the second language, translating all required information, following that in English. Numeric characters that are identical in both languages need not be repeated.
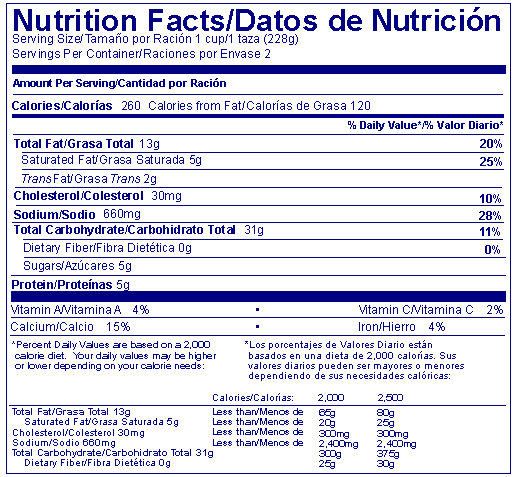
Answer: When the only accepted common or usual name for a food is in a language other than English (e.g., salsa, chili con carne, croissants, rigatoni) use of this common or usual name does not necessitate dual language declaration. However, if the name of the food is intended to bring the article to the attention of a person who does not speak English (e.g., Frijoles Pintos), all required information must be presented in the foreign language.
Answer: When a package contains two or more packaged foods that are intended to be eaten individually, such as a variety pack of breakfast cereals or when packages may be used interchangeably for the same type of food, such as round ice cream containers, the manufacturer may choose to include separate Nutrition Facts labels for each food product, or may use an aggregate Nutrition Facts label.

Answer: The manufacturer may choose to use: (1) a separate Nutrition Facts label for each variety of cookie in the package, (2) an aggregate label (i.e., a single Nutrition Facts label including nutrient content information and % DVs in separate columns for each variety), or, (3) if it is likely that one person would eat an assortment of the cookies at the same time, a composite label that provides one set of nutrition information based on a weighted average of all of the cookies in the assortment.
Answer: No, the statement of identity on the PDP along with the statement of identity above each column of nutrient values in the aggregate Nutrition Facts label will provide adequate information for the consumer to determine which nutritional values in the aggregate label apply to the contents of the package.
Answer: "As packaged" refers to the state of the product as it is marketed for purchase. "As prepared" refers to the product after it has been made ready for consumption (e.g., ingredients added per instructions and cooked such as a cake mix that has been prepared and baked or a condensed or dry soup that has been reconstituted).
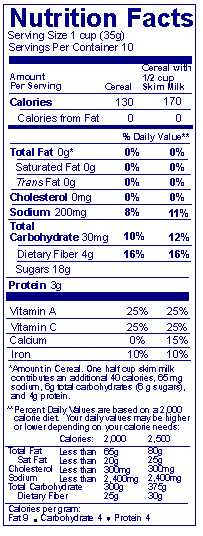 If a manufacturer chooses to do so, how may a food be labeled if the labeled food is commonly combined with another food before eating?
If a manufacturer chooses to do so, how may a food be labeled if the labeled food is commonly combined with another food before eating?
Answer: The Nutrition Facts label must state the nutrients in the food "as packaged" (i.e., before consumer preparation). However, manufacturers are encouraged to add a second column of nutrition information showing calories, calories from fat and the % DV for the combination of foods eaten. Quantitative amounts (i.e., g/mg) need only be given for the packaged food. However, as shown in this example, a footnote can be added to indicate the amount of nutrients in the added food. Alternatively, the quantitative amounts of the prepared food may be included immediately adjacent to those for the packaged food (e.g., "Sodium 200 mg, 265 mg"). 21 CFR 101.9(e)
Answer: The dual listing of serving size and servings per container is not required when providing a second column of nutrient information. The only requirement is to list the serving size and servings per container that are based on the Reference Amounts Customarily Consumed (RACC) for the product. 21 CFR 101.9(b)(9) and 21 CFR 101.9(e)
Answer: Yes. Section 101.9(b)(11) states that if the product is promoted on the label or labeling for a use that differs in quantity from the RACC by 200% or greater, dual declaration would be required. FDA considers recipes on the label as "promoting" a use of the food. The regulations (21 CFR 101.9(b)(11)) specifically exempt bulk products used primarily as ingredients (e.g., flour, sugar, oils) or traditionally used for multi-purposes (e.g., eggs, butter) from dual declaration requirements.
Answer: Such a label would have two columns with a heading "Cereal" and "Cereal with ½ cup (or ¼ cup) _____ milk" where the blank is filled in with the type of milk.
Answer: Dual declaration is optional.
Answer: Only if the recipe calls for 200% or more of the RACC of the product for each serving of the food created by the recipe. When the recipe calls for an amount less than 200% of the RACC, such information could be voluntarily listed. However, nutrition information for a specific recipe may be presented outside of the Nutrition Facts label.
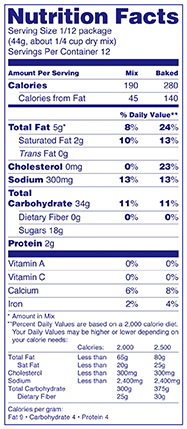 If a manufacturer chooses to do so, what is an example of the Nutrition Facts label for a food requiring further preparation by the consumer?
If a manufacturer chooses to do so, what is an example of the Nutrition Facts label for a food requiring further preparation by the consumer?
Answer: When the nutrient values in the column for the product prepared according to package directions would be identical to the column for the product as packaged (e.g., the only ingredients added during preparation are ingredients such as water), manufacturers may omit the second column and include the amount made as part of the serving size declaration. For example, a dry beverage mix could declare: "Serving Size: 1 tsp dry powder (4 g)(makes 1 cup prepared)."
Answer: Yes. The nutrient content of the food, not available label space, is the determining factor.
Answer: The type size and layout requirements are the same as that required for the full format.
Answer: The intent of the simplified format was to minimize the amount of information required to be on the label. While the agency discourages the listing of optional nutrients, present at insignificant amounts, in the simplified format, the regulations do not prohibit such listing. When non required nutrients (e.g., calories from fat, saturated fat, trans fat, cholesterol, dietary fiber, sugars, vitamin A, vitamin C, calcium or iron) are voluntarily listed as zero, the footnote required by 21 CFR 101.9(f)(4) is not required.
Answer: No, since use of the simplified format is optional all required information must be presented when the full format is used.
Answer: This statement, which must list all nutrients required by the full format that are present at insignificant amounts, must be included when: (1) nutrition claims are made; or (2) vitamins and minerals are added; or (3) naturally occurring nutrients that are not required on the full format (e.g., potassium) are voluntarily declared.
Answer: Yes. However, as noted in the previous question and answer, when a claim is made, the statement "Not a significant source of _____________" (with the blank filled in with the name(s) of any nutrient(s) identified in 21 CFR 101.9(f) and calories from fat that are present in insignificant amounts) must be included at the bottom of the nutrition label. 21 CFR 101.9(f)(4)
Answer: When the full format is presented in a tabular display, the statement "Not a significant source of ____________" should be placed beneath the vitamins and minerals and be separated by a hairline. When the simplified format is presented in a tabular display, the statement should be separated by a bar under the nutrients declared.
Answer: 6 point
 Is there a Nutrition Facts format for a food in which most nutrients are present in insignificant amounts?
Is there a Nutrition Facts format for a food in which most nutrients are present in insignificant amounts?
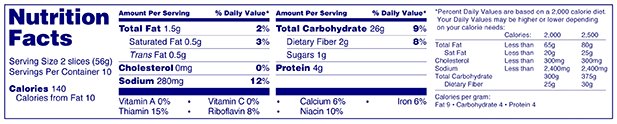
Answer: A simplified Nutrition Facts label may be used if at least eight of the following nutrients are present in insignificant amounts: Calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, sugars, protein, vitamin A, vitamin C, calcium and iron (slightly different rules for labeling foods intended for children less than 2 years). The five core nutrients, shown in bold in the adjoining example, must always appear on all Nutrition Facts labels regardless of amounts present in the food. In addition, any of the nutrients required on the full Nutrition Facts label that are naturally present or are added to the food must be "declared on the simplified Nutrition Facts label.
21 CFR 101.9(f) - List of nutrients; 101.9(f)(1) - "Insignificant" defined; 101.9(c) - "Insignificant" levels listed for nutrients
Answer: These are the amounts that are permitted to be shown as zero on the Nutrition Facts label (e.g., less than 5 calories may be expressed as 0 calories) except that for total carbohydrate, dietary fiber, and protein, it is the amount that can be declared as "less than 1 g" on the Nutrition Facts label. 21 CFR 101.9(c)
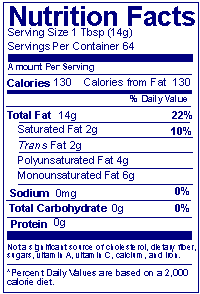 When should a statement be used on simplified format labels to list nutrients present at insignificant amounts?
When should a statement be used on simplified format labels to list nutrients present at insignificant amounts?
Answer: A "simplified format label" must include a statement listing "zero" level nutrients when nutrients are added to the food or voluntarily declared on the Nutrition Facts label, and when claims are made on the label. In this example, the manufacturer voluntarily lists polyunsaturated and monounsaturated fat, and therefore must add the statement "Not a significant source of _________" with the blank filled in by the names of nutrients present at insignificant levels.
Answer: No. The simplified format only requires the statement "Percent Daily Values are based on a 2,000 calorie diet" regardless of the size of the package. If the term "Daily Value" is abbreviated in the heading as "DV," the statement must indicate that "DV" means "Daily Value" (e.g., "Percent Daily Values (DV) are based on a 2,000 calorie diet").
Answer: FDA is requiring that trans fatty acids be listed in nutrition labeling in response to a petition from the Center for Science in the Public Interest and to published human studies that show that intake of trans fatty acids, similar to the intake of saturated fatty acids, increases low density lipoprotein-cholesterol (LDL-C) ("bad cholesterol") in the blood. An elevated LDL-C increases the risk of developing coronary heart disease. Reports published by the Institute of Medicine of the National Academy of Sciences (IOM/NAS) and the Federal government have recommended that Americans limit their intake of trans fat and other cholesterol-raising fats while consuming a nutritionally adequate diet. For Americans to follow these recommendations, they must know the amount of trans fatty acids in the individual foods that they eat. Therefore, FDA is requiring that this information be provided in nutrition labeling to assist consumers in maintaining healthy dietary practices. (68 FR 41434, July 11, 2003)
Answer: The Agency's regulatory chemical definition of trans fatty acids is "all unsaturated fatty acids that contain one or more isolated double bonds in a trans configuration." Trans vaccenic acid, a trans fatty acid with a single double bond, and other trans fatty acids of ruminant origin with either a single double bond or nonconjugated double bonds are included in this definition. Trans fatty acids with conjugated bonds are not included because they do not meet the Agency's definition. Thus, trans fatty acids, regardless of origin, that meet the above definition are to be included in the label declaration of trans fat. Further, using FDA's regulatory chemical definition, the categories "trans fatty acids" and "conjugated fatty acids" are mutually exclusive. The definition of trans fatty acids, excluding fatty acids with conjugated double bonds, is consistent with the way that cis isomers of polyunsaturated fatty acids are defined. (68 FR 41434 at 41461, July 11, 2003.)
Answer: Yes. The listing of trans fatty acids is mandatory even when mono- and polyunsaturated fatty acids are not listed. 21 CFR 101.9(c), (c)(2)(ii), (c)(2)(iii), and (c)(2)(iv).
Answer: Trans fatty acids should be listed as "Trans fat" or "Trans" on a separate line under the listing of saturated fat in the Nutrition Facts label (see figure). The word "trans" may be italicized to indicate its Latin origin. Trans fat content must be expressed as grams per serving to the nearest 0.5-gram increment below 5 grams and to the nearest gram above 5 grams. If a serving contains less than 0.5 gram, the content, when declared, must be expressed as "0 g." (21 CFR 101.9(c)(2)(ii)).
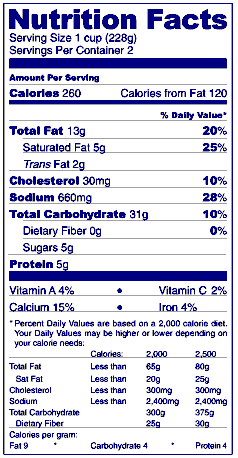
Answer: For conventional food products (those food products other than dietary supplements), declaration of "0 g" of trans fat is not required for such products that contain less than 0.5 g of total fat in a serving and no claims are made about fat, fatty acid or cholesterol content. If trans fat is not listed, the statement "Not a significant source of trans fat" may be placed at the bottom of the table of nutrient values in lieu of declaring "0 g" of trans fat. If these claims are present, then the statement "Not a significant source of trans fat" is not an option and the declaration of "0 g" of trans fat is required. 21 CFR 101.9(c)(2)(ii)
The labeling of dietary supplements is different than the labeling of conventional foods. Certain nutrients in conventional foods, when not present or when present at levels that the agency has determined to be "zero" (see 21 CFR 101.9(c)), must be listed as zero on conventional food labels. However, when those same nutrients are not present in dietary supplements or present in dietary supplements at levels that the agency has determined, for conventional foods, to be "zero," such nutrients must not be listed on dietary supplement labels. Amounts of "0 g" and "Not a significant source..." statements are not allowed in the nutrition labeling of dietary supplements (i.e., Supplement Facts). Consequently, when the amount of trans fat in a dietary supplement is less than 0.5 gram per serving, trans fat must not be listed on the Supplement Facts label. 21 CFR 101.36(b)(2)(i)
Answer: Although the updated Nutrition Facts label will now list the amount of trans fat in a product, be aware that it does not have a % DV for trans fat. While scientific reports have confirmed the relationship between trans fat and an increased risk of CHD, none has recommended an amount of trans fat that FDA could use to establish a DV. Without a DV, a % DV cannot be calculated. As a result, trans fat will be listed with only a gram amount.
Answer: Yes. Food manufacturers are allowed to list amounts of trans fat with less than 0.5 gram (½ g) as 0 (zero) on the Nutrition Facts label. As a result, consumers may see a few products that list 0 gram trans fat on the label, while the ingredient list will have "shortening" or "partially hydrogenated vegetable oil" on it. This means the food contains very small amounts (less than 0.5 g) of trans fat per serving.
Answer: Nutrient content claims are statements that are made on the food label package that indicate that the product contains a range from low to high of the amount of a specific nutrient. Examples: "Low Fat" and "High in Fiber." At this time, FDA has insufficient scientific information to establish NCCs for trans fat. Such claims are permitted, however, for saturated fat and cholesterol.
Answer: The Food and Drug Administration (FDA) issued an advance notice of proposed rulemaking (ANPRM) in the Federal Register Register (Food Labeling: Trans Fatty acids in Nutrition Labeling; Consumer Research to Consider Nutrient Content and Health Claims and Possible Footnote or Disclosure Statements; 68 FR 41507; July 11, 2003) to solicit information and data that potentially could be used to establish new NCCs about trans fat, to establish qualifying criteria for trans fat in current NCCs for saturated fat and cholesterol, lean and extra lean claims, and health claims that contain a message about cholesterol raising fats, and, in addition, as disclosure and disqualifying criteria to help consumers make heart-healthy food choices. The agency also requested comments on whether to consider statements about trans fat, either alone or in combination with saturated fat and cholesterol, as a footnote in the Nutrition Facts label or as a disclosure statement in conjunction with claims to enhance consumers' understanding about such cholesterol-raising lipids and how to use the information to make healthy food choices. Information and data obtained from comments and from consumer studies conducted by FDA may be used to help draft a proposed rule that would establish criteria for certain nutrient content or health claims or require the use of a footnote, or other labeling approach, about one or more cholesterol-raising lipids in the Nutrition Facts label to assist consumers in maintaining healthy dietary practices.
Answer: FDA is unlikely to take regulatory action for minor errors. However, such errors should be corrected during the next printing of labels.
Answer: Always.
Answer: Under 21 CFR 101.9(g)(9), FDA may permit alternative means of compliance or additional exemptions to deal with special situations. Firms in need of special allowances should make their request in writing to the Office of Nutritional Products, Labeling, and Dietary Supplements, HFS-800, 5100 Paint Branch Pkwy., College Park, MD 20740. The letter should: (1) specify that you are requesting an exemption or special provision under 21 CFR 101.9(g)(9), (2) identify the particular product(s) that are the subject of the request, (3) state the reason(s) why it is technologically infeasible or impracticable to adhere to the regulations for such products, and (4) identify the proposed alternative procedure. If possible, include an example of the proposed label(s).
Answer: The same labeling laws apply to all categories of retail sale, including mail orders. Foods sold by mail order must be fully labeled.
Answer: Correcting label mistakes in any manner is acceptable if the final label is correct and complies with all regulations at the time of retail sale. The stickers should not cover other mandatory labeling, and should adhere tightly.
Answer: No, it is the responsibility of the manufacturer or importer of a food to comply with current food labeling regulations.
Answer: Manufacturers must use the information provided in the regulation to determine a specific serving size for their products. The process consists of three steps:
The first important step in establishing an appropriate serving size is to determine if your product is in a single serving container. Products packaged and sold in small units are required to be labeled as single-serving containers; the specifications for these products are described in 21 CFR 101.9(b)(6). If your product is a single serving, it must be labeled in accordance with the labeling requirements for single-serving containers in 21 CFR 101.9(b)(6).
The serving size is expressed as a common household measure followed by the equivalent metric quantity in parenthesis (e.g., "½ cup (112 g)"). Acceptable household measures are listed in order of appropriate use in 21 CFR 101.9(b)(5). Rounding rules for metric quantities and a few additional format options are included in 21 CFR 101.9(b)(7).
Answer: The nutrition information on the label is based on the household unit closest to the RACC. In this case it would be based on 54 grams, which would be declared as the weight of the label serving size. The RACC is used as the starting point to determine the serving size for the foods in each product category and to govern claims.
Answer: The RACC for a partially cooked pasta product is the amount of partially cooked pasta that makes one RACC of cooked pasta (140 grams). 21 CFR 101.12(c)
Answer: Pickled vegetables are categorized with "pickles, all types" with a RACC of 30 grams.
Answer: The agency realizes that the categories in Table 2 "Reference Amounts Customarily Consumed" may not include all foods marketed in the U.S. Therefore, in order to allow manufacturers to provide nutrition information on currently marketed product labels, the manufacturer should write the agency and send in information regarding the primary usage, amount customarily consumed, and any other information as requested for a petition as discussed in section 21 CFR 101.12(h). FDA will provide a "suggested RACC" for the product that may be used to meet the manufacturer's immediate needs to nutrition label its products. While the agency will provide a "suggested RACC" so as to allow the manufacturer to nutrition label its products at this time, FDA believes that it will be necessary at a later date to undertake notice and comment rulemaking to formally establish a RACC. Alternatively, the manufacturer or any other interested party may petition FDA at any time to establish a RACC as specified in 21 CFR 101.12(h).
Answer: The "suggested RACCs" to date are shown below. The labeled serving size for these products would be expressed in a household unit followed by the metric equivalent in parentheses.
| Powdered, flavored candy | 15 g |
| Colored, flavored syrup-filled wax candy | 15 mL |
| Ice | 4 ounces |
| Dried tomatoes (halved, sliced, minced, bits) | 5 g |
| Dried tomatoes in oil (halved, sliced, minced, bits) | 10 g |
| Eggroll, dumpling, wonton or potsticker wrappers | 60 g |
| Egg whites (fresh, frozen, dried) | ~ 1 large egg |
| Sugared eggs, sugared egg yolks | ~ 1 large egg |
| Flavoring oils | 1 t |
| Fruit chutney | 1 T |
| Dried yeast | 0.5 g |
| Baking cocoa, carob powder | 2 T |
| Coconut milk | 1/3 cup |
| Dried, e.g., sun-dried tomatoes, dried mushrooms, dried seaweed | 10 g |
| Dried seaweed sheets | 3 g |
| Vegetable spreads (eggplant caponata, olive spread) | 2 T |
| Sprouts | 10 g |
Answer: The serving size declaration is made up of two parts: a "household measure term" followed by its metric equivalent in grams (g). For beverages, the household measures may be declared as either fluid ounces, cups, or fractions of a cup with the metric equivalent in milliliters (mL). The examples below show permitted declarations.
| Food | Examples |
|---|---|
| Cookies | "1 cookie (28 g)" or "1 cookie (28 g/1 oz)" |
| Milk, juices, soft drinks | "8 fl oz (240 mL)," or "1 cup (240 mL)" for multiserving containers, or the container (e.g., "1 can") for single serving containers |
| Grated cheese | "1 tablespoon (5 g)" or "1 tablespoon (5 g/0.2 oz) |
21 CFR 101.9(b)(2), 21 CFR 101.9(b)(5), 21 CFR 101.9(b)(7), and 21 CFR 101.12(b)
Answer: Yes, the RACC is used to derive a serving size for a particular product. The following example shows how to use the RACC to determine the serving size for a 16 oz (454g) pizza:
1/3 X 454g = 151g
¼ X 454g = 113g
Note that 151g is closer than 113g to the RACC for pizza (140g)
Example: "Serving Size 1/3 pie (151g)"
Therefore, the serving size is " 1/3 pizza (151g)" for this example, whereas the RACC is 140g for all pizzas. Note: Sections 101.9(b)(2)(i) (discrete units), 21 CFR 101.9.(b)(2)(ii) (large discrete units), and 21 CFR 101.9(b)(2)(iii) (bulk products) describe how to use the RACC to derive a serving size. 21 CFR 101.12(b)
Answer: FDA added a label statement column to the RACC tables to provide manufacturers with examples of how serving sizes could appear on product labels. Exact values were initially provided as part of these statements, but have since been removed because some manufacturers incorrectly believed that the exact label statements were required even if the values were inaccurate for their specific products. Manufacturers should realize that the label statement column is not all inclusive and merely provides a few examples of possible label statements. Manufacturers should use an appropriate household measure and the corresponding metric weight or volume actually measured for their specific product.
Answer: For serving sizes halfway between two numbers of units, the serving size should be rounded up to the higher value (21 CFR 101.9(b)(5)(ix)). For example, the RACC for cookies is 30 g. If the product is a bag of 12 g cookies, then 2 units weigh 24 g, and 3 units weigh 36 g. Thus, 2.5 candies would weigh exactly 30 g, and the serving size would be rounded to the next incremental value: "3 cookies (36 g)."
Answer: The following example shows how to calculate the serving size for a biscuit mix product and similar products that require further preparation:
Use the form "Serving Size __ cup (__ g)," the blanks filled in with correct values for the product. 21 CFR 101.12(b)&(c)
Answer: It is not necessary to adjust the size of your cookies to fit the RACC. For example, if four cookies weigh 28 grams (and five cookies weigh 35 grams), declare the number of cookies nearest the RACC and label with the exact weight of that number of cookies for the serving size: "Serving size 4 cookies (28g)" or "4 cookies (28g/1 oz)." 21 CFR 101.12(b)
Answer: For cups, these fractions of a cup are allowed household measures: ¼ cup, 1/3 cup, ½ cup, 2/3 cup, ¾ cup, 1 cup, 1 ¼ cup, etc. If serving sizes are declared in fluid ounces, declare the serving size in whole numbers (such as 4 fl oz, 5 fl oz, 6 fl oz, etc). For tablespoons, the following fractions of a tablespoon are allowed: 1, 1 1/3, 1 ½, 1 2/3, 2, and 3 tablespoons. For teaspoons, the fractions of a teaspoon shall be expressed as 1/8, ¼, ½, ¾, 1, or 2 teaspoons. 21 CFR 101.9(b)(5)(i)
Answer: These fractions must be used in serving sizes for foods such as cakes or pies: "½", " 1/3", "¼", "1/5", "1/6", "1/8", "1/9", "1/10", "1/12" and smaller fractions that can be arrived at by further division by 2 or 3. 21 CFR 101.9(b)(2)(ii)
Answer: The slices are treated as "discrete units." One slice is a single serving if it weighs from 67% to less than 200% of the RACC. Larger slices (weighing more than 200% of RACC) may be declared as a serving if the whole slice can reasonably be eaten at a single-eating occasion. For slices weighing between 50%-67% of the RACC, the serving size may be declared as either one or two slices. For slices weighing less than 50% of the RACC, the serving size is the number of slices closest to the RACC. 21 CFR 101.9(b)(2)(i) 21 CFR 101.12
Answer: For packages containing from two to five servings, round the number of servings to the nearest ½ serving. Examples: "2 servings," "2½ servings," "3 servings," "3½ servings," "4 servings," "4½ servings," and "5 servings." For packages containing five or more servings, round the number of servings to the nearest whole serving. Examples: "5 servings," "6 servings," "7 servings." Rounding should be indicated by the term "about" (e.g., "about 6 servings"). 21 CFR 101.9(b)(8)
Answer: Although the RACC for mixed dish products is one cup, this amount is for the prepared product. The serving size, however, must represent the product as packaged. This will be the amount of the product, expressed in a household measure, that will make one cup when prepared according to package directions. For example, the serving size for a dry seasoned rice mix will be less than one cup since rice expands during cooking. The gram weight in the parenthetical expression will be the weight of the household measure of dry mix.
Answer: The serving size and servings per container for unpopped popcorn is based on the amount of the product as packaged or purchased needed to make the RACC of the prepared product. A second column of nutrition information based on the as prepared basis may also be presented.
Answer: Generally, serving sizes cannot be declared on the basis of fractions of a package. The exception is for unprepared products where the entire contents of the package mix is used to prepare one large discrete unit that is usually divided for consumption (e.g., cake mix, pizza kit) (21 CFR 101.9(b)(5)(v)). For example, a mix for a sheet cake may declare: "1/12 package (40 g/about 1/3 cup mix)." This option is not allowed for other dry mixes or other products. However, a fraction of the package may be used as part of the visual unit of measure when ounces is used as the primary household measure (21 CFR 101.9(b)(5)(iii)). For example, the serving size listed on a 1 lb (16 oz) box of spaghetti could be: "2 oz (56 g/ 1/8 box)."
Answer: Single serving containers and individually packaged products within multi-serving containers must use a description of the individual container or package (21 CFR 101.9(b)(5)(iv)): "1 can (360 mL)" or "2 boxes (38 g)," and products in discrete units must use a description of the individual unit (21 CFR 101.9(b)(5)(iv)): "2 candies (22 g)" or "1 slice (45 g)."
Answer: Products consisting of two or more distinct ingredients or components packaged and presented to be consumed together (e.g., dry macaroni and cheese mix, cake and muffin mixes with separate ingredient packages, pancakes and syrup) may declare serving size and nutrition information either: (a) for each component or (b) as a composite. For products where one of the components is represented as the main ingredient, there are provisions for representing the amount of the main ingredient and proportioned minor ingredients (21 CFR 101.9(b)(5)(i)-(iii)): "2 pancakes with syrup (160 g)" or alternatively "2 pancakes (110 g)" and either "syrup for 2 pancakes (50 g)" or "__ tbsp syrup (50 g)" if 50 g of syrup makes __ tbsp. In addition, these products may also use ounces (21 CFR 101.9(b)(5)(vii)): "4 oz (112 g/about 2/3 cup macaroni and 2 tbsp dry cheese mix)" or alternatively "3 oz dry macaroni (84 g/about 2/3 cup)" and "1 oz dry cheese mix (28 g/about 2 tbsp)."
Answer: The RACC for nuts is 30 grams edible portion. The serving size for peanuts with shells would be the household measure closest to 30 grams of nuts without shells. In order to reduce consumer confusion regarding the serving size, a clarifying statement can be used. For example, the serving size statement for your product might read: "½ cup nuts without shells (30 g/ about 1 cup nuts with shells )."
Answer: The serving size for pickled vegetables is based on the drained weight of the product because the liquid is not usually consumed with these type products. For canned vegetables, the liquid is included in the determination of serving size.
Answer: Yes. Section 21 CFR 101.9(b)(10) permits the voluntary listing of nutrition information per 100 grams or 100 mL of the food as packaged or purchased. A column may also be presented with nutrition information "per 1 oz" or "per 1 fl oz" as packaged or prepared.
Answer: The regulations allow a second column of nutrition information to be declared for a food providing that it is not misleading to consumers. The serving size and first column of nutrition information for these products would be based on their use as a mixed dish, but the second column could be based on their use as an appetizer.
Answer: Yes, manufacturers may use a second column to declare information based on a different serving size. The first column under the Nutrition Facts label would show the serving size, servings per container, and nutrition information based on a 30 gram RACC for the pickled vegetable and the second column could show nutrition information based on the RACC for the product use as a vegetable side dish.
 What are the exemptions for single-serving containers?
What are the exemptions for single-serving containers?
Answer: Single serving containers may omit the "servings per container" declaration. In addition, most single serving containers may omit the metric equivalent portion of the serving size declaration. However, if it is voluntarily included, it must be consistent with the net quantity of contents value. The serving size for single-serving containers must be a description of the container such as: "Serving Size: 1 package" for food in bags, "Serving Size: 1 container" for foods in plastic containers, or "Serving Size: 1 can" as appropriate. Only those few foods that are required to declare drained weights must include the metric equivalent as part of the serving size declaration (e.g., "Serving size: 1 can drained (__g)"). 21 CFR 101.9(b)(5)(iv), 21 CFR 101.9(b)(7) and 21 CFR 101.9(d)(3)(ii)
Answer: Single-serving containers are discussed in 21 CFR 101.9(b)(6). Products that are packaged and sold individually are considered to be single servings if they contain less than 200% of the RACC for the product category. Above 200% of the RACC, it is the manufacturer's option to label the product as a multi-serving container or as a single-serving container if it can reasonably be consumed at a single eating occasion. For example, the RACC for brownies is 40 g. All brownies that are packaged and sold individually and that weigh less than 80 g must be labeled as a single serving. If the manufacturer believes it is reasonable for an individually packaged brownie that weighs more than 80 g to be consumed at one time, such a brownie may also be labeled as one serving.
Answer: If a product has a RACC of 100 g or 100 mL or larger and is packaged and sold individually, it must be labeled as a single-serving if it contains 150% or less of the RACC. However, packages for such products containing between 150% and 200% of the RACC may be labeled as one or two servings at the manufacturer's option.
For example, the RACC for potato salad is 140 g. Containers of potato salad that are packaged and sold individually and that weigh 210 g or less must be labeled as a single serving. Containers weighing between 210 g and 280 g may be labeled as 1 or 2 servings. However, the serving size for a product labeled as two servings is based on the household measure and not on the weight of ½ package.
Answer: The serving size statement for multi-serving containers must use the hierarchy of common household measures (21 CFR 101.9(b)(5)(i)-(iii)), whereas single-serving containers are required to use a description of the individual container or package (21 CFR 101.9(b)(5)(iv)). Multi-serving packages must list the metric equivalent to the household measure and the number of servings in the container; however this is optional information on single-serving containers. If the metric equivalent is listed on single-serving containers, it must match the net contents declaration for the product. An example of a single-serving container would be a 360 mL can of soda that is packaged and sold individually. The serving size for this product would be "1 can" or "1 can (360 mL)," and the number of servings would be "1" or not listed at all. By contrast, the serving size for a one liter soda bottle (1000 mL) would be "8 fl oz (240 mL) or "1 cup (240 mL)," and the number of servings would be listed as "about 4."
Answer: Yes. The serving size for beverages in single-serving containers is the total contents of the container. Thus, the serving size would be listed as "1 bottle," but the contents could vary greatly (e.g., 8 fl oz, 12 fl oz, 16 fl oz, etc.). Since the RACC for beverages is 240 mL, the serving size for multi-serving beverage containers such as the commonly available one-liter bottle would be either "1 cup (240 mL)" or "8 fl oz (240 mL)."
Answer: A 130 gram muffin weighs 236% of the RACC for muffins. Products that weigh more than 200% of the RACC may be labeled as one serving if the entire contents of the package can reasonably be consumed at a single eating occasion. Therefore, there are two options for the serving size declaration for this large muffin: "1 muffin (130 g)" or "½ muffin (65 g)."
Answer: Products that are packaged and sold individually are considered to be single servings if they contain less than 200% of the RACC shown in 21 CFR 101.12. For packages that contain 200% or more of the RACC, it is the manufacturer's option to label the product as a single serving if the entire contents can reasonably be eaten at one time. 21 CFR 101.9(b)(6)
Answer: The answer depends on the size of the RACC. For foods with RACC less than 100g (solid foods) or 100mL (liquids), packages must contain at least 200% of the RACC to be labeled as 2 servings. For foods with RACCs of 100g or 100mL or more, you may choose to label packages containing more than 150% but less than 200% of the RACC as either one or two servings. 21 CFR 101.9(b)(6) and 21 CFR 101.12(b)
Answer: No. Rounding to the nearest 0.5 servings is allowed between 2 and 5 servings. Below 2 servings, the number of servings must be listed as "1" or "about 2." For example, the RACC for egg rolls is 140 g. Since the RACC is greater than 100 g, a package of egg rolls containing more than 150% but less than 200% of the RACC can be labeled as 1 or 2 servings. For example, a package of egg rolls weighs 225 g and contains 3 egg rolls (75 g each). The manufacturer may choose to label the product as 1 serving (3 egg rolls (225 g)). Alternatively, if the manufacturer chooses to label the product as more than 1 serving, the serving size would be "2 egg rolls (150 g)." The number of servings, determined as the total contents divided by the serving size, would be 1.5 and would be rounded to "about 2."
Answer: Serving sizes for products in discrete units (e.g., muffins, sliced bread, and individually-packaged products in multi-serving packages) are discussed in 21 CFR 101.9(b)(2)(i). The serving size options depend on the RACC for the product and the weight of a single discrete unit.
FDA also provided additional specific provisions for (1) products (such as pickles) that naturally vary in size; (2) products made up of two or more foods, packaged and intended to be consumed together; and (3) products containing several, fully labeled, single serving units.
Answer: For products with RACCs of 100 mL or larger, the serving size for discrete units that contain 67% or more but less than or equal to 150% of the RACC is 1 unit. For beverages, this range is 160.8 mL to 360 mL. Thus, "1 bottle" would be the serving size for beverages packaged in 6 fl oz (180 mL) bottles.
Answer: The pre-portioned slices are treated like all other discrete units. The 55 g piece of cake is less than 50% of the RACC for heavy weight cakes (50% of 125 g = 62.5 g); therefore the serving size will be the number of units closest to the RACC. Two pieces weigh 110 g, and 3 pieces weigh 165 g; therefore, the serving size would be "2 pieces (110 g)".
Answer: Serving sizes for products in large discrete units usually divided for consumption (e.g., cake, pie, pizza, melon, cabbage) are discussed in 21 CFR 101.9(b)(2)(ii). The serving size depends on the RACC for the product and on the fraction of the large discrete unit. The serving size is expressed using the allowed fraction ("friendly fraction") that is closest to the RACC.
For example, the RACC for pizza is 140 g. A 16 oz (454 g) pizza can be divided in half (one piece = 227 g), thirds (one piece = 151 g), fourths (one piece = 113 g), etc. The closest fraction is 1/3; therefore the serving size would be " 1/3 pizza (151 g)."
Allowable fractions include 1/2, 1/3, 1/4, 1/5, 1/6, or smaller fractions that can be generated by further division by 2 or 3. An additional example would be: 1/8 (i.e., 1/4 divided by 2). Thus, fractions such as 1/7, 1/11, 1/13, and 1/14 are not allowed.
Answer: Serving sizes for non-discrete bulk products (e.g., breakfast cereal, flour, sugar, dry mixes, concentrates, pancake mixes, macaroni and cheese kits) are discussed in 21 CFR 101.9(b)(2)(iii). The serving size depends on the RACC for the product and on the household measure. The serving size is expressed using the allowed household measure that is closest to the RACC.
For example, the RACC for snacks is 30 g. If a bag contains a mixture of nuts and caramel popcorn that weighs 23 g per cup, then 1¼ cup weighs 28.75 g and 1 1/3 cup weighs 30.7 g. The closest household measure is 1 1/3 cup; therefore the serving size would be "1 1/3 cup (31 g)."
Allowable household measures include (a) cups as ¼, 1/3, ½, 2/3, ¾, 1, 1¼, 1 1/3, etc, (b) tablespoons as 1, 1 1/3, 1½, 1 2/3, 2, and 3, and (c) teaspoons as 1/8, ¼, ½, ¾, 1, and 2. In addition, piece, slice, tray, jar, fraction, and ounce may be used in accordance with the provisions of 21 CFR 101.9(b)(5).
Answer: In these cases, manufacturers may use an ounce declaration (21 CFR 101.9(b)(5)(vii)). For example, the RACC for prepared macaroni and cheese is 1 cup. If a 12 oz package (9 oz dry macaroni and 3 oz dry cheese mix) makes 3 cups of prepared macaroni and cheese, then the serving size for the composite product could be expressed as "4 oz (112 g/about 2/3 cup macaroni and 2 tbsp dry cheese mix)." Alternatively, the manufacturer may provide nutrition information separately for each component. Thus, the serving size could also be expressed as "3 oz dry macaroni (84 g/about 2/3 cup)" and "1 oz dry cheese mix (28 g/about 2 tbsp)."
Answer: For products that require further preparation, where the entire contents of the package are used to prepare a large discrete unit usually divided for consumption, the serving size is the amount of the unprepared product used to make one "RACC for the unprepared product." The "RACC for the unprepared product" is the amount of the unprepared product that is required to make the fraction of the prepared product closest to the RACC of the prepared product. For example, a prepared medium-weight cake has a RACC of 80 grams. If 480 grams of cake mix makes 900 grams of prepared cake, then 1/12 of the prepared cake (75 g) is the closest fraction to the 80 gram RACC for medium weight cakes. Therefore, the RACC for the unprepared cake is 1/12 of 480 g, or 40 g. The serving size could be listed as "1/12 package (40 g/about 1/3 cup mix)."
Answer: Common household measures are discussed in 21 CFR 101.9(b)(5). Manufacturers should first try to express serving sizes for their products using cups, tablespoons, or teaspoons (21 CFR 101.9(b)(5)(i)). Second, if cups, tablespoons, and teaspoons are not appropriate, then whole units and fractions of large whole units should be used, such as pieces, slices, tray, or jar (21 CFR 101.9(b)(5)(ii)). Finally, if other options fail (usually because the product size naturally varies to a considerable degree), manufacturers should use ounces with an appropriate visual unit of measure (21 CFR 101.9(b)(5)(iii)).
For example, small pastas, such as macaroni, can be measured by cup: "__ cup (__ g)." Larger discrete pastas, such as lasagna, can be measured by the piece: "__ lasagna noodles (__ g)". A few pastas, such as spaghetti, may need to use ounces: "__ oz (__ g/visual unit of measure)." Visual units of measure could include descriptive phrases such as "1/8 box " or "about 1 ¼-inch circle of spaghetti."
Answer: Yes, if a manufacturer, packer, distributor or retailer chooses to nutrition label a product that is exempt under section 21 CFR 101.9(j), all applicable labeling regulations must be followed.
Answer: No, only the package that bears the claim is required to provide nutrition labeling.
Answer: Generally speaking, a food that involves no interstate commerce (i.e., it is not manufactured from ingredients that have moved in interstate commerce or itself is not distributed in interstate commerce) would not be subject to FDA regulation. However, FDA notes that interstate commerce is interpreted very broadly and, additionally, many states model their requirements after FDA's.
Answer: All imported products are required to have nutrition labeling unless the manufacturer/packer/distributor qualifies for an exemption.
Answer: The exemptions in 21 CFR 101.9(j) apply only to nutrition labeling requirements when the food bears no claim or other nutrition information.
Answer: No. The firm is exempt provided that no claims are made. A firm whose total gross sales for all products, food and non-food, is $501,000, with only $49,000 of this figure representing sales of food, is also exempt. Under the NLEA, firms who have an annual gross sales made or business done in sales to consumers that is not more than $500,000 or have annual gross sales made or business done in sales of food to consumers of not more than $50,000 are exempt 21 CFR 101.9(j)(1)(i). The following chart illustrates the exemption:
| SALES IN FOOD | TOTAL SALES (FOOD & NON-FOOD) | STATUS |
|---|---|---|
| $50,000 or less | $500,000 or less | EXEMPT |
| $50,000 or less | $500,001 or more | EXEMPT |
| $50,001 or more | $500,000 or less | EXEMPT |
| $50,001 or more | $500,001 or more | NOT EXEMPT |
Answer: Products manufactured for a company that is not exempt must bear nutrition labeling. The company whose name appears on the label is responsible for providing nutrition information. Company "X" is not required by law to provide the nutrition information to the private labeler. However, company "X" may wish to develop nutrition information for their product line and provide it to their customers for use on the label.
Answer: It is up to each company to maintain records, such as tax returns, to support such an exemption. FDA will not maintain such records.
Answer: The agency defines "brokered sales" as the sale of foods shipped in bulk form that are not for distribution to consumers but are for use solely in the manufacture of other foods or that are to be processed, labeled, or repackaged at a site other than where originally processed or packed. Accordingly, any brokered sale would not need to be considered in determining eligibility for the small business exemption.
Answer: Yes. As long as the retailer is simply repacking the food into smaller containers and placing the small business's name and address on the packaged food (i.e., the package label bears no name or logo that would tie the product to the larger retailer), the food would retain any exemption it was eligible for under 21 CFR 101.9(j)(1) or (18).
Answer: If the retailer is eligible for the exemption in 21 CFR 101.9(j)(1) (based on gross sales), product purchased from a large manufacturer but repacked by the retailer would be exempt from nutrition labeling, as long as the package label bears no name or logo that would tie the product to the manufacturer. However, to be eligible for the exemption in 21 CFR 101.9(j)(18), the product must meet the definition of low volume products (based on the total number of units of the product sold by the large manufacturer in the United States).
Answer: The exemption for low volume food products is based on the average number of full time equivalent employees (FTE's) and the number of units of product sold in the United States.
Answer: No. Firms eligible for the exemption based on gross sales and firms with less than 10 FTE's and less than 10,000 units do not have to file with the FDA. However, such firms can choose to do so voluntarily in order to establish a record that they are claiming an exemption. Also, all importers must file.
Answer: There is a separate exemption from nutrition labeling for foods sold in restaurants of any size, provided the food does not bear a claim (21 CFR 101.9(j)(2)). These foods do not need the small business exemptions. However, to the extent that a restaurant distributes food products for sale outside the restaurant (e.g., through grocery stores), such products may be eligible for an exemption from nutrition labeling under the small business exemptions.
Answer: Foods which are served or sold for use only in restaurants and other establishments in which food is served for immediate consumption are exempt from nutrition labeling. However, if there is a reasonable possibility that the product will be purchased directly by consumers (e.g., club stores), nutrition information is required. 21 CFR 101.9(j)(2)(iii) and 21 CFR 101.9(j)(2)(iv)(B)
Answer: Individual serving size packages that are served to consumers and make a claim are required to have nutrition labeling (e.g., light salad dressing).
Answer: It is up to the manufacturer to determine its own exemption status, and such a statement can not be used to avoid compliance with the regulations.
Answer: Food sold in a restaurant or other retail establishment (e.g., a bakery or delicatessen) that is sold from behind a counter and placed in a wrapper, carry-out box, or other non-durable container whose sole purpose is to facilitate handling would not be considered "packaged food" and would not need to bear a net weight statement, ingredient declaration, or the other labeling required of packaged foods. However, if consumers make their selections based on the food in its packaged form (e.g., the food is wrapped or boxed by the retailer and sold from a self-service case in a corner of a restaurant, or across the aisle from an in-store deli), the food must bear all required information.
Answer: This exemption is based on 3 primary criteria: 1) when the food is consumed, 2) the location in which the food is processed and prepared, and 3) the extent to which the food is processed and prepared (i.e., must be ready-to-eat and of the type served in restaurants).
Bakeries and delis that sell foods for immediate consumption (e.g., where the deli or bakery has facilities for customers to sit and consume the food on the premises) are considered analogous to restaurants and all foods sold in such establishments are exempt under 21 CFR 101.9(j)(2).
When foods are not for immediate consumption, they may be exempt if they meet all of the criteria listed in 21 CFR 101.9(j)(3). That is, when the food is ready-to-eat and is processed and prepared primarily on the premises of the establishment from which it is sold, it is exempt - regardless of how it is sold (i.e., from behind a counter or in pre-portioned packages from a self-service shelf). However, if the food is not primarily processed and prepared on-site, nutrition labeling is required.
To meet the criteria for being "primarily processed and prepared on-site", the food must be augmented on site in a manner that changes the nutrient profile of the food (i.e., filling, icing, enrobing). Washing and garnishing with nuts, onions or seeds would fall under the definition of "primarily processed and prepared" if the added foods change the nutrition profile of the finished product. Custom cakes are exempt.
If pre-formed dough, pre scaled/molded and par baked dough are merely proofed and baked or simply thawed, the product is considered to be "standardized" and nutrition labeling is required.
Foods which are not prepared on premises and that are portioned to consumer specifications on-site are not required to have nutrition labeling (e.g., 1 lb of potato salad; 2 lb cheese, 1 lb assorted cookies, 5 rolls). However, if these items are packaged and offered for sale in another section of the store (e.g., refrigerator case; self service bins), nutrition labeling is mandatory. 21 CFR 101.9(j)(3)(iv)
Answer: Candy sold at the manufacturing site is not required to have nutrition labeling. Also, individual candies offered from behind a counter for consumer selection (i.e., packaged to consumer specification) are not required to have nutrition labeling. However, consumer packages of candy offered for sale at the satellite stores must have nutrition labeling. The same applies to bakeries that sell product at satellite stores.
Answer: The regulations provide for an exemption for foods that contain insignificant amounts, as defined in 21 CFR 101.9(j)(4), of all of the nutrients and food components required to be included in the nutrition label. Exempted foods include coffee beans (whole or ground), tea leaves, plain instant unsweetened instant coffee and tea, condiment-type dehydrated vegetables, flavor extracts, and food colors. Some spices contain levels of nutrients that would not meet the criteria of "insignificant" and would require nutrition labeling.
Answer: A product would be exempt from nutrition labeling if it contains insignificant amounts of all the nutrients required to be on the label, so long as no nutrient content or health claims are made for the product.
Answer: Under FDA labeling regulations the term Mineral Water is a statement of identity and does not trigger mandatory nutrition labeling if there is no nutrient content claims about a particular mineral and if all required nutrients are present at insignificant levels.
Answer: If a nutrient for which there is an RDI or DRV is referenced on the label, nutrition information is required. However, if state regulations require declaration of nutrients which are not provided for on the nutrition label (e.g., fluoride, arsenic), nutrition labeling cannot accommodate such nutrients and nutrition labeling is therefore not required.
Answer: Nutrition Facts labels for foods specifically for children less than 4 years do not provide % Daily Values for the macronutrients or footnotes as required in 21 CFR 101.9(d)(9). Also, foods specifically for children less than 2 years of age must not present information on calories from fat and calories from saturated fat and quantitative amounts for saturated fat, polyunsaturated fat, monounsaturated fat and cholesterol. In both cases, % Daily Value is declared only for protein, vitamins, and minerals.
| Fruit dessert for children less than 2 years old |
Fruit dessert for children ages 2 years to 4 years |
|---|---|
 |
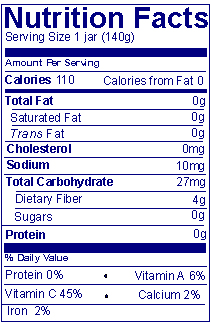 |
| 21 CFR 101.9(j)(5)(i) | 21 CFR 101.9(j)(5)(ii) |
Answer: Food packages with a surface area of 40 sq. in. or less available for labeling may place the Nutrition Facts label on any label panel (not limited to the information panel), may omit the footnote required in 21 CFR 101.9(d)(9) if an asterisk is placed at the bottom of the label with the statement "Percent Daily Values are based on a 2,000 calorie diet," and, may also use the tabular display label format.
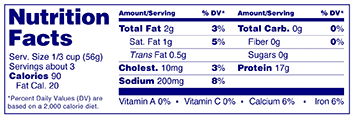
Answer: A linear (string) format may be used on food packages with 40 sq. in. or less total surface area available for labeling if the package shape or size cannot accommodate the nutrition information placed in columns on any label panel.

Answer: Food packages with a surface area of 40 sq. in. or less available for labeling may use the following abbreviations in the Nutrition Facts label:
| Label Term | Abbreviation | Label Term | Abbreviation |
|---|---|---|---|
| Serving size | Serv size | Cholesterol | Cholest |
| Servings per container | Servings | Total carbohydrate | Total carb |
| Calories from fat | Fat cal | Dietary fiber | Fiber |
| Calories from saturated fat | Sat fat cal | Soluble fiber | Sol fiber |
| Saturated fat | Sat fat | Insoluble fiber | Insol fiber |
| Monounsaturated fat | Monounsat fat | Sugar alcohol | Sugar alc |
| Polyunsaturated fat | Polyunsat fat | Other carbohydrate | Other carb |
 What is the exemption for small food packages?
What is the exemption for small food packages?
Answer: Small packages (less than 12 sq. in. total surface area available to bear labeling) may be printed with a telephone number or an address to obtain nutrition information. This exemption (using a telephone number or address in place of the Nutrition Facts label) is permitted only if there are no nutrient content claims or other nutrition information on the product label or in labeling and advertising. 21 CFR 101.9(j)(13)(i)
Answer: Small packages (less than 12 sq. in. total surface area available to bear labeling) may use type sizes no smaller than 6 point or all uppercase type of not less than 1/16 inch for all required nutrition information. 21 CFR 101.9(j)(13)(i)(B)
Answer: If space is limited on the label, there is flexibility to adjust non-required graphic elements to help fit the nutrition label to the available space. The required graphic elements are those that are specified in 21 CFR 101.9(d).
Answer: In determining the total surface area available to bear labeling, flanges and ends (tops and bottoms) of cans, shoulders and necks and caps of bottles and jars, and folded flaps and other unusable area may be excluded; as provided for in 21 CFR 101.1(c) and 21 CFR 101.2(a)(1). However, packages that provide label information on tops, bottoms, or necks should include those areas when calculating available label space. The available label space includes the principal display panel and is not limited to currently labeled areas.
Answer: When normal handling by the consumer would result in the bottom of the box being easily seen, such as frozen food boxes. The bottom of boxes stored end up would not be considered "available to bear labeling" since consumers do not look at these areas during normal handling. Likewise, the bottoms of cans and jars are not normally seen and would not be calculated when determining "space available to bear labeling."
Answer: Section 101.9(j)(13)(i) states clearly that the area available for labeling is based on the total surface area available to bear a label.
Answer: No. When determining what format is required, space occupied by vignettes, design and other non-mandatory label information must be considered as available label space.
Answer: If the package has less than 12 square inches of space available to bear labeling because of the irregular container surface and no claims are made, nutrition labeling requirements may be met by providing an address or phone number where consumers could obtain the information.
Answer: Section 101.9(j)(16) allows foods sold from bulk containers to display the required nutrition information on the outside of the container or on posters, counter cards, tags, or similar measures. The containers these foods are put into when sold to the consumer do not need to bear nutrition labeling as long as the required nutrition information is displayed at point-of-purchase (i.e., plainly in view by the bulk containers).
Answer: Yes.
Answer: The regulations require that nutrition information be displayed to consumers on the labeling of the container plainly in view. Therefore, this method of labeling would be acceptable if the underside of the lid were displayed at all times and another means used to protect the contents of the drum.
Answer: Yes. When foods are received by a retail store in bulk form and repacked for sale to consumers as a packaged food, the package must meet all mandatory labeling requirements.
Answer: The number of servings in a bulk container will vary according to the fill of the container, and such a number is of little or no usefulness to consumers. FDA would be unlikely to object to a statement that the "Servings per container" are "varied" on bulk food containers or on random weight portions of foods repackaged by the retailer.
Answer: The retailer is responsible for displaying the nutrition information in the required format on or adjacent to the bulk container. The information may be obtained/provided by either the supplier or retailer. The decision as to who actually develops the information is up to those parties involved.
Answer: Yes. Subject, of course, to the exemptions for small businesses.
Exemptions/Voluntary Nutrition Labeling of Raw Fruits, Vegetables and Fish (21 CFR 101.9(j)(10) and 21 CFR 101.42, 21 CFR 101.43, 21 CFR 101.44, 101.45)
Answer: On July 25, 2006 (71 FR 42031), corrected August 15, 2006 (71 FR 47439), 2006, FDA published a final rule to update the names and nutrition values of the top 20 raw fruits, vegetables, and fish. The 20 foods for each group are identified in 21 CFR 101.44. The same list is to be used nationwide. The 20 most frequently consumed raw fruits are: Apple, avocado (California), banana, cantaloupe, grapefruit, grapes, honeydew melon, kiwifruit, lemon, lime, nectarine, orange, peach, pear, pineapple, plums, strawberries, sweet cherries, tangerine, and watermelon. The 20 most frequently consumed raw vegetables are: Asparagus, bell pepper, broccoli, carrot, cauliflower, celery, cucumber, green (snap)beans, green cabbage, green onion, iceberg lettuce, leaf lettuce, mushrooms, onion, potato, radishes, summer squash, sweet corn, sweet potato, and tomato. The 20 most frequently consumed raw fish are: Blue crab, catfish, clams, cod, flounder/sole, haddock, halibut, lobster, ocean perch, orange roughy, oysters, pollock, rainbow trout, rockfish, salmon (Atlantic/coho/Chinook/sockeye, chum/pink), scallops, shrimp, swordfish, tilapia, and tuna.
Answer: Yes. The names and descriptions of these foods should clearly identify them as distinct from the foods among the most frequently consumed list for which FDA has provided data (21 CFR 101.45(c)(1)). Nutrition labeling values for foods not on FDA's lists are subject to the compliance provisions of 21 CFR 101.9(g).
Answer: When providing nutrition information on the package, even when nutrition labeling is otherwise voluntary, the information should be presented in a format that is consistent with the format requirements in 21 CFR 101.9(d).
Answer: The NLEA provides for voluntary nutrition labeling of "raw agricultural commodities and raw fish." The FD&C Act defines "raw agricultural commodities" as any food in its raw or natural state, including all fruits that are washed, colored, or otherwise treated in their unpeeled natural form prior to marketing. Therefore, fruit and vegetables that receive little or no processing and no heat treatment, regardless of whether the fruit and vegetables are waxed, are subject to the voluntary program. In addition, for ease of administration the agency has chosen to draw a practical line in terms of retail selling practices and program implementation by including raw fruit and vegetables that are sold in the produce section and that are peeled, trimmed, cut and/or packaged with no added ingredients (e.g., carrot sticks, mixed salad greens) in the voluntary program when no claims are made for the product. When claims are made, nutrition labeling is required on the package unless the required nutrition information is provided on a poster or other means as specified in 21 CFR 101.45.
Accordingly, fresh herbs and nuts (e.g., walnuts, peanuts) that have no added ingredients, such as salt, and that are sold in the produce section would be exempt from nutrition labeling under the voluntary program. However, when shelled or unshelled nuts or produce are processed in a manner other than mixing with other raw produce items, peeling, trimming , or cutting, (e.g., dried fruit, roasted nuts, frozen melon balls), nutrition labeling is required under 21 CFR 101.9.
Answer: When processed foods, such as salad dressings or croutons, are added to packages of raw vegetables or fruits, the product is considered to be a multi-ingredient processed packaged food and is no longer part of the voluntary program. Therefore, nutrition labeling is mandatory for the entire contents of the package. (Subject, of course, to the exemption for ready-to-eat food that is primarily processed or prepared at the retail location and the small business exemptions.)
Answer: Because restaurant salads may be served with the dressing on the side or the croutons in a side package, packages of salads prepared in the retail establishment would be considered ready-to-eat when the only preparation needed by the consumer is adding the dressing or croutons. In contrast, products that require a significant amount of assembly or preparation (e.g., a pizza kit) would generally not be considered ready-to-eat.
Answer: No. Nutrition labeling is still required for both the greens and the salad dressing. However, 21 CFR 101.9(h)(1) allows separately packaged ingredients that are intended to be eaten at the same time to be labeled individually or with a composite value. Therefore, the greens and salad dressing can be labeled individually. If the nutrition label on the packet is visible at the point of purchase, the information on the dressing need not be reprinted on the outer bag.
Answer: Yes. These products are multi-ingredient processed food products. Therefore, nutrition labeling is mandatory.
Answer: Raw single-ingredient fish that are packaged by the retailer, whether fresh or frozen, fall under the voluntary nutrition labeling program. However, for the retail store to be in compliance with the voluntary program, the nutrition labeling information must be available at point of purchase (i.e., be displayed in close proximity to the product) of both the fresh and frozen fish. It may be necessary for some retail stores to display signs/brochures with the nutrient data for fish in the frozen food section as well as the fresh fish section of the store. In contrast, raw frozen fish that are packaged by a manufacturer (e.g., packaged in a box with a printed label and brand name) come under the mandatory nutrition labeling program.
Answer: Pasteurized crab meat that is not shelf-stable and is sold on ice or refrigerated is included under the voluntary nutrition labeling program, whereas canned crab meat that is shelf-stable must bear nutrition labeling.
Answer: Plain, thermally processed shelled or unshelled lobster, crab, and shrimp are included in the voluntary nutrition labeling program when sold in either the fresh fish or deli sections of the store. However, consistent with earlier answers for fruit and vegetable products, when a food is composed of more than one ingredient, some of which are not included in the voluntary program (such as a seasoning mix or cocktail sauce), it must bear nutrition labeling. These added ingredients would generally alter the nutrient content of the product so that the nutrienst values posted for the voluntary program would no longer accurately represent the finished product. However, if the finished product meets the criteria for a ready-to-eat food, primarily processed and prepared at the location from which it is sold (e.g., steamed, spiced shrimp prepared in-house), it may be exempt from nutrition labeling under 21 CFR101.9(j)(3).