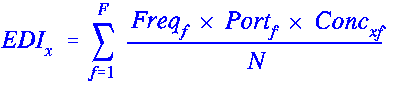
|
U.S. Food & Drug Administration Center for Food Safety & Applied Nutrition Office of Premarket Approval September 1995 (Effective June 18, 2001, Office of Premarket Approval is now Office of Food Additive Safety. See updated contact information) |
The latest version of this guidance issued August 2006. Below is an earlier version.
The US Food and Drug Administration (FDA) regulates substances either intentionally added to food to accomplish a proven technical effect or inadvertently added through contamination, processing, or packaging.(1) The premarket approval process for food additives requires an estimate of the probable consumer exposure to the additive to determine whether its use or presence in a food at a given concentration is safe. The intent of this document is to provide an understanding of the databases and methodologies used by the Office of Premarket Approval (OPA) in FDA's Center for Food Safety and Applied Nutrition to estimate exposure to food additives and other substances (e.g., chemical contaminants) found in the diet. This document is primarily directed at petitioners for food additive regulations. Illustrative examples of the calculations that the technical reviewers of OPA's Chemistry Review Branch (CRB)(2) perform to obtain the legally-required estimate of probable exposure for substances added to food are included. It should also be useful to other parties interested in the means by which exposures to food additives and chemical contaminants can be estimated.
The key determinant in the safety evaluation of a substance found in or added to the diet is the relation of its probable human exposure to the level at which adverse effects are observed in toxicological studies.(3) Simply, "the dose makes the poison." The implications of this adage as it pertains to food can be illustrated with two clear examples. While "pure" water can be viewed as the safest of foods, excessive intake can lead to a potentially fatal electrolyte imbalance. Conversely, pure concentrated sulfuric acid destroys human tissue, but it is affirmed as "Generally Recognized as Safe" (GRAS) by the FDA in the Code of Federal Regulations (CFR)(4) when used to control pH during the processing of alcoholic beverages or cheeses. Clearly, the use and the dose (i.e., exposure) are overriding considerations when discussing the safety of a component of food.
The definition of a food additive in section 201 (s) of the Federal Food, Drug, and Cosmetic Act, as amended (hereinafter referred to as "the Act") refers to substances whose intended use results directly or indirectly in the substance becoming a component of food. The FDA refers to direct food additives as those added to a food to accomplish an intended effect. Indirect additives are those that unintentionally, though predictably, become components of food. Components of plastic packaging materials that can migrate to food are indirect additives.
The differences between direct and indirect additives are such that different methodologies are necessary to prepare estimates of probable human exposure. It is relatively straightforward to determine how much of a direct additive could be present in any given food. Indirect additives, on the other hand, cannot be treated in a parallel manner due to the great variety of packaging materials (glass, paper, coated papers, plastics, laminates, and adjuvants used in their manufacture), and the great variability in the use of packaged foods. Also, the fact that these additives are not intentionally added to food (which is to say that "use levels" in food cannot be defined), makes direct comparisons with methods used for estimating exposures to direct additives inappropriate.(5)
This document deals only with estimating exposure to direct additives and chemical contaminants. The procedures used to estimate exposure to chemical contaminants in food (including naturally occurring toxicants, such as mycotoxins) are essentially the same as those used for direct additives. Thus, contaminants will be considered in the discussion of direct additive exposure estimation. The procedures discussed herein are equally applicable to color additives, GRAS substances, prior-sanctioned ingredients, and pesticide residues.(6)
Direct food additives are regulated in 21 CFR 172 (GRAS ingredients are regulated in 21 CFR 182 and 184). Secondary direct additives, a sub-class of direct additives, are primarily processing aids. These materials, which are used to accomplish a technical effect during the processing of food but are not intended to serve a technical function in the finished food, are regulated in 21 CFR 173. Secondary direct food additives are like indirect additives in that only residues of the additive, or its components, are typically found in the food. The estimate of daily intake (EDI) for a secondary direct additive, however, is generally calculated in the same manner as those for direct additives and will be considered in this document.
Chemical contaminants are substances that are present in food or food additives either unavoidably or unintentionally. Typically, there are two types of chemical contaminants that are encountered: (1) those present in food additives (generally, from manufacture), and; (2) those present in food itself (due to manufacture, natural, or environmental contamination, e.g., lead, aflatoxin, or polychlorinated biphenyls). The practical difference between estimating exposure for contaminants and additives is the derivation of the concentration of the substance. Contaminant concentrations are usually determined experimentally, while additive concentrations or use levels are proposed by a petitioner(7) for specified uses of a food additive. The different procedures used for estimating exposure to additives and contaminants will be discussed in the remainder of this document, with detailed examples in the last section.
There are a number of sources available to OPA for data used in estimating exposure to substances in the diet,(8) including:
Each of these sources has advantages and limitations. For a number of reasons, including cost and availability, breadth of data, and ease of data manipulation, OPA relies primarily on data taken from food consumption surveys. OPA has not used data from body burden/excretion measurements or weights and analyses of foods for estimating exposure. In the following sections, OPA's use of the data from consumption surveys, disappearance data, and market basket surveys will be outlined.
OPA's use of food consumption survey data for estimating exposure to substances in the diet involves the linking of food intakes with independently-determined concentrations of the substances in foods. Because distributions of food intakes are generally available from a food consumption survey, estimates for mean exposure or for any point on the population distribution (e.g., 50th or 90th percentile) are possible.
OPA regularly uses national food consumption surveys to estimate dietary exposure to substances. These surveys measure food intake by one or more methods: 24-hour intake recall, food intake record (also called food diary), and food frequency record or recall. For the first two methods, participants recall or record the amounts and types of each food eaten during the day. For the food frequency methods, participants record or recall only the number of occasions each food was consumed over a time period that may vary from a few days to more than a year.(9) These eating-occasion frequencies are multiplied by an appropriate portion size (based on age-sex considerations) to obtain estimates of the daily food intake. Useful surveys include consumption data for foods eaten both at home and away from home.
The USDA has surveyed food use by US households since 1936. In the 1960s, the surveys were expanded to include food intake by individuals. Traditionally, these surveys appraised the nutritional adequacy of American diets rather than the safety of food with respect to additives or contaminants. However, the information on food intakes found in the USDA surveys is now frequently used to assess exposure to additives and contaminants.
Marketing research groups in the United States, such as the Market Research Corporation of America (MRCA) and the National Purchase Diary (NPD) have also surveyed the food consumption patterns of individuals and households for the food industry. Surveys of this type are not common throughout the rest of the world, but the World Health Organization (WHO) has emphasized the need for such data in a publication prescribing its guidelines for monitoring intakes of chemical contaminants.(10) A review of food consumption surveys throughout the world was published by the Food and Agriculture Organization (FAO) in 1985.(11)
The FASEB report issued by its Life Sciences Research Office in 1988 examined in detail the theory behind the calculation of exposure estimates.(12) This report, prepared for the US FDA, listed several food consumption databases for use in estimating food intakes. These included national food consumption surveys conducted by the US government, such as the USDA's Nationwide Food Consumption Survey (NFCS) and Continuing Survey of Food Intake by Individuals (CSFII), as well as the National Health and Nutrition Examination Survey (NHANES), and commercially sponsored surveys, such as the MRCA Menu Census and the NPD Eating Trends surveys. OPA has historically relied on the USDA's 3-day Nationwide Food Consumption Surveys and the Market Research Corporation of America's Surveys (herein referred to as the MRCA Survey(13)). The USDA 3-day surveys, the CSFII, and the MRCA 14-day surveys are further described below.
3-Day USDA/NFCS Survey and the CSFII
Summary reports from the US Department of Agriculture's 3-day Nationwide Food Consumption Survey of 1977-78 were published in 1982 as "Foods Commonly Eaten by Individuals". The survey was repeated during 1987 and 1988, but, due to statistical problems resulting from low response rates in certain sub-populations, the results have not yet been published in summary report form.(14) The information was collected in two steps: the first involved an interviewer-recorded recall of all of the foods consumed during the first day; the second part was 2-day a recall/report completed by the participant. Detailed instructions, including measuring cups, spoons, and a ruler, were given to the participants to aid in accurately reporting their intakes. The participants were selected to avoid geographical bias, and the survey was conducted over a twelve month period to avoid any seasonal bias in eating habits.
The data in the summary reports are presented in tables, each addressing the intake of a broad food class, such as meat and poultry, or an associated sub-class, such as fried chicken. All of the reported intake figures are on an eaters-only(15) basis. The percentage of eaters in each classification is included. The raw data from USDA surveys have been accessible to reviewers at CRB; however, such access, until recently, has not been routine. For many years, the summary report food intake data from the 1977-78 survey have been used in estimating exposure.
The information available from this survey includes: 1) the total number of individuals consuming the food on one, two, or all three of the survey days; 2) quantities of each food type consumed, averaged over the three days or averaged for individuals consuming the food on one, two, or three days; and 3) the quantity consumed per eating occasion. The data pertaining to quantities consumed are presented both as overall averages and as a percentile distribution, ranging from 5th to 99th percentile intakes. Maximum quantities consumed on one day, three days, and per eating occasion are also included in the same formats.
The 1987-88 NFCS was conducted using the same data collection and reporting techniques used in the 1977-78 survey. The raw data from the 1987-88 NFCS, and the related 1989-90, 1990-91, and 1991-92 CSFII's, are accessible to CRB through a proprietary software package designed to use the data in producing exposure estimates.(16) The CSFII planned for 1994-1996 will be a two day survey, as will future NFCS surveys. Non-sequential days will be surveyed in order to assure that additional intra-individual bias is not introduced into the survey by the first day's food choices influencing choices made on the second survey day.
The Market Research Corporation of America's 14-day food-frequency survey had until recently been a primary source of food intake information for OPA. The survey, which is updated annually, is intended to examine the number of eating occasions per day for any food consumed over a 14-consecutive day period. The most recent data available to OPA were compiled during the five year period from 1982 to 1987. This survey, which included over 25,000 participants, was also conducted in a manner to avoid geographical and seasonal eating biases.
The MRCA survey data are recorded by a single member of each surveyed household, usually the female head. For each member of the household, the number of eating occasions for each specific food consumed, both in-home and away-from-home, is recorded. Following the survey period, the recorder would submit the age, sex, weight, and diet status for each member of the household. The frequencies of eating occasions thus obtained need to be linked to appropriate portion size data to obtain the food intakes necessary for estimating exposure to substances in food (see discussion below in "Modeling exposure analysis".) This form of data collection allows estimates to be prepared for specific age/sex/demographic groupings, as well as for specific conditions, such as diabetics.
In the United States, information on the weight of commodities entering commerce is available annually from the Economic Research Service of the USDA.(17) Quantities are measured by deducting data on exports, year-end inventory, and non-food use from data on production, imports, and beginning inventories. These data are sometimes referred to as "disappearance" data because they represent the disappearance of food into the marketing system. Annual disappearance figures for a food commodity can be divided by the national population and by 365 days to obtain a "per capita" estimate of the food that is available for consumption per day (see example: Estimated Daily Intake for an Amino Acid in Sweet Pickles).
The food industry also measures the disappearance of specific products into the market. These data are usually gathered to observe marketing trends and lack the specificity needed for use by regulatory bodies. When the data obtained reflect disappearance of all of the product being monitored, such as total alcohol or carbonated beverage consumption, crude estimates of per-capita intake can be made.
Food disappearance data may overestimate actual per capita consumption because they include spoilage and waste accumulated in the marketing system and in the home, and some food that is not available for human consumption, such as turkey parts used in pet foods.(18) For example, in 1987-88, the estimate of food energy available for consumption per capita per day in the United States was about twice the estimate of mean food energy intake by the US population based on reported food intake in a national food consumption survey.(19)
For certain years, annual poundages of some substances (food additives and ingredients) produced and used solely for addition to food in the United States have been compiled as a part of the National Academy of Sciences (NAS) Survey of Industry on the Use of Food Additives (National Research Council, 1972, 1975, 1977, 1982, and 1987(20)). The reliability and validity of these data depend heavily on the completeness of the voluntary industry response for a given substance. A correction factor is applied to account for under-reporting in the NAS surveys before the adjusted poundage is determined for each substance of interest. This factor is related to the historical percentage of companies responding to the survey; for the 1982 and 1987 NAS poundage updates the correction factor is 0.6.
Disappearance data cannot be used to estimate exposure for targeted sub-populations (e.g., young children, diabetics, other age/sex groups), and dietary exposure cannot be expressed on a body weight basis. Because of the limitations inherent in this type of data, OPA uses disappearance data only in conjunction with exposures obtained using data from different sources (primarily to check the reasonableness of an estimate), or when no other data concerning a substance are available, if it can be inferred that the substance of interest is widely distributed in the food supply.
FDA conducts a market basket study annually (The Total Diet Study(21)) to monitor trends in the levels of certain nutrients and contaminants in representative diets of various age-sex groups. These diets, compiled from over 200 foods, have been developed from national food consumption data. To estimate exposure using data from such a market basket study, commonly eaten foods representing the whole diet of the target population(s) are purchased from the marketplace, prepared for eating (e.g., cooked), and then analyzed to determine the concentrations of the substances of interest in each food. Food consumption data from another source are then merged with the food composition data to obtain the desired estimate.
The advantage of the market basket approach is that chemical analyses are performed directly on the foods. There is, however, a major drawback associated with the routine use of this approach for determining the exposure to food additives and contaminants in the diet: the total number of foods analyzed must be limited because of cost and time restrictions. Additionally, a food that is unusually high in the component of interest may not be included in a market basket. Also, obtaining the distribution of exposures to a substance over the consumer population is not feasible.
OPA typically estimates exposure by linking food intakes from surveys to independently determined concentrations of the substances of interest. The concentration data used depend on the nature of the specific exposure assessment. For a premarket safety evaluation of a food additive, the proposed levels of the substance in targeted foods are generally used. For substances already in the food supply and for naturally occurring or accidental contaminants, OPA obtains concentration data from various sources, such as agency records (FDA, EPA, USDA), users of the substance, the scientific literature, or chemical analyses of foods in which the substance is known to be used or can be found.
OPA evaluates the quality of the concentration data used for estimating exposure. For estimating exposure to additives and contaminants, analytical sampling methodology, the precision and sensitivity of the method, and method validation procedures are considered. Additional considerations are made when estimating exposure to contaminants. For example, it is important to recognize that a contaminant is generally not distributed uniformly in a living organism: its concentration in plant roots or animal flesh may differ significantly from that in the leaves of the plant or the skin of the animal. Therefore, the concentration of the contaminant in the edible portion(s) of the organism as prepared for consumption is estimated or determined before OPA uses the data to estimate exposure.
Trace levels of materials in foods often fall below an analytical limit of detection and are typically reported as "non-detects." A number of papers have been published discussing the treatment of data sets containing non-detects for use in estimating exposures.(22) Values of zero, one half the limit of detection, the limit of detection, or some other derived distribution of values have been assigned to non-detects. OPA uses all of these methods, selecting the most appropriate in each case on the basis of the quality and quantity of data available. To gauge the effect of non-detect values on an exposure assessment, the estimation process may be completed twice: once using zero as the non-detect concentration to determine the low end of the estimate range and again using the limit of detection as the non-detect concentration to determine the high end of the estimate range. The spread in this range is useful as a guide in assessing the importance of non-detect concentration values in a given exposure estimate.
Two factors are required for estimating exposure to a food substance. The first is the daily intake of the foods in which the substance is used or can be found. The second is the concentration or use level of the substance in each food. (A simple example of an exposure estimate is outlined in Exposure to a Volatile Antimicrobial.) In the early 1970s, data on food intake by individuals were available from only two nationwide food consumption surveys: the 1965 USDA NFCS and the Market Research Corporation of America's (MRCA) Menu Census survey. In conjunction with the FDA, the NAS developed a model for estimating exposure that combined the MRCA data with the USDA/NFCS information. The MRCA data provided information on the frequency of consumption of foods over a 14-day period; the USDA/NFCS data provided portion size information. The use of intakes derived from multiple-day survey of individuals was considered more representative of long-term intake than single-day survey-derived estimates. The relationship of food consumption frequency, portion size, and substance concentration data to the EDI for a single individual is captured in the following equation.
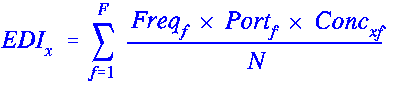
where:
F = Total no. of foods in which x can be found
Freqf = No. of eating occasions for food f over N survey days
Portf = Average portion size for food f
Concxf = Concentration of the substance x in the food f
N = No. of survey days
OPA typically estimates exposures to substances that may be consumed by the national population or a large sub-population. The most useful exposure information is derived from a distribution of exposures for the target population, rather than a single exposure figure. If OPA were assessing the safety of a food containing a substance that showed toxic effects in pregnant animals, the distribution of food intakes for women of child-bearing age would be used for the exposure analysis. For a national population, OPA is concerned with the potential for high exposure to food additives or contaminants for individuals who consume substantial quantities of the food(s) that may contain these substances. Information on food-intake distributions provides a means for estimating exposures to components of foods for the fraction of the population that can be considered "heavy" consumers of the foods of interest. OPA defines "heavy" consumers as those individuals who consume the food at or above the 90th percentile of the food intake distribution.
For the premarket evaluation of the safety of a food additive or to assess the risk associated with long-term exposure to a contaminant, OPA evaluates data concerned with lifetime (chronic) exposures to the substance. Food intake data derived from a multiple-day food consumption survey are used. OPA assumes that consuming a variety of foods over a multi-day period reflects the probable eating pattern of a population over a long period of time. The concentration values for the substance are chosen to reflect the levels likely to be found over time. A regulatory upper use limit could be used for a food additive that is expected to be consumed at a relatively constant level over time. OPA may decide to use an upper limit in a specific exposure assessment because average levels might underestimate the intakes of heavy consumers who consume the treated product frequently, or have a preference for a brand treated with the highest concentration. On the other hand, for a persistent contaminant such as lead or heavy metals, OPA would use an analytically determined mean concentration, including non-detects, to reflect probable exposure conditions.
OPA must also focus on very short-term, or even single, exposures, especially for contaminants associated with acute toxic effects. To estimate exposure in these cases, food intakes from single eating occasions or from one-day intakes (sometimes referred to as person-day intakes) would be used. Using a substance concentration from the high end of the distribution of measured levels would ensure that the risk assessment associated with the exposure assessment would be conservative for a heavy consumer of food containing the substance. When food intake data are not available, or are known to be underreported in food intake surveys (e.g., alcoholic beverages), eating scenarios may be developed for use in estimating exposure (an eating scenario is illustrated in the example Special Cases - Dietary Scenarios.)
The EPA guidelines for exposure assessment, published in 1992, concisely state: "Exposure assessments are done for a variety of purposes, and for that reason, cannot be easily regimented into a set format or protocol. Each assessment, however, uses a similar set of planning questions, and by addressing these questions the assessor will be better able to decide what is needed to perform the assessment and how to obtain and use the information required."(25) After defining the nature of the risk associated with the substance under consideration, the sub-populations at risk, and the food in which the substance may be found, information on the intakes of the foods affected and the concentration of the substance in each of those foods is compiled. At this point, the manner in which this information is used becomes paramount. Although the model for estimating exposure requires that incremental exposure to the substance from each food be summed to determine the total exposure to the substance, in reality this operation may be very different depending on whether raw or summary food intake data are available.(26)
When the raw data from a food consumption survey are available, reported food intakes for each individual in the survey can be combined with substance concentrations to estimate individual exposure to the substance. A distribution of the exposures for a target sub-population can then be derived and used in the risk assessment. OPA uses this methodology whenever possible because estimates of exposure obtained this way are more accurate than those obtained with summary intake data and should be considered the "state of the art." The proprietary software available to OPA for accessing the raw data from the 1987-88 NFCS, and the 1989-90, 1990-91, and 1991-92 CFSII's permits such an approach.
When only summary food intake survey data are available, assumptions must be made about how to combine exposures from the intake of the individual foods (or food groupings) containing the substance. An example of food intake summary data is presented in Table 1.
| Percent eaters | Food Intake (Eaters-only) | ||
|---|---|---|---|
| mean (g/p/d) | 90th percentile (g/p/d) | ||
| Food A | 65 | 25 | 45 |
| Food B | 23 | 15 | 35 |
If the concentration of substance x is 10 µg/g in food A and 25 µg/g in food B, the exposure to x from the consumption of each food is shown in Table 2 below.
| Concentration (µg/g) | Exposure to X (Eaters-only) | ||
|---|---|---|---|
| mean (µg/p/d) | 90th percentile (µg/p/d) | ||
| Food A | 10 | 250 | 450 |
| Food B | 25 | 375 | 875 |
The mean and 90th percentile exposures to substance x from the consumption of foods A and B cannot be taken from Table 2. The eaters-only exposure from food A in Table 2 cannot be added to that from food B because the 65% of the population that eats food A is not identical to the 23% of the population that eats food B. To sum individual exposures, the exposures must be placed in terms of the total sample. Total sample mean exposures can be summed. In the above example, the total sample mean exposure to substance x (provided it is only found in foods A and B) would be 249 µg/p/d (65% of 250 plus 23% of 375).
To convert this total sample mean exposure to an eaters-only exposure, the percentage of the population that is consuming either foods A or B (or both) must be determined. The percentage of eaters of A or B (or both) in this example lies between 65 and 88%, as shown in Figure 1.
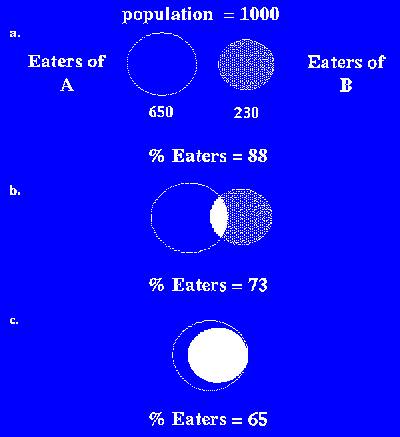
a.) No overlap - Largest eater population
b.) Partial (statistical) overlap
c.) Total overlap - Smallest eater population
In case a., the population of eaters of food A and the population of eaters of food B are different. Hence, the percentage of the total population that eats either Food A or B is 88 (65% + 23%). This scenario represents the eaters of "either-or" type foods, e.g., coffee or tea, soda or diet soda, etc. In case b., a consumer's choice of one food, e.g., lasagna or chocolate ice cream, is unrelated to the choice of another food. The overlap of the two populations is a statistical sampling equal to the product of the two percentages of eaters (0.65 x 0.23 = 0.15).(27) Hence, the percentage of the total population that eats A or B (or both) is 73 (88%(28) - eaters of A and B (15%)). In case c., a consumer who has chosen to eat food A will also eat food B. For example, dieters might use packets of artificial sweetener (food B) and drink diet soft drinks (food A). The percentage of eaters of A or B in case c. would be 65%.(29)
The size of the eating population must be calculated on a case-by-case basis. Case a. is less likely to occur when many foods contain the substance of interest because almost everyone will consume one or the other of the treated (or contaminated) foods. The most conservative estimates of exposure are made by assuming the lowest possible eating population (case c.). In most cases, assuming the statistical blend (case b., above) should provide a reasonable estimate of the size of the eating population and hence a reasonable estimate of exposure (see example Multiple-Use Additives - Probable Exposure for an Emulsifier).
OPA estimates upper percentile exposure to substances in the diet to account for individuals who are considered heavy consumers of specific foods (the 90th, 95th and 97.5th percentile exposures are used by various regulatory bodies in the world; OPA typically uses the 90th percentile). Approaches for estimating upper percentile dietary exposure from summary intake data on individual foods or food groupings are discussed in this section.
An intake estimate for a specific percentile for each food represents the intake of that food for a specific population. Thus, summation of these percentile exposures from individual foods is incorrect and should be avoided, particularly in estimates for high consumption groups (e.g., the intake for corn at the 90th percentile and the intake for pizza at the 90th percentile would almost certainly not be representative of any one consumer).
Examination of food frequency and other types of food consumption surveys conducted in the United States, shows that intake at the 90th percentile for most commonly-consumed foods is roughly 2 times the mean intake for that food, and intake at the 95th percentile is roughly 4 times the mean intake. Thus, a crude approximation of intake at the 90th percentile of a substance can be obtained by doubling the calculated mean intake and at the 95th percentile by quadrupling the mean.
Computer-based Monte Carlo simulations have been used by OPA to calculate specific percentile intakes for substances using summary data.(30) These simulations are used to evaluate models in which one or more inputs (here, food intakes and substance concentrations) can be defined by a distribution of values. Rather than using a single value (e.g., a mean or 90th percentile food intake) for such an input, a computerized Monte-Carlo simulation selects a value at random from the distribution of possible values for the input, uses that value to calculate the outcome of the model, stores the result, and then repeats the procedure a pre-determined number of times (iterations), using new values of the input taken from the distribution for each iteration. The resulting output from this procedure is a range of possible outcomes for the model. A probability distribution function is prepared from the range. An exposure at a designated percentile may be obtained directly from the distribution function.(31) Because Monte Carlo modeling is a probabilistic technique that can use all the available food intake and concentration data, more accurate estimates at upper percentiles than those obtained using point values will result (see results in the example Multiple-Use Additives - Probable Exposure for an Emulsifier).
The strength of the Monte Carlo modeling technique is that it allows for a more realistic estimation of exposure for the mean and for given centiles of a population of eaters when more than one food source for a substance is available than does summing individual food exposures from summary data. When modeling exposure with a Monte Carlo simulation, food intakes, use levels (or concentrations), and eater/non-eater variables can be accessed as distributions (or "Yes/No toggles" in the case of eater/non-eater), using the complete range of available information. Estimates of exposure are derived through Monte Carlo modeling for substances that are used or found in a number of foods; consumers food choices may be considered through the use of correlation functions. As an example, it is possible to test the scenario in which all peanut butter eaters are assumed to be jelly eaters (a reasonable assumption for the subpopulation of young children).
Go to the examples for this document
Go to the footnotes for this document
Hypertext updated by dms/hrw/emw 2006-AUG-29