
Volume 6, No. 7, July 2008
Features
American College of Obstetricians and Gynecologists
ACOG Practice Bulletin No. 94: Medical Management of Ectopic Pregnancy
Overview: In the United States, ectopic pregnancy accounts for 2% of all first-trimester pregnancies and 6% of all pregnancy-related deaths; it is the leading cause of maternal death in the first trimester. Early detection of ectopic pregnancy can lead to successful management without surgery. Methotrexate, a folic acid antagonist, can be used successfully to treat early, nonruptured ectopic pregnancy. The purpose of this document is to review the risks and benefits of the use of methotrexate in the management of ectopic pregnancy.
Summary of Recommendations and Conclusions:
The following conclusion is based on good and consistent evidence (Level A):
- In comparing systemic methotrexate with tube-sparing laparoscopic surgery, randomized trials have shown no difference in overall tubal preservation, tubal patency, repeat ectopic pregnancy, or future pregnancies.
The following recommendations and conclusions are based on limited or inconsistent evidence (Level B):
- An increase in serum hCG of less than 53% in 48 hours confirms an abnormal pregnancy.
- With an hCG level of 5,000 mIU/mL or higher, multiple doses of methotrexate may be appropriate.
- Methotrexate can be considered in those women with a confirmed, or high clinical suspicion of, ectopic pregnancy who are hemodynamically stable with an unruptured mass.
- Failure of the hCG level to decrease by at least 15% from day 4 to day 7 after methotrexate administration is considered treatment failure requiring therapy with either additional methotrexate administration or surgical intervention.
- Posttreatment hCG levels should be monitored until a nonpregnancy level is reached.
The following conclusion is based primarily on consensus and expert opinion (Level C):
- If the initial hCG level is less than 200 mIU/mL, 88% of patients experience spontaneous resolution.
ACOG Practice Bulletin No. 94: Medical Management of Ectopic Pregnancy . Obstet Gynecol 2008 111: 1479-1485. http://www.ncbi.nlm.nih.gov/pubmed/18515537
ACOG Committee Opinion No. 410: Ethical Issues in Genetic Testing
Abstract: Genetic testing is poised to play an increasing role in the practice of obstetrics and gynecology. To assure patients of the highest quality of care, physicians should become familiar with the currently available array of genetic tests and the tests’ limitations. Clinicians should be able to identify patients within their practices who are candidates for genetic testing. Candidates will include patients who are pregnant or considering pregnancy and are at risk for giving birth to affected children as well as gynecology patients who, for example, may have or be predisposed to certain types of cancer. The purpose of this Committee Opinion is to review some of the ethical issues related to genetic testing and provide guidelines for the appropriate use of genetic tests by obstetrician–gynecologists. Expert consultation and referral are likely to be needed when obstetrician–gynecologists are confronted with these issues.
ACOG Committee Opinion No. 410: Ethical Issues in Genetic Testing.
Obstet Gynecol 2008 111: 1495-1502. http://www.ncbi.nlm.nih.gov/pubmed/18515541
ACOG Committee Opinion No. 409: Direct-to-Consumer Marketing of Genetic Testing
Abstract: Marketing of genetic testing, although similar to direct-to-consumer advertising of prescription drugs, raises additional concerns and considerations. These include issues of limited knowledge among patients and health care providers of available genetic tests, difficulty in interpretation of genetic testing results, lack of federal oversight of companies offering genetic testing, and issues of privacy and confidentiality. Until all of these considerations are addressed, direct or home genetic testing should be discouraged because of the potential harm of a misinterpreted or inaccurate result.
ACOG Committee Opinion No. 409: Direct-to-Consumer Marketing of Genetic Testing.
Obstet Gynecol 2008 111: 1493-1494. http://www.ncbi.nlm.nih.gov/pubmed/18515540
ACOG Committee Opinion No. 408: Professional Liability and Gynecology-Only Practice
Abstract: Fellows of the American College of Obstetricians and Gynecologists may choose to limit the scope of their practices to gynecology. The College considers early pregnancy care (often up to 12–14 weeks of gestation) to be within the scope of gynecology and gynecologic practice. Liability insurers who provide coverage for “gynecology-only” practices should provide coverage for clinical practice activities that involve the management of early pregnancy and its complications.
ACOG Committee Opinion No. 408: Professional Liability and Gynecology-Only Practice.
Obstet Gynecol 2008 111: 1491. http://www.ncbi.nlm.nih.gov/pubmed/18515539
New interactive site for clinicians serving women with disabilities
ACOG has released a recorded slide program, Reproductive Health Care for Women with Disabilities , which assists women's health care clinicians with office skills to assist with their care of women with physical, developmental or sensory disabilities giving specific information for reproductive health care. Available now are the first 2 parts of a six part series:
Part 1 includes an overview of the program, The Scope of Disability in Women, Sexuality, and Psychosocial Issues.
Part 2 includes The GYN Examination and GYN Health Screening.
This program was recorded by Raymond L Cox, Jr., MD, MBA, FACOG and Caroline Signore, MD, MPH, FACOG. Elisabeth Quint, MD, FACOG served as faculty chair.
http://streaming.acog.org/WomenWithDisabilities/American Family Physician**
Food Allergies: Detection and Management
ABSTRACT: Family physicians play a central role in the suspicion and diagnosis of immunoglobulin E-mediated food allergies, but they are also critical in redirecting the evaluation for symptoms that patients are falsely attributing to allergies. Although any food is a potential allergen, more than 90 percent of acute systemic reactions to food in children are from eggs, milk, soy, wheat, or peanuts, and in adults are from crustaceans, tree nuts, peanuts, or fish. The oral allergy syndrome is more common than anaphylactic reactions to food, but symptoms are transient and limited to the mouth and throat. Skin-prick and radioallergosorbent tests for particular foods have about an 85 percent sensitivity and 30 to 60 percent specificity. Intradermal testing has a higher false-positive rate and greater risk of adverse reactions; therefore, it should not be used for initial evaluations. The double-blind, placebo-controlled food challenge remains the most specific test for confirming diagnosis. Treatment is through recognition and avoidance of the responsible food. Patients with anaphylactic reactions need emergent epinephrine and instruction in self-administration in the event of inadvertent exposure. Antihistamines can be used for more minor reactions.
Kurowski K, Boxer RW, Food Allergies: Detection and Management, Am Fam Physician. 2008;77(12):1678-1686, 1687-1688. http://www.aafp.org/afp/20080615/1678.html
AHRQ
“Real Men Wear Gowns”
The Agency for Healthcare Research and Quality (AHRQ) has joined with the Advertising Council to launch a national public service campaign designed to raise awareness among middle-aged men about the importance of preventive medical testing. The new campaign—"Real Men Wear Gowns"—tells men over 40 to learn which preventive screening tests they need to get and when they need to get them. According to AHRQ's Medical Expenditure Panel Survey, men are 25 percent less likely than women to have visited the doctor within the past year and are 38 percent more likely than women to have neglected their cholesterol tests. Data from the Centers for Disease Control and Prevention indicate that men are 1.5 times more likely than women to die from heart disease, cancer, and chronic lower respiratory diseases.
The campaign encourages men to visit a comprehensive Web site, www.ahrq.gov/realmen/. The site provides the recommended ages for preventive testing (as well as a list of tests), a quiz designed to test knowledge of preventive health care, tips for talking with the doctor, a glossary of consumer health terms, and links to online resources where men can find more medical information.
Ask A Librarian - Diane Cooper, M.S.L.S. / NIH
Health education materials available online in a variety of languages
Sally J. Bremner, Health Sciences Librarian of the Alaskan Health Sciences Information Service, provided information on an exciting new service offered by the National Library of Medicine on MedlinePlus. Follow the link below to check out the searchable offerings:
http://www.nlm.nih.gov/medlineplus/languages/languages.html
Behavioral Health Insights - Peter Stuart, IHS Psychiatry Consultant
Chronic Pain Management 101 – Saying No to Patients
Vignette: Ms. Howard has been followed in your clinic for several years for a variety of complaints including chronic neck and arm pain. She has a history of rotator cuff surgery which did not improve substantially her pain status. Her rehab participation could be characterized by the words “erratic to none.” She also has a history of periodic drinking episodes, depression and several overdoses while intoxicated, and previous tolerance development to narcotics and drug-seeking behavior. She is involved in a chaotic relationship. You do believe she has some physiologic basis for her pain but her compliance with your recommendations is erratic at best and you have on several occasions assisted her in detoxing from narcotics only to have her go to another provider and be restarted on them. She can be quite dramatic when claiming to suffer from pain and has accused you previously of “not doing enough to help me with my pain.” She is also on an antidepressant and gabapentin and cannot tolerate NSAIDs due to GI complications. You have a pain contract in place.
She presents today telling you she needs a refill of her Percocet 1 week before they were supposed to run out. She has been taking “1 or 2 extra a day because I was cleaning house last week and twisted my shoulder again.” She has a fairly recent history of prior “lost meds” that she has been counseled on in line with the pain treatment contract she signed. She appears mildly anxious and exhibits some discomfort with the shoulder even when non-obtrusively observed. The daily quantity is sufficient enough that you are concerned about withdrawal symptoms.
(At this point most providers start grinding their teeth) How do you approach her? Turf her to the psychiatrist or chronic pain committee (if you’re fortunate enough to have either?) What about her current needs?
In the murky world of real human beings, this amalgam of physical, social and emotional issues is not uncommon. The patient is likely to genuinely need some type of pain management, but is clearly out of control of her life and her self-care. She has demonstrated concerning tolerance development and there certainly is an addiction component active. She is clearly stepping outside of the boundaries set by the treatment contract. What is a reasonable goal here? Do we, as providers, have a commitment to provide compassionate and effective care no matter what the circumstances? If so, what exactly does that look like in this case? Is the appropriate overall approach “Harm Reduction” or “Abstinence-Based” or something else?
For me in situations like this it is helpful to go back to some basic clinical principles: First, that the overall clinical situation always trumps any absolute clinical rules about specific issues (i.e. if the pain contract says “no more meds after you lose an RX”). Borrowing a little from rabbinic tradition, each patient presents a unique set of predispositions, current symptoms and possible outcomes that require the provider to exercise discernment and judgment in determining what the appropriate course of action is.
For this patient, that means understanding that my goal is long-term pain relief and life management – the path I’m looking for keeps the patient in my practice, but also provides enough incentive for her to make some necessary changes for her benefit. Second, sometimes the right answer is “No”. As physicians, the principles of beneficence and minimal malfeasance are in play here. She is presenting in a situation where contingency management can be helpful in shifting behavior. In this case it might be worth using the positive contingency of getting her pain meds to decrease her discomfort both from withdrawal symptoms and pain in order to get her attending rehab and counseling. The challenge here is often a systems one – lack of access to these services at the time of presentation. **If the system is broken and doesn’t work well – it is incredibly important to acknowledge that reality.**
An appointment in rehab is available in 3 days time. In order to involve her in the process you negotiate with her to walk to rehab, schedule the appointment in person, and return to your office at which time you will provide her with an Rx for the 3 days. Further refills will be contingent on showing up at rehab and will be filled after she has her rehab appointment. Here is where saying “no med refill until . . .” can be very important.
Alternatively, you believe the big issue here is that she is not managing her meds appropriately (she is not following the contract, you have given her prior “chances” to learn the system, the system has done its part by providing her with the full Rx when she comes in, and is available to her if she has been debating about increasing her dose at home). In this case, the challenge becomes saying “No” without breaking the treatment relationship.
Third, “No” can be said in a supportive way. The principles for saying “No” in a supportive way are as follows: 1) Start with empathizing and putting yourself in the patient’s shoes, for example “I hear from what you’re saying and can see by looking at you that you are very uncomfortable right now, and I’m guessing you’re also worried about what withdrawal is going to be like if you don’t get something. Is that close to what’s going on?” 2) Align yourself alongside the patient and look at the problem together and the potential consequences of different approaches together, for example “Help me look at the options from your perspective. First option – I just refill the meds, no further questions asked, what happens then? And after that? And what’s happened before when we’ve done that? And how was that experience? Second option – I tell you no more meds . . . (repeat) Third option – can you think of a third option? “ 3) Summarize the options preferably using similar language to the patient, 4) tell the patient what you personally are comfortable doing and why, and 5) acknowledge that the patient may angry, frustrated, annoyed, scared, etc. and that you appreciate and understand that response and will continue working with them on their care.
Part 4 is the most difficult part because it is where you may be choosing an option different than the one the patient prefers. It is very important to be prepared for strong reactions. In Ms. Howard’s case she initially tells you to “F*** off – I’ll just go somewhere else then.” You reply, “Yes, I think I’d be upset and scared too, and at the same time I hope you will continue to work with me.” If the strong feelings can be safely tolerated and tested with the patient you have just successfully improved the relationship bond with the patient and the ability of the patient to tolerate further conflict with you – for the best interest of the patient.
In summary, the key ingredients here are an ability to see the big picture with the patient, to align yourself alongside the patient rather than against, and to support movement towards health sometimes by setting firm limits and boundaries but always in a context of compassion and caring. What else does this approach require? Time and focused attention . . .and if you can’t hold the system accountable for providing these basic human needs, you’re not going to get far in holding the patient accountable.
Breastfeeding - Suzan Murphy
A new look at what works to support breastfeeding in hospitals
June 13, 2002, Centers for Disease Control and Prevention (CDC) published in its weekly Morbidity and Mortality Weekly Report (MMWR), “Breastfeeding-Related Maternity Practices at Hospitals and Birth Centers – United States, 2007.” The article is the result of the first national Maternity Practices in Infant Nutrition and Care (mPINC – called “m-pink”) survey. The survey used questions from 7 categories of practice that are known to support breastfeeding, such as those found in the Ten Steps for Baby Friendly Hospitals. The categories and typical questions were:
- Labor and delivery – Do mothers and newborns routinely experience skin-to-skin contact and early breastfeeding initiation?
- Breastfeeding assistance – Is assessment, recording and instruction provided to new families on infant feeding? For example, are pacifiers not provided to breastfeeding infants?
- Mother- newborn contact – Is the separation of mother and newborn avoided?
- Newborn feeding practices – What and how breastfed babies are fed while in the hospital or birthing center? For example, is supplementation common and why?
- Breastfeeding support after discharge – Are resources provided for the new family when they are discharged? Where can they go for assistance if problems or concerns arise?
- Nurse/birth attendant breastfeeding training and education – What kind of breastfeeding support training and on-going education does staff receive?
- Structural and organizational factors related to breastfeeding –
- Does the facility have a breastfeeding policy and how is it communicated to staff?
- Is there support for breastfeeding employees?
- Does the facility receive free infant formula?
- Is prenatal breastfeeding education available?
- Is there coordination of lactation care?
The survey was mailed to 3,143 hospitals and 138 birth centers - of these, 2,690 hospitals and 121 birthing centers responded. The survey was structured to be completed by the person most knowledgeable of the facility’s intra-partum practices related to breastfeeding. To encourage true answers to survey questions, the responders and their administration were assured that their location and name would be kept confidential. The only potential identifier was the name of the state where the facility was located. The data was summarized by state and region.
The results of the survey were based on a maximum score of 100. For each category from above, the mean subscale scores from highest to lowest was:
- 2. Breastfeeding assistance
77 4. Newborn feeding practices
70 3. Mother-newborn contact
- 7. Structural and organizational factors related to breastfeeding
- 1. Labor and delivery
- 6. Nurse/birth attendant breastfeeding training and education
40 5. Breastfeeding support after discharge
State survey results strongly correlated with their breastfeeding prevalence rates. This suggests that where there are more evidenced-based breastfeeding support maternity practices in place, there are positive impacts on breastfeeding. Unfortunately, the reverse is also true.
For more information the survey project and survey questions, please see www.cdc.gov/mmwr
June 13, 2008/vol 57/No. 23, and www.cdc.gov/mpinc
CDC, Breastfeeding-Related Maternity Practices at Hospitals and Birth Centers --- United States, 2007 MMWR Weekly, June 13, 2008 / 57(23);621-625.
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5723a1.htm
Recall of Mommy’s Bliss Nipple Cream
FDA informed consumers not to use or purchase Mommy's Bliss Nipple Cream, marketed by MOM Enterprises, Inc., because the product contains potentially harmful ingredients that may cause respiratory distress or vomiting and diarrhea in infants. The product is promoted to nursing mothers to help soothe and heal dry or cracked nipples. Potentially harmful ingredients in the product are chlorphenesin and phenoxyethanol. Chlorphenesin relaxes skeletal muscle and can depress the central nervous system and cause slow or shallow breathing in infants. Phenoxyenthanol, a preservative that is primarily used in cosmetics and medications, can also depress the central nervous system and may cause vomiting and diarrhea, which can lead to dehydration in infants. Mothers and caregivers should seek immediate medical attention if their child shows signs and symptoms of a decrease in appetite, difficulty in awakening, limpness of extremities or a decrease in an infant's strength of grip and a change in skin color.
Read the entire 2008 MedWatch Safety Summary, including a link to the FDA News Release regarding this issue at: http://www.fda.gov/medwatch/safety/2008/safety08.htm#BlissCCC Corner Digest
Nicely laid out hard copy - A compact digest of last month’s CCC Corner
If you want a copy of the CCC Digest mailed to you each month, please contact nmurphy@scf.cc
Domestic Violence - Denise Grenier, Tucson / Rachel Locker, Warm Springs
WHO study links domestic violence and poor health; DV an urgent global health priority
BACKGROUND: This article summarises findings from ten countries from the WHO multi-country study on women's health and domestic violence against women.
METHODS: Standardised population-based surveys were done between 2000 and 2003. Women aged 15-49 years were interviewed about their experiences of physically and sexually violent acts by a current or former intimate male partner, and about selected symptoms associated with physical and mental health. The women reporting physical violence by a partner were asked about injuries that resulted from this type of violence.
FINDINGS: 24,097 women completed interviews. Pooled analysis of all sites found significant associations between lifetime experiences of partner violence and self-reported poor health (odds ratio 1.6 [95% CI 1.5-1.8]), and with specific health problems in the previous 4 weeks: difficulty walking (1.6 [1.5-1.8]), difficulty with daily activities (1.6 [1.5-1.8]), pain (1.6 [1.5-1.7]), memory loss (1.8 [1.6-2.0]), dizziness (1.7 [1.6-1.8]), and vaginal discharge (1.8 [1.7-2.0]). For all settings combined, women who reported partner violence at least once in their life reported significantly more emotional distress, suicidal thoughts (2.9 [2.7-3.2]), and suicidal attempts (3.8 [3.3-4.5]), than non-abused women. These significant associations were maintained in almost all of the sites. Between 19% and 55% of women who had ever been physically abused by their partner were ever injured.
INTERPRETATION: In addition to being a breach of human rights, intimate partner violence is associated with serious public-health consequences that should be addressed in national and global health policies and programmes.
Ellsberg M , Jansen HA , Heise L , Watts CH , Garcia-Moreno C ; WHO Multi-country Study on Women's Health and Domestic Violence against Women Study Team . Intimate partner violence and women's physical and mental health in the WHO multi-country study on women's health and domestic violence: an observational study. Lancet. 2008 Apr 5;371(9619):1165-72. http://www.ncbi.nlm.nih.gov/pubmed/18395577
Elder Care News - Bruce Finke, Elder Care Initiative
Several factors can quickly identify mortality risk among frail elderly persons living in the community
The Agency for Healthcare Research and Quality provides the following synopsis of a study that was partially funded through AHRQ:
Community-based long-term care programs such as PACE (Program of All-Inclusive Care for the Elderly) can help frail, chronically ill elderly people who would ordinarily enter nursing homes stay in the community. Asking about certain risk factors during routine clinical care can identify which of these frail community-dwelling elderly are at risk of dying, according to a new study.
The researchers predicted time to death using data on risk factors (demographic characteristics, coexisting medical conditions, and functional status), which they obtained from a geriatric assessment performed at the time of study enrollment. The risk scoring system scored male sex as 2 points; age 75-84, 2 points; 85 and older, 3 points; dependence for help with toileting, 1 point; dependence for partial or full help with dressing, 1 and 3 points, respectively; cancer, 2 points; congestive heart failure, 3 points; chronic obstructive pulmonary disease, 1 point; and renal insufficiency, 3 points.
In the validation group, respective 1 and 3-year mortality rates were 7 and 18 percent in the lowest risk group (0-3 points), 11 and 36 percent in the middle-risk group (4-5 points), and 22 and 55 percent in the highest-risk group (more than 5 points). The eight-variable index is easy to use and includes variables that can be obtained in the course of a routine clinical exam.
The ability of the index to predict mortality risk among the frail elderly reinforces the importance of considering multiple domains in assessing the prognosis of older patients.
The AHRQ summary is available at: http://www.ahrq.gov/research/jun08/0608RA10.htm .
Abstract and PubMed Citation at: Carey EC, Covinsky KE, Lui LY, Eng C, Sands LP, Walter LC. Prediction of mortality in community-living frail elderly people with long-term care needs. J Am Geriatr Soc. 2008 Jan;56(1):68-75. Epub 2007 Nov 20. http://www.ncbi.nlm.nih.gov/pubmed/18031487
Living wills should be updated, since preferences for life-prolonging treatments change when health status changes
Abstract:
As a substantial body of research attests, the acceptability of life-prolonging treatment (e.g., tube feeding) tends to be greater among people in worse health than among healthier ones. Because a decision for or against a life-prolonging treatment represents a choice between two prospects-life (usually in poor health) and death-we propose a decision model, Prospect Theory, as a theoretical account of this phenomenon. Prospect Theory postulates that pairs of distant prospects are less distinguishable than pairs of closer ones. Thus, to healthy individuals, the prospects of death and life in poor health would both be remote, and therefore, the distinction between them, small. To less healthy individuals, however, the difference between the same pairs of prospects would appear greater, and therefore, life-prolonging treatment may be more acceptable. In a cross-sectional study of 304 community-dwelling people, aged 60 years and over in the Philadelphia area, USA, preferences for 4 life-prolonging treatments in 9 health scenarios were examined in relation to participants' current health, operationalized as number of deficits in physical functioning. As predicted, less healthy people expressed stronger preferences for all life-prolonging treatments compared with healthier ones, with differences greatest in the worse-health scenarios. Preferences also varied by health scenario, with any treatment preferred in the better health scenarios. Treatment preferences did not differ by type of treatment, depressed mood or any demographic characteristic except race, with African-Americans expressing stronger treatment preferences. Implications for advance care planning are discussed.
Winter L , Parker B. Current health and preferences for life-prolonging treatments: an application of prospect theory to end-of-life decision making. Soc Sci Med. 2007 Oct;65(8):1695-707. Epub 2007 Jul 25. http://www.ncbi.nlm.nih.gov/pubmed/17655996
Family Planning
Injectable contraception: what should the longest interval be for reinjections?
BACKGROUND: Progestin-only injectable contraceptives continue to gain in popularity, but uncertainty remains about pregnancy risk among women late for reinjection. The World Health Organization (WHO) recommends a "grace period" of 2 weeks after the scheduled 13-week reinjection. Beyond 2 weeks, however, many providers send late clients home to await menses. STUDY DESIGN: A prospective cohort study in Uganda, Zimbabwe and Thailand followed users of depot-medroxyprogesterone acetate (DMPA) for up to 24 months. Users were tested for pregnancy at every reinjection, allowing analysis of pregnancy risk among late comers. RESULTS: The analysis consists of 2290 participants contributing 13,608 DMPA intervals. The pregnancy risks per 100 women-years for "on time" [0.6; 95% confidence interval (CI), 0.33-0.92], "2-week grace" (0.0; 95% CI, 0.0-1.88) and "4-week grace" (0.4; 95% CI, 0.01-2.29) injections were low and virtually identical.
CONCLUSION: Extending the current WHO grace period for DMPA reinjection from 2 to 4 weeks does not increase pregnancy risk and could increase contraceptive continuation.
Steiner MJ , Kwok C, Stanback J, Byamugisha JK, Chipato T, Magwali T, Mmiro F, Rugpao S, Sriplienchan S, Morrison C. Injectable contraception: what should the longest interval be for reinjections? Contraception. 2008 Jun;77(6):410-4. Epub 2008 Apr 10.
http://www.ncbi.nlm.nih.gov/pubmed/18477489
Depo Now: preventing unintended pregnancies among adolescents and young adults
PURPOSE: We compared the immediate administration of DMPA (Depo Now) to the immediate use of short-term hormonal methods that served as a "bridge method" until later DMPA initiation. We examined whether Depo Now, as compared to initiating with a bridge method (pills, transdermal patch, or vaginal ring), resulted in greater DMPA continuation at six months. METHODS: Young women aged 14 to 26 years seeking to use DMPA were randomized (nonblinded) after meeting eligibility criteria to either the Depo Now (n = 101) or bridge method (n = 232) group. Depo Now subjects received their first injection of DMPA at the conclusion of their first visit provided each was medically suitable and had a negative urine pregnancy test regardless of menstrual cycle day. Those assigned to the bridge method group were allowed to choose their starting contraceptive method and it was provided at the first visit. All subjects were told to return to the clinic in 21 days to repeat the urine pregnancy test, and among those who were assigned to use a bridge method, to receive their first injection of DMPA. All subjects were followed to their third injection, or about 6 months later.
RESULTS: Those randomized to a bridge method were 1.8 (1.1, 2.9) times more likely than Depo Now subjects to return for their 21-day repeat pregnancy test, but only 55% (n = 125) of these young women actually received their first DMPA injection. Continuation rates at the third injection were 29.7% (n = 30) for those in the Depo Now group and 21.1% (n = 49) for those assigned to the bridge method (p = .09). Three factors were significantly associated with adherence to the third injection: randomized to Depo Now group, knowing more women who use DMPA, and returning to clinic for the 21-day repeat pregnancy test visit. Finally, 28 pregnancies were diagnosed during the study period, and those in the bridge method group were almost 4.0 (1.2, 13.4) times more likely to be diagnosed with a pregnancy than those in the Depo Now group. CONCLUSIONS: Immediate administration of DMPA is associated with improved adherence to DMPA continuation and fewer pregnancies.
Rickert VI , Tiezzi L, Lipshutz J, León J, Vaughan RD, Westhoff C. Depo Now: preventing unintended pregnancies among adolescents and young adults. J Adolesc Health. 2007 Jan;40(1):22-8. http://www.ncbi.nlm.nih.gov/pubmed/17185202
CCC Editorial Comment:
The first article provides reassurance that a woman who is late, even as late as four weeks, for her scheduled Depo-Provera injection faces a low risk of unintended pregnancy and should receive the next injection without additional barriers. The second article provides more support for a “quick start” approach to Depo-Provera initiation. A “quick start” protocol can also be used for resuming Depo Provera use for those who are more than 2 to 4 weeks past the recommended date for their next dose.
Featured Website - David Gahn, IHS MCH Portal Web Site Content Coordinator
IHS Head Start Oral Health Resources
The IHS Head Start Program has developed a Best Practices document and Toolkit to assist in work with AI/AN Head Start Programs and those who visit the website will find many resources to assist in their work with Head Start, specifically AI/AN Head Start Programs.
http://www.ihs.gov/NonMedicalPrograms/HeadStart/index.cfm?module=fa_oralhealth_dc
Frequently Asked Questions
Progesterone for the Prevention of Recurrent Spontaneous Preterm Birth
Q. My patient is asymptomatic with a documented history of one prior spontaneous preterm birth at 33 weeks who is now 18 weeks gestation by a good LMP and an ultrasound at 12 weeks. Should she be give progesterone therapy?
A. Yes, but first make sure she agrees to return for weekly till 36 weeks of pregnancy.
Background
The incidence of preterm birth has risen progressively over the last decade from 9% to 12% of all births in the United States. Preterm birth is the second leading cause of infant mortality in this country and a significant proportion of survivors have residual disabilities. Despite multiple trials of tocolytics, antibiotics, and other preventive strategies, no effective method of preventing preterm birth has been found.
Recently, prophylactic treatment of high risk women with a history of one or more prior spontaneous preterm births with progestational compounds have demonstrated efficacy (1, 2). A prior meta-analysis (3) has also demonstrated a significant reduction in the rate of preterm delivery with the use of 17 alpha hydroxyprogesterone caproate (17P). The American College of Obstetrics and Gynecology (ACOG) has recommended that when progesterone is used, it be restricted to women with a documented history of a previous spontaneous birth at less than 37 weeks of gestation (4). Extensive experience with progesterone has shown it not to be a teratogen (5), and its use in this protocol will not involve administering it during organogenesis in the first trimester.
As a referral center, ANMC renders care to a large number of women who have experienced preterm birth, and an effective preventive treatment would be most advantageous to this population. Referral of these women and/or their infants to Level III centers for delivery and prolonged level III newborn intensive care generates significant expenditures for the institution that could be avoided by prevention of the problem.
Eligible Patients:
- Asymptomatic women with a documented history of one or more prior spontaneous preterm births (less than 35 weeks gestation) who are identified prior to 20 weeks gestation, who are dated by ultrasound prior to 20 weeks, and who agree to return for weekly injections from 16 weeks (may enroll up to 20 weeks) to 36 weeks of pregnancy.
- Consult with Maternal Fetal Medicine to establish eligibility and monitor outcomes.
Ineligible Patients:
- Women with a history of prior preterm birth due to a known cause such as a uterine malformation or cervical insufficiency requiring cervical cerclage.
- Women who present with symptoms of preterm labor (symptomatic uterine contractions, short cervix on ultrasound, uterine bleeding, ruptured membranes) after 20 weeks gestation.
- Women with a multi-fetal gestation.
- Women with a known fetal anomaly.
- Women with a prior indicated preterm birth (as a result of severe preeclampsia, placenta previa, fetal demise, or threatening maternal medical illness).
Drug Treatment Protocol:
- Weekly intramuscular injection of 17-hydroxyprogesterone caproate (17P) 250 mg* from 16 through 36 weeks of gestation.
- Fetal growth ultrasounds every 4 weeks while the patient is being treated.
Outcomes for Quality Assurance:
- Incidence of preterm birth prior to 35 weeks.
- Infant weights and Apgar scores.
- Incidence of preterm labor requiring inpatient treatment but not resulting in preterm birth.
- Any adverse maternal effects while receiving the therapy.
CCC Editorial Comment:
Short cervix, multiple gestation, preterm labor
There is as yet no consistent evidence that this drug is effective in women with preterm labor, a short cervix, or other high risk conditions (6). On the other hand, progesterone supplementation did NOT reduce the rate of preterm birth in multiple gestations randomly assigned to receive this therapy (7).
References:
- Meis PJ, et al. Prevention of recurrent preterm delivery by 17 alpha hydroxyprogesterone caproate. N Engl J Med 2003; 348:2379-85. http://www.ncbi.nlm.nih.gov/pubmed/12802023
- Da Fonseca EB, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2003; 188:419-24.
- Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Brit J Obstet Gynaecol 1990; 97:149-57.
- ACOG Committee Opinion No.291. Use of progesterone to prevent preterm birth. Obstet Gynecol 2003; 102:1115-6.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 6 th edition, 2002, page 674. Lippincott Williams and Wilkins. Philadelphia.
- Meis PJ. 17 hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol 2005 May;105(5):1128-35.
- Rouse DJ et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007 Aug 2;357(5):454-61. http://www.ncbi.nlm.nih.gov/pubmed/17671253
*Available through many pharmacies as 10mL vials (250mg/mL) at a cost of approximately $120/vial (if >6 vials are ordered). Be sure to check out availability before you discuss the possible therapy with your patient.
Indian Child Health Notes - Steve Holve, Pediatrics Chief Clinical Consultant
Quote of the month: “If you rest, you rust.” Helen Hayes
Articles of Interest
Cardiovascular Monitoring of Children and Adolescents with Heart Disease
Receiving Medications for Attention Deficit/Hyperactivity Disorder: A Scientific
Statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing
Circulation 2008;117;2407-2423; originally published online Apr 21, 2008;
http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.189473/DC1
Cardiovascular Monitoring and Stimulant Drugs for Attention-Deficit Hyperactivity Disorder - Policy Statement of the American Academy of Pediatrics
Published online on May 28, 2008
http://www.aap.org/new/ecg-adhd.htm
American Academy of Pediatrics/American Heart Association Clarification of Statement on Cardiovascular Evaluation and Monitoring of Children and Adolescents with Heart Disease Receiving Medications for ADHD
Published online on June 3, 2008
http://americanheart.mediaroom.com/index.php?s=43&item=422
In late April the American Heart Association (AHA) released new guidelines for children receiving stimulant medication for Attention Deficit Hyperactivity Disorder (ADHD) The AHA recommended that all children beginning treatment with stimulant medications, and those already on stimulant medication, should have an electrocardiogram (ECG) to search for occult heart disease that could place them at increased risk for sudden cardiac death. This recommendation caused anxiety in many parents and frustration in many psychiatrists and pediatricians.
The AHA recommendation has significant procedural and financial implications. It is estimated that 2.5 million children are receiving stimulant medication now. Obtaining an ECG on each one would entail significant costs. For some patients a further barrier was the suggestion that the ECG should be reviewed by a practitioner experienced in reading pediatric ECG. This would limit the reading of these studies to pediatric cardiologists and a small subset of pediatricians.
Within a month the American Academy of Pediatrics (AAP) fired back. The AAP published its own policy statement on stimulants and ADHD on May 28, 2008. Highlights included.
- Sudden Cardiac Death (SCD) in persons taking medications for ADHD is a rare event occurring at rates no higher than in the general population of children and adolescents
- Stimulant medication can cause mild elevations of blood pressure and heart rate but there is no evidence they increase the risk of SCD
- There is no evidence that the routine use of ECG screening before beginning medication for ADHD treatment would prevent SCD.
- Substantial evidence exists concerning the efficacy and safety of ADHD treatment with stimulant medications.
- Requiring an ECG before stimulant treatment could create a barrier to timely therapy. Limiting children’s access to effective treatment for ADHD could have serious implications, because there are substantial risks of not treating ADHD.
The AAP recommended that the cardiac evaluation of children taking stimulant medication should not change from their previous policy in 2000. The recommendations were as follows:
1. The AAP continues to recommend a careful assessment of all children, including those starting stimulants, using a targeted cardiac history (e.g., patient history of previously detected cardiac disease, palpitations, syncope, or seizures; a family history of sudden death in children or young adults; hypertrophic cardiomyopathy; long QT syndrome) and a physical examination, including a careful cardiac examination.
2. Given current evidence, the AAP encourages primary care and subspecialty physicians to continue currently recommended treatment for ADHD, including stimulant medications, without obtaining routine ECGs or routine subspecialty cardiology evaluations for most children before starting therapy with these medications.
To resolve confusion between the two professional societies the AAP and AHA issued a joint statement on June 3, 2008. The relevant portion is listed below:
- Obtaining a patient and family health history and doing a physical exam focused on cardiovascular disease risk factors are recommended by the AAP and AHA for assessing patients before treatment with drugs for ADHD.
- It is reasonable for a physician to consider obtaining an ECG as part of the evaluation of children being considered for stimulant drug therapy, but this should be at the physician’s judgment, and it is not mandatory to obtain one.
- Treatment of a patient with ADHD should not be withheld because an ECG is not done
Pediatric Chief Clinical Consultant Editorial Comment:
The AAP statement of May 28, 2008 is reasonable guidelines that practitioners should embrace. Patients should be screened by history for potential cardiac risk factors and have a physical exam that focuses on cardiovascular system. Patients with known or suspected heart disease may need an ECG and further specialty evaluation.
Infectious Disease Updates Rosalyn Singleton, MD
Teen Vaccines: How are we doing?
Since 2005, three new vaccines have been licensed and recommended specifically for teens: Tdap, HPV and meningococcal conjugate vaccine.
- Tdap – for 11-18 year olds with >5 years since last Td vaccine,
- HPV – 3 doses for 9-26 year old females
- Meningococcal conjugate (Menactra®, MCV4) – for 11-18 year olds
CDC published the first estimates of national vaccination coverage among teens aged 13-17 years in U.S.-2006 in Aug. 2007 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5634a3.htm Among 13-17 year olds, 10.8% had received 1 dose of Tdap and 60.1% had received 1 dose of Td or Tdap since age 10 years; 11% had received meningococcal vaccine. According to recent CDC data, 23% of females aged 13-17 years had received 1+ doses of HPV vaccine.
Indian Health Service started reporting Adolescent Immunization rates for American Indian and Alaska Native adolescents 13-17 years of age from IHS/contract facilities in 12/31/07. Adolescent coverage data from 12/31/07 and 3/31/08 are presented here:
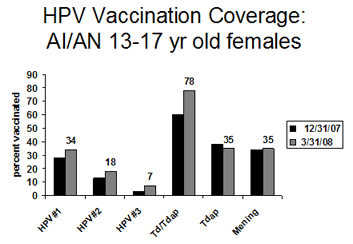
Comments: The initial Indian Health Service adolescent vaccine coverage rates for new vaccines compare favorably with national coverage rates; however, the national rates reflect an earlier time period. These rates reflect a robust delivery system; however, it will be challenging to administer 2 nd and 3 rd doses in a timely manner to teens. Providers can pull up lists of teens due for their 2 nd or 3 rd HPV doses in the RPMS Immunization Package by running a list of girls who have “received HPV vaccine” and are “due for HPV vaccine”.
Recent literature on American Indian/Alaskan Native Health Michael L. Bartholomew, MD
Pertussis-Associated Hospitalizations in American Indian and Alaska Native Infants
Before the introduction of the pertussis vaccine in the 1940s, the US incidence of pertussis was approximately 150 cases per 100,000 population or roughly 175,000 cases per year 1. By the 1980-90’s the incidence declined to approximately 1 case per 100,000 population. Since 1980, the incidence of pertussis has steadily increased. Waning immunity in the adolescent and adult populations are felt to be contributing factors to this increase 2.
This study investigates the burden of pertussis in American Indian and Alaska Native (AI/AN) infants from 1980 to 2004 and 2000 to 2004 through the analysis of discharge/hospitalization data from the Indian Health Service (IHS)/Tribal health care system. Infant pertussis hospitalizations were examined by sex, age group (infants less than 6 months and 6-11 months) and IHS region (Northern Plains, Southern Plains, Southwest, and Alaska). Rates of infant pertussis hospitalizations for 2000-2004 were compared to the pertussis hospitalizations of the general U.S. infant population in 2003.
In IHS, there were 483 infant pertussis hospitalizations between 1980 and 2004. This equals an annual pertussis hospitalization rate of 132.7 per 100,000 AI/AN infants (95% CI = 121.3 to 145.2). The highest numbers of hospitalizations were in infants less than 6 months of age (427 hospitalizations); equaling a rate of 234.5 per 100,000 infants. Rates in males and females were similar by age. The Southwest region had the highest rate (187.9 per 100,000) of pertussis hospitalizations as compared to Alaska (92.0), Northern Plains (103.3) and the Southern Plains (87.8).
In comparison, the average annual pertussis hospitalization rate for AI/AN between 2000 and 2004 was higher than the 2003 general US infant population pertussis hospitalization rate (100.5 vs. 67.7). Additionally, Alaska (156.9) and Southwest (130.9) regions had higher hospitalization rates than the Northern (59.1) and Southern (59.2) Plains during the same time period.
The authors concluded that the burden of pertussis is greatest in AI/AN infants less than 6 months of age predominantly those residing in the Southwest and Alaska regions. Despite levels of immunization rates equal or slightly less than the general population, environmental and social influences likely contribute to the higher disease burden in the AI/AN population; similar to what is seen with respiratory syncytial virus infections in Alaska Native infants 3. Prevention of pertussis by timely administration of DTaP at 6 to 8 weeks of age and promotion of Tdap vaccination in adults and adolescents are strongly encouraged.
Murphy TV, Syed SB, Holman RC, Haberling DL, Singleton RJ, Steiner CA, Paisano EL, Cheek JE. Pertussis-Associated Hospitalizations in American Indian and Alaska Native Infants. J Pediatr. 2008;152:839-43. http://www.ncbi.nlm.nih.gov/pubmed/18492528
References:
- Centers for Disease Control and Prevention. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pert.pdf
- American Academy of Pediatrics. Pertussis. In: Pickering LK, Baker CJ, Long SS, McMillan JA, eds. Red Book: 2006 Report of the Committee on Infectious Diseases. 27 th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2006:498-520.
- Holman RC, Curns AT, Cheek JE, Bresee JS, Singleton RJ, Carver K, Anderson LJ. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics 2004 Oct;114(4):e437-44. http://www.ncbi.nlm.nih.gov/pubmed/15466069
Information Technology
New RPMS Scheduling Package Released
The Indian Health Service Office of Information Technology and the Clinical Scheduling for Windows Application Team announce the following application release: Clinical Scheduling for Windows, v2.0 (RPMS namespace: BSDX) A release notice from the IHS Office of Information Technology has been sent to all RPMS site managers, and site managers are advised to notify users once the application has been installed.
GUI Scheduling is a supplement to the character based RPMS PIMS Scheduling package allowing the user a fast and easy approach to scheduling and managing appointments. Users of this package will be able to enjoy color-coded appointment types and view clinics side by side to see what times are available for scheduling. The package supports most of the daily scheduling activities including Rescheduling, Cancellations, Check-In and Walk-ins. Displaying and printing Clinic Schedules, Reminder Letters, Cancellation Letters and Patient Letters are also available. The current release fixes a number of issues with version 1.0 that limited the application's usability and deployment.
Thank you to the following beta test sites for their assistance with this release.
- Chemawa Indian Health Center (Western Oregon Service Unit)
- Fort Peck Service Unit (Poplar, Montana)
- Warm Springs Health & Wellness Center (Warm Springs, Oregon)
- WW Hastings (Tahlequah, Oklahoma)
For user support please contact the RPMS help desk at support@ihs.gov or call (888) 830-7280.
PCC+ v2.6 (Well Child Module) Released
Introduction
The PCC+ v2.6 Well Child Module (WCM) will become an “add-on module” to the current version of PCC+. The objective of the Well Child Module is to use information technology to standardize well child care throughout the Indian Health Service and to lay the groundwork for the inclusion of age-specific guidelines and reminders for well child care into the Electronic Health Record (EHR). The well child module is designed to be in compliance with a set of national guidelines and standards that were provided by a group of senior pediatricians in the IHS. As a rule, guidelines and standards are taken from nationally recognized sources such as Bright Futures, the American Academy of Pediatricians, the Ages and Stages Child Monitoring Program, and the IHS Patient Education advisory group.
Background
Well Child care is one of the most common yet clinically complex services. Typically, the well child “record” is captured on a series of special encounter forms with each form corresponding to a specific age from birth through adolescence. Each form contains age-specific guidelines for developmental screening, anticipatory guidance, examinations, immunizations, nutritional counseling, and patient education. The ideal situation is that children are scheduled for well child appointments and the forms correspond to the age(s) of the child being seen however; experience tells us that this type of precision is rarely, if ever achieved.
The objectives of the PCC+ Well Child Module are to:
- Standardize well child care throughout the IHS
- Present the correct age-specific guidelines to the provider at the time of encounter using current nationally recognized guidelines
- Enable the components of the Well Child Module to be incorporated and presented in a multi-modal format so that it can be used by the Electronic Health Record (EHR), PCC+ and Health Summary.
In addition to the objectives above, hybrid components will be available that will enable providers to enter well child information (i.e. ASQ scores) directly into the RPMS system via a desktop module.
Features of the new Well Child Module (WCM)
1.) Knowledgebase and Knowledgebase Manipulator
From the moment a child is born until age 21, there are thousands of age-specific guidelines and reminders that apply to well child care. The PCC+ WCM contains a knowledgebase in RPMS that serves as a repository for age-specific guidelines and reminders. The Knowledgebase Manipulator is a tool used by WCM that will enable providers to edit the knowledgebase and to determine exactly which age-specific guidelines and reminders will be displayed on PCC+ encounter forms and in the near future, the HER application.
2.) ASQ Screening
The Ages and Stages questionnaire is a commercial instrument for monitoring childhood development (0 to 5). The ASQ contains a set of 19 age-specific questionnaires for the child’s parent to answer. The questionnaire is then scored by well child care personnel and results are entered into the RPMS system, either by data entry personnel or via a WCM GUI application at the point of care. ASQ results are stored as “measurements” in RPMS and are displayed on the new Well Child Health Summary.
3.) Informal Development Screening
For sites that are not able to conduct ASQ screening on every visit, the WCM can be configured to display representative milestones from the Denver Developmental Screening Test (DDST) and these milestones will display on the PCC+ encounter form. The child’s age determines exactly which milestones will display as well as the percentage of children at that age who are expected to pass a particular milestone.
4.) Intervention Reminders
Special exams and interventions are due throughout childhood. The WCM will provide the following age-specific exams and reminders:
- Special risk exams, such as TB screening.
- Age-specific exams, such as strabismus and scoliosis.
- General screening exams such as lead levels.
- Autism screening questions.
- Immunizations, past immunization given (history), and forecasting lists will be automatically generated by WCM & displayed on the encounter form.
5.) Anticipatory guidance
Anticipatory guidance is a cornerstone of well child care. There are literally thousands of age-specific topics, general patient education topics, and nutrition counseling topics available for display. Using the Knowledgebase Manipulator the WCM will enable providers to display only topics and standards they choose to display on encounter forms. Topics include substance abuse, behavioral health, community interaction, oral health, etc…
6.) PCC+ Growth Grids
Users of traditional PCC+ have been able to display growth grids and immunization lists. PCC+ 2.6 will continue to print the grids and lists to be used as patient handouts.
7.) Data Entry Mnemonic
Since the new WCM captures new data elements, it will increase the workload of data entry personnel. To compensate, the new WCM includes a new “intelligent” mnemonic that will be synchronized with the actual patient visit. Using the new “WCE” mnemonic, only information that is specifically associated with a well child visit will present to the data entry clerk, thus increasing the efficiency of data entry personnel.
8.) New Well Child Health Summary component
The new Well Child Health Summary component will display age-specific guidelines and reminders specific to Well Child care.
Summary of the new Well Child Module (SCM)
The new version of PCC+ will include a Well Child Module that is intended to standardize well child care in the IHS and lay the groundwork for inclusion of well child care age-specific guidelines and reminders into the EHR application. The WCM leverages the power of information technology and automated decision support in two specific ways to:
- Capture and encapsulate data gathered during well child care encounters that previously was only collected piecemeal over an extended amount of time.
- Customize and present age-specific guidelines and reminders to the pediatrician at the time of encounter.
Several other validated screening tools exist such as the Arizona PEDS® instruments used by pediatricians in Arizona. New autism screening tools (i.e. mCHAT®) are also becoming available. We intend to add these screening options to future versions of the WCM.
Acknowledgements go to many and include the test sites:
Southern Indian Health Council in Alpine, CA
SEARHC in Sitka, AK & Juneau, AK (both sites participated)
Cherokee Indian Hospital Authority in Cherokee, NC - first test site
For more information on the WCM, please contact Clarence Smiley at clarence.smiley@ihs.govInternational Health Update - Claire Wendland, Madison, WI
New evidence on lead’s long-term effects
Lead has been back in the news lately. A potent neurotoxin, lead damages the brain by altering neurotransmitter release in a way that leads to accelerated apoptosis (cell death). It is a known toxin for adults, but appears to be a particularly bad actor in the developing brain. Though it hasn’t typically been a problem in the rural parts of Indian country, urban AI/AN people living in dilapidated housing stock are at risk for chronic lead toxicity. Two new articles based on longitudinal research from the Cincinnati Lead Study (CLS) may heighten the stakes of the debate over acceptable childhood lead levels.
The CLS birth cohort was recruited in the womb between 1979 and 1984 from urban inner-city neighborhoods known to have high lead levels. These children got detailed exposure histories from the prenatal period on, frequent neuropsychiatric exams and serum lead levels, and have now been followed into young adulthood. One of the two new reports shows a small but significant correlation between childhood lead levels and adult arrest records in this cohort. After careful adjusting for potential confounders, John Wright and colleagues found that for every 5 mcg/dl increase in prenatal (maternal) and childhood blood lead, total arrests and arrests for violent crime went up by roughly forty percent. In the second report, Kim Cecil and colleagues used MRI to assess brain volume in 157 members of the CLS cohort. Regression analysis demonstrated a linear dose-dependent correlation between childhood serum lead levels and reduction in adult brain volume, a result that was highly statistically significant. Most interesting, the reduction was specific to grey matter in the anterior cingulate cortex and portions of the prefrontal cortex. (White matter and CSF volume were not affected.) These are the regions responsible for judgment, focusing attention, regulation of mood, and executive functions. Grey-matter volume loss in these areas was also much more pronounced among men than women, even at similar lead levels. This finding suggests a mechanism for the links previously explored between childhood lead exposure and adult anti-social behavior, criminal activity, and poor intellectual performance.
Some of you may be scratching your heads over why this is an international health problem. Unfortunately, the measures put in place to mitigate lead toxicity in the United States years ago – screening programs, mandated transitions to unleaded gasoline, abolition of lead-based paints and ceramic glazes – never happened in much of the Third World. In fact, some gasoline sold today in South America, the Middle East and Africa has more lead additives than leaded gas in our country ever did; paint sold for residences, toys, and playground equipment still contains high levels of lead in Africa and several countries in Asia. In Central and South America, unregulated industrial processes are the major culprits. Some experts argue that an unrecognized epidemic of low- to moderate-level lead poisoning is occurring in much of the Third World, and a few small pilot studies of lead levels tend to confirm this assessment. Improvements in screening, policy and enforcement are urgent.
Cecil KM et al. Decreased brain volume in adults with childhood lead exposure. PLoS Medicine 5(5):e112, 2008 http://www.ncbi.nlm.nih.gov/pubmed/18507499
Wright JP et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Medicine 5(5):e101, 2008 http://www.ncbi.nlm.nih.gov/pubmed/18507497MCH Alert
American Academy of Pediatrics Releases Statement
on Strategies to Improve Adolescent Health Care
Achieving Quality Health Services for Adolescents provides recommendations and
criteria for assessing the quality of adolescent health care and discusses the
need for comprehensive efforts to improve the quality of primary care delivered
to adolescents in the United States. The policy statement, developed by the American
Academy of Pediatrics' Committee on Adolescents, focuses on quality issues that
relate to staying healthy -- preventive care themes. Topics include
adolescent health status and risky behaviors, primary care access and utilization,
access to quality care for adolescents, emerging quality measures for adolescent
care, and opportunities and challenges for primary care. Conclusions and recommendations
are included. The policy
statement is available at: http://aappolicy.aappublications.org/cgi/content/full/pediatrics;121/6/1263.
Report Presents Findings from the 2007 National Youth Risk Behavior
Survey
"Since 1991, the prevalence of many health-risk behaviors among students
nationwide has decreased. However, many students continue to engage in behaviors
that place them at risk for the leading causes of mortality and morbidity," state
the authors of a report published on June 6, 2008, in MMWR Surveillance Summaries.
The national Youth Risk Behavior Survey (YRBS) is the primary source of data
to measure 15 Healthy People 2010 objectives and three leading health indicators.
This report provides the 2010 target and data from the 2007 national YRBS for
all 15 objectives.
The Youth Risk Behavior Surveillance System (YRBSS) includes a national school-based
survey conducted by the Centers for Disease Control and Prevention and state
and local school-based surveys conducted by state and local education and health
agencies. This report summarizes results from the national survey, 39 state surveys,
and 22 local surveys conducted among students in grades 9-12 during 2007.
The authors found that
- Among students in grades 9-12 nationwide during 2007, 11% had never or
rarely worn a seatbelt when riding in a car driven by someone else. - During the 30 days before the survey, 29% of students had ridden in a
car or other vehicle driven by someone who had been drinking alcohol. - 18% had carried a weapon, and more than 5% had not gone to school
because they felt they would be unsafe at school or on their way to or
from school. - During the 12 months before the survey, almost 7% of students had
attempted suicide. In addition, 75% had ever drunk alcohol, and more
than 4% had ever used methamphetamines. - About 48% of students had ever had sexual intercourse, 35% were
currently sexually active, and more than 38% of currently sexually
active students had not used a condom during last sexual intercourse. - Twenty percent of students had smoked cigarettes during the 30 days
before the survey, 35% had watched television 3 or more hours per day on
an average school day, and 13% were obese. - During the 7 days before the survey, almost 79% of students had not
eaten fruits and vegetables five or more times per day, about 34% had
drunk soda or pop at least one time per day, and more than 65% had not
met recommended levels of physical activity.
The authors conclude that "more effective school health programs and
other policy and programmatic interventions are needed to reduce risk and improve
health outcomes among youth."
Eaton DK, Kann L, Kinchen S, et al. 2008. Youth risk behavior surveillance --
United States, 2007. MMWR Surveillance Summaries 57(SS04):1-131. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5704a1.htm .
More information is available from the following MCH Library resource:
http://www.mchlibrary.info/guides/schoolhealth.html
Panel Reviews National Study Plan on Environmental and Genetic Effects
on Health in Children
The National Children's Study Research Plan: A Review presents a scientific assessment
of a national longitudinal study to examine the effects of environmental influences
(including chemical, biological, and psychosocial) on the health and development
of approximately 100,000 children across the United States, following them from
before birth until age 21. The report, published by the National Academies Press,
is based on a review conducted by the National Research Council in collaboration
with the Institute of Medicine to ensure that the National Children's Study (NCS)
is scientifically rigorous and is being carried out with the best available methods.
Topics include NCS goals, conceptual framework, and core hypotheses; priority
outcome and exposure measures; study design, data collection, and analysis; ethical
procedures and community engagement; and conclusions and recommendations. An
executive summary, references, and biographical sketches of the review panel
members are included. The report is available at http://www.nap.edu/catalog.php?record_id=12211 (requires
sign-in for free PDF download).
The National Children’s Study, to be conducted in selected counties across the U.S., includes several counties with significant Native American representation. The National Children’s Study website can be reviewed at: http://www.nationalchildrensstudy.gov/
Bright Futures for Women’s Health and Wellness Releases new
Tools on Emotional Health
The Bright Futures for Women’s Health and Wellness (BFWHW) series includes
new tools to address the connection between women's mental and physical well-being
and encourage better health across the lifespan. The tools, produced by the Health
Resources and Services Administration's Office of Women's Health, focus on three
main concepts: appreciating oneself, finding balance and purpose in life, and
connecting with others. Each tool is designed for a specific audience including
women, young women, community groups, and primary care health professionals.
All BFWHW tools are wellness-focused, gender-specific, and evidence-based.
New tools in the BFWH series are available from the MCH Library's Web site as follows:
-
A Young Woman's Guide to Emotional Wellness
http://mchlibrary.info/BFWHW/BF_young_women_Revised_707.pdf
-
A Woman's Guide to Emotional Wellness
http://mchlibrary.info/BFWHW/BF_Woman_revised_707.pdf
-
A Community Organization’s Guide to Promoting Emotional Wellness
http://mchlibrary.info/BFWHW/BF_Community_Revised_707.pdf
-
A Health Care Provider's Guide to Promoting Women's Emotional Wellness
http://mchlibrary.info/BFWHW/BF_Clinicians_Revised_707-2.pdf
A companion one-page flyer for health professionals to use in waiting and exam rooms to support client-professional dialogue about emotional wellness is also available http://mchlibrary.info/BFWHW/BF_Flyer.pdf.
Print copies from this series may be ordered from the HRSA Information Center Web site at http://ask.hrsa.gov.
MCH Headlines - Judy Thierry HQE
Bright Futures Web Site Updated
The Bright Futures Web site has been renovated for the 3rd edition, including free access to the Guidelines posted by chapter and hyperlinked references. Bright Futures is a national health promotion and disease prevention initiative that addresses children's health needs in the context of family and community. In addition to use in pediatric practice, many states implement Bright Futures principles, guidelines and tools to strengthen the connections between state and local programs, pediatric primary care, families, and local communities. Whether you are a health care or public health professional, a parent, or a child advocate, Bright Futures offers many different resources for your use in improving and maintaining the health of all children and adolescents. Access the site online at: http://brightfutures.aap.org.
Resources and Guidance for Fatherhood Programs (10 Promising Practices)
An increasing number of programs focus on improving fathers' involvement with children and families. What features of fatherhood programs really matter? A new National Responsible Fatherhood Clearinghouse brief authored by Child Trends examines experimental evaluations of fatherhood and parenting programs to identify ten promising practices:
- Teaching methods and materials that are culturally appropriate for fathers being served.
- Staff members who believe in the program and have relevant training and coaching.
- A high staff-participant ratio.
- One-on-one relationships between staff and participants.
- Clear, specific program goals.
- Theory-based approaches that have influenced parenting behaviors in other contexts.
- Varied teaching methods that focus on fathers as individuals.
- Sufficient time to complete important core program activities.
- Incentives to engage fathers and families.
Additional resources at:
Now Available: Baby Oral Health Program
The Department of Pediatric Dentistry at the University of North Carolina at Chapel Hill has developed an educational program titled Baby Oral Health Program (BOHP). As part of this program, an infant and toddler oral health kit has been developed to provide the entire dental team with the necessary background information, technique demonstration and educational materials to begin an effective BOHP as an extension to its current practice. This preventive program promotes early oral health values and the establishment of a dental patient for life. It is now available to all dental practitioners. This kit provides a hands-on package that includes 1) a “how to do and why” DVD, 2) clinical forms for the first dental visit, 3) resource handouts on this topic, and 4) an Educational Flip Chart outlining the important concepts that guide infant and toddler oral health education for caregivers in the dental office. In addition, a two hour CE credit can be earned through self-study of the BOHP Kit©. Information is available online at: www.bohp.unc.edu.
Child Maltreatment Institute: Enhancing Leadership for Child Maltreatment Prevention
Apply now for PREVENT’s upcoming state-of-the-art Child Maltreatment Institute. Application deadline for is August 1, 2008. The Institute will provide experienced, multi-organizational teams access to expert faculty in child maltreatment and primary prevention and the opportunity to network with other leaders from across the country. Trained coaches will use an action learning approach to facilitate and support teams in producing concrete outcomes such as:
- a detailed, team-based 6-month action plan focused on developing an effective program that stimulates organizational and social change
- a Logic Model that outlines the transition from the 6-month plan into a comprehensive primary prevention program over a 5-year period
- a framework for program evaluation with measurable objectives
- communication strategies and message development for media and legislative advocacy
- approaches for achieving program sustainability
The PREVENT Child Maltreatment Institute consists of two (2) intensive three-day, on-site sessions separated by six months of team project work at home with an experienced coach. The first three-day on-site session will be November 16-19, 2008 at the Sheraton Chapel Hill Hotel, Chapel Hill, North Carolina. The second on-site session will be conducted in May 2009.
Multi-organizational teams of up to 6 people will be selected based on their experience working together, demonstration of leadership in child maltreatment prevention AND readiness to take an increased leadership role in making social and organizational changes to prevent child maltreatment. The Institute will cover lodging and most meals for up to 4 team members (up to 2 additional members are welcome but must cover their own lodging; their meals will be provided by PREVENT). Selected teams are responsible for travel and a one-time non-refundable $750 team registration fee. For more information and to submit an application, please see the attached flyer and visit http://prevent.unc.edu/education/.
The PREVENT Child Maltreatment Institute is supported by the Doris Duke Charitable Foundation and is operated by The University of North Carolina Injury Prevention Research Center, a partner in the National Training Initiative for Injury and Violence Prevention (NTI). PREVENT was launched in 2003 with support from the National Center for Injury Prevention and Control at CDC and has trained more than 900 violence practitioners in 44 states in the primary prevention of different types of violence.
Medical Mystery Tour
A diabetic patient who is finally losing weight
As you may recall last month we discussed……
…. a 21 yo G1 P0 presents with persistent nausea and vomiting for 7 weeks. The patient was diagnosed with type 2 diabetes 4 years ago and has had fair control with and an oral hypoglycemic agent. She stopped her hypoglycemic when she learned she was pregnant. Her glucose has been well controlled with diet and exercise during her pregnancy. The patient has a history of migraine that often presents with concomitant nausea.
Exam reveals a 7 % loss from her pre-pregnancy body weight which she states was without trying to lose weight. Otherwise the patient’s fundus is palpable 3 fingerbreadths below her umbilicus.
Laboratory evaluation reveals no evidence of urinary tract infection, but she does have ketonuria.
Ultrasound confirms a single 16 week female fetus with normal amniotic fluid and a Grade I anterior fundal placenta.
We established that pregnancy is not an ideal time for weight loss. Significant weight loss should be confined to the preconception and postpartum periods, but still had these two questions:
To review:
- Some degree of nausea with or without vomiting occurs in most pregnancies, typically with onset at five to six weeks of gestation, then peaking at nine weeks, and usually abating by 16 to 18 weeks of gestation. Hyperemesis gravidarum represents the severe end of the spectrum of symptoms.
- The diagnosis of hyperemesis gravidarum is made clinically in a woman with onset of persistent vomiting accompanied by weight loss exceeding 5 percent of prepregnancy body weight and ketonuria in the first trimester, unrelated to other causes.
- The standard initial evaluation of pregnant women with persistent vomiting includes measurement of weight, orthostatic blood pressures, serum free T4 concentration, serum electrolytes, urine ketones, and an ultrasound examination to exclude gestational trophoblastic disease and multiple gestation.
#1 What are some of the treatment options for this patient?
Here are some basic treatment recommendations
- Women who are significantly dehydrated should receive intravenous fluids. We suggest a short period of gut rest during hydration, followed by reintroduction of oral intake with liquids and low fat foods.
- Women should try to become aware of and avoid environmental triggers and foods which might provoke their nausea and vomiting. Acupuncture and acupressure have not been shown to significantly reduce nausea and vomiting. However, given the absence of harm and the strong placebo effect, some patients may benefit from a trial of acupressure wrist bands. Ginger also appears to have beneficial effects and ginger containing foods, such as ginger lollipops, may be helpful in women with mild nausea and vomiting.
- Where available, pyridoxine-doxylamine succinate combination therapy is recommended for initial pharmacologic treatment of hyperemesis gravidarum. If this drug is not available, we suggest pyridoxine, adding doxylamine succinate if pyridoxine alone is not effective.
- If nausea and vomiting persist, promethazine is also suggested.
- Corticosteroids are not recommended for treatment of hyperemesis.
#2 If conservative measures are successful, does a PICC line facilitate treatment of hyperemesis gravidarum?
No. In fact, it may be dangerous. In Holmgren et al, 42 women hospitalized with hyperemesis gravidarum (HEG) were assigned to treatment with medication alone, 33 to a peripherally inserted central catheter (PICC) line, and 19 to a nasogastric (NG) or nasoduodenal (ND) tube. Of those managed with a PICC line, 66.4% (P<.001) required treatment for infection, thromboembolism, or both. In addition, neonatal complications including small for gestational age (SGA), admission to neonatal intensive care, termination of pregnancy because of HEG, and fetal loss were increased in the women who had a PICC. A complete discussion of Holmgren et al can be found in this month’s Abstract of the Month.
Have you taken advantage of the free CME we offer on this topic? If not, this next part is definitely low hanging fruit. Just go to this module, review the material, answer a few quick questions, hit the submit button and voila free CME credits…..plus the module offers a great set of materials for future reference.
Nausea and Vomiting in Pregnancy
http://www.ihs.gov/MedicalPrograms/MCH/M/PNC/NVP01.cfm
Holmgren C, Aagaard-Tillery KM, Silver RM, Porter TF, Varner M. Hyperemesis
in pregnancy: an evaluation of treatment strategies with maternal and neonatal
outcomes. Am J Obstet Gynecol. 2008;198:56.e1–56.e4
http://www.ncbi.nlm.nih.gov/pubmed/18166306
Medscape*
A sample of recent free CME offerings on Medscape:
Female Sexual Dysfunction: A Clinical Update
http://www.medscape.com/viewprogram/14727
Statement from USPSTF: Screening for Iron Deficiency Anemia -- Including
Iron Supplementation for Children and Pregnant Women
http://cme.medscape.com/viewprogram/7033
Antibiotic Prophylaxis Reduces Risk for Postpartum Perineal Wound Complications
http://cme.medscape.com/viewarticle/575875
Uncovering Rheumatoid Arthritis: A Diagnosis and Co-Management Case in Primary
Care
http://cme.medscape.com/viewprogram/12670
Management of Herpes Simplex Infections Reviewed
http://cme.medscape.com/viewarticle/575879
Guidelines Issued on Pertussis, Tetanus, Diphtheria Prevention in Pregnant
Women and Newborns
http://cme.medscape.com/viewarticle/574587
Adult Asthma: Individualizing and Optimizing Care
http://cme.medscape.com/viewprogram/14649
General Information about Medscape:
Ask the Experts topics in Women's Health and OB/GYN Index, by specialty,
Medscape
http://www.medscape.com/pages/editorial/public/ate/index-womenshealth
OB GYN & Women's Health Clinical Discussion Board Index, Medscape
http://boards.medscape.com/forums?14@@.ee6e57b
Clinical Discussion Board Index, Medscape
Hundreds of ongoing clinical discussions
available
http://boards.medscape.com/forums?14@@.ee6e57b
Free CME: MedScape CME Index by specialty
http://www.medscape.com/cmecenterdirectory/Default
*NB: Medscape is free to all, but registration is required. It can be accessed from anywhere with Internet access. You just need to create a personal username and password.
Menopause Management
Low-Dose Estradiol Spray to Treat Vasomotor Symptoms
OBJECTIVE: To investigate the safety and efficacy of a transdermal estradiol (E2) spray in women with postmenopausal vasomotor symptoms.
METHOD: A randomized, double-blind, placebo-controlled, multicenter, parallel-group clinical trial was conducted. Postmenopausal women (N=454) with at least eight moderate-to-severe hot flushes per day applied daily, one, two, or three E2 (90 microliter spray contains 1.53 mg E2) or matching placebo sprays. The primary efficacy endpoints were mean change from baseline in frequency and severity of moderate-to-severe hot flushes at weeks 4 and 12.
RESULTS: All three E2 groups showed a significant decrease in hot flushes at weeks 4 and 12 compared with their placebo groups (P<.010). The mean change in frequency at week 12 was eight fewer flushes per day for women in the E2 groups and between four and six fewer flushes for women in the placebo groups. Women in the three- and two-E2 spray groups demonstrated significant (P<.050) reductions in severity score at weeks 4 and 12; women in the one-spray group showed significant reductions at week 5. At week 12, the majority (74-85%) of women on E2 showed at least a 50% hot flush frequency reduction as compared with 46% in the placebo group. The systemic E2 delivery rates at week 12 were approximately 0.021 mg/d, 0.029 mg/d, and 0.040 mg/d for the one-, two-, and three-spray doses, respectively. Common adverse events were similar to those previously reported with other transdermal products. Treatment-related application site reaction rate was similar to placebo (1.3% compared with 1.8%).
CONCLUSION: The three dose levels of E2 spray achieved efficacy at 0.021-0.040 mg/d delivery rates. The spray is a well-tolerated, new, convenient method of delivering low-dose E2 transdermally.
Buster JE, Koltun WD, Pascual ML, Day WW, Peterson C. Low-Dose Estradiol Spray to Treat Vasomotor Symptoms: A Randomized Controlled Trial. Obstet Gynecol. 2008 Jun;111(6):1343-1351. http://www.ncbi.nlm.nih.gov/pubmed/18515518
Midwives Corner - Lisa Allee, CNM, 4 Corners Regional Health Center, Red Mesa, AZ
ACNM Four Corners Chapter is sponsoring Centering Pregnancy Training
September 6 & 7, 2008 in Chinle!
This event is being paid for by a grant from the March of Dimes to support Centering Pregnancy on the Navajo Nation and, therefore, will be FREE to all who would like to provide Centering Pregnancy prenatal care to women and families on the Navajo Nation. Please try to assemble a team of people from your site including midwives, nurses, nurses’ aides, physicians, other potential group co-facilitators, administrators who can support a Centering program, support staff who will be involved in promoting Centering and scheduling patients in groups, etc. Think big picture. The training is limited to 30 participants, so please register your team early!
This training is fun and empowering for those who attend and then that fun and empowerment ripples out to the women and families who are blessed with the opportunity to participate in Centering groups. Come and join in this amazing, powerful form of care!
For more information, contact Lisa Allee, CNM at lisa.allee@ihs.gov.
Navajo News - Jean Howe, Chinle
Forty one (41) Indian Health Care Providers complete SANE/SAFE course in Window Rock
Chinle Service Unit recently sponsored a successful SANE/SAFE course at the Navajo Nation Museum June 9-13, 2008. The course instructor was Diana Faugno, RN, MSN, CNP, who provides this SANE/SAFE education across the country. The course focused on forensic medical examination techniques for adults and adolescent survivor of sexual assault. The course also provided the licensed health care providers with the didactic training and resources needed for the certification as sexual assault nurse examiner or sexual assault forensic examiner.
There were 41 participates in Window Rock, Arizona with representatives from the various service units from across the different Indian Health Systems, Navajo, Phoenix, Aberdeen and Billings Area. There were presentations from the Navajo Police Department and Dine Nation Prosecutor’s Office, U.S. Attorney’s Office from Phoenix, Chinle’s Victim Advocate’s Office and Crime Lab who provided protocols, policy and procedures from their offices regarding sexual assault and how the SANE/SAFE providers can best facilitate forensic evidences through the examination.
Nurses Corner - Sandra Haldane, HQE
What Is Forensic Nursing?
Forensic Nursing is the application of nursing science to public or legal proceedings; the application of the forensic aspects of health care combined with the bio-psycho-social education of the registered nurse in the scientific investigation and treatment of trauma and/or death of victims and perpetrators of abuse, violence, criminal activity and traumatic accidents.
The forensic nurse provides direct services to individual clients, consultation services to nursing, medical and law related agencies, and expert court testimony in areas dealing with trauma and/or questioned death investigative processes, adequacy of services delivery, and specialized diagnoses of specific conditions as related to nursing.
The above is excerpted from the International Association of Forensic Nurses web site.
For more information about how to become a Sexual Assault Nurse Examiner (SANE) visit the IAFN web site at: http://www.iafn.org/index.cfm .
Need technical assistance? Visit the Sexual Assault Forensic Examiner Technical Assistance web site at http://www.safeta.org/.
Office of Women's Health, CDC
Guiding Principles for Development of ACIP Recommendations for Vaccination During Pregnancy and Breastfeeding
This document provides guidance to help standardize procedures for policy formulation and presentation of the rationale and recommendations for vaccination of pregnant and breastfeeding women. Topics in Guiding Principles include 1) guidance for structure of the background section, 2) guidance for structure and language of recommendations, 3) clarification of the definitions of precautions and contraindications in the context of pregnant and breastfeeding women, 4) suggestions for approaches to policy decision-making in the absence of adequate data, and 5) description of a consistent process to gather expert opinion. These principles will be applied to future ACIP vaccine statements and routine updates of existing statements in which vaccination of pregnant and breastfeeding women is considered.
Text version - http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5721a3.htm
PDF version (p. 580) - http://www.cdc.gov/mmwr/PDF/wk/mm5721.pdf
Healthy Eating Index (HEI) Scores Among Adults, 60 Years of Age and Over, by Sociodemographic and Health Characteristics: United States, 1999–2002
Seventy-two percent of older adults met the guidelines for cholesterol intake and 56% met the recommendation for diet variety, but less than one-third met the recommendations for HEI’s five food groups. Only 17% of older adults consumed a ‘‘good’’ quality diet. Males had higher scores for some components, but females had higher scores for others. Females with a BMI of 30 or higher ate fewer servings of dairy products, consumed a higher percentage of calories from total and saturated fat, and had a lower quality diet than those whose BMI was less than 30. http://www.cdc.gov/nchs/data/ad/ad395.pdf
HIV/AIDS Surveillance Slide Sets
Slides focusing on HIV/AIDS in women (estimated number and proportion of AIDS cases, AIDS rates and cases, those living with HIV infection and AIDS, by transmission category, by race and ethnicity, age at diagnosis, diagnosis rates, etc); general epidemiology; race and ethnicity, trends, and adolescents and young adults.
Women - http://www.cdc.gov/hiv/topics/surveillance/resources/slides/women/index.htm
General Epidemiology - http://www.cdc.gov/hiv/topics/surveillance/resources/slides/epidemiology/index.htm
Race and Ethnicity - http://www.cdc.gov/hiv/topics/surveillance/resources/slides/race-ethnicity/index.htm
Trends - http://www.cdc.gov/hiv/topics/surveillance/resources/slides/trends/index.htm
Adolescents and Young Adults - http://www.cdc.gov/hiv/topics/surveillance/resources/slides/adolescents/index.htm
Visit the CDC Office of Women’s Health website at www.cdc.gov/women for information on these topics, including how to sign up for their monthly newsletter with links to women’s health articles, conference and grant announcements and other useful information.Oklahoma Perspective Greggory Woitte – Hastings Indian Medical Center
ACOG Committee on Indian Affairs visits 3 Oklahoma Area Sites
The ACOG Committee on Indian Affairs visited 3 sites in Oklahoma Area (Claremore, Tahlequah, and Talihina) in early June. The ACOG Committee has a longstanding relationship with Indian Health Service and performs an annual site visit to a one region on a rotating basis to assess care provided to Native Women, share best practices, and make suggestions for improvement.
Learn more about the Oklahoma Area
Osteoporosis
New evidence that Calcium supplementation may work, at least while you take them…
BACKGROUND: The effect of supplementation with calcium alone on risk fractures in a healthy population is not clear.
OBJECTIVE: The objective was to determine whether 4 y of calcium supplementation would reduce the fracture risk during treatment and subsequent follow-up in a randomized placebo-controlled trial.
DESIGN: The participants were aged <80 y at study entry (mean age: 61 y), were generally healthy, and had a recent diagnosis of colorectal adenoma. A total of 930 participants (72% men; mean age: 61 y) were randomly assigned to receive 4 y of treatment with 3 g CaCO(3) (1200 mg elemental Ca) daily or placebo and were followed for a mean of 10.8 y. The primary outcomes of this analysis were all fractures and minimal trauma fractures (caused by a fall from standing height or lower while sitting, standing, or walking).
RESULTS: There were 46 fractures (15 from minimal trauma) in 464 participants in the calcium group and 54 (29 from minimal trauma) in 466 participants in the placebo group. The overall risk of fracture differed significantly between groups during the treatment phase [hazard ratio (HR): 0.28; 95% CI: 0.09, 0.85], but not during the subsequent posttreatment follow-up (HR: 1.10; 95% CI: 0.71, 1.69). Minimal trauma fractures were also less frequent in the calcium group during treatment (HR: 0; 95% CI: 0, 0.50).
CONCLUSION: Calcium supplementation reduced the risk of all fractures and of minimal trauma fractures among healthy individuals. The benefit appeared to dissipate after treatment was stopped.
Bischoff-Ferrari HA , Rees JR , Grau MV , Barry E , Gui J , Baron JA . Effect of calcium supplementation on fracture risk: a double-blind randomized controlled trial. Am J Clin Nutr. 2008 Jun;87(6):1945-51. http://www.ncbi.nlm.nih.gov/pubmed/18541589
Patient Information
Food Allergies
http://www.aafp.org/afp/20080615/1687ph.html
Nutrition, Physical Activity, and Cancer: What You Should Know
http://www.aafp.org/afp/20080601/1579ph.html
Vaccines for Your Child
http://www.aafp.org/afp/20080601/1571ph.html
Perinatology Picks - George Gilson, Maternal Fetal Medicine, ANMC
Longer PROM associated with more infections
OBJECTIVE: This study was undertaken to define the time thresholds of increased risk for infectious maternal morbidities with relationship to length of ruptured membranes at term.
STUDY DESIGN: We designed a retrospective cohort study of all women with premature rupture of membranes beyond 37 weeks' gestation at a single institution. Dichotomized time thresholds of length of ruptured membranes before delivery were examined in 2-hour increments using bivariate and multivariable analyses to assess the rates of chorioamnionitis and endomyometritis. RESULTS: Among the 3841 women meeting inclusion criteria, increased rates of chorioamnionitis and endomyometritis were noted at time thresholds of 12 hours (adjusted odds ratio 2.3 [95% confidence interval, 1.2-4.4]) and 16 hours (adjusted odds ratio 2.5 [95% confidence interval, 1.1-5.6]), respectively.
CONCLUSION: We found that when length of time of ruptured membranes before delivery is examined via dichotomized time thresholds, the risks of chorioamnionitis and endomyometritis are significantly increased at 12 hours and 16 hours, respectively. These time thresholds derived from dichotomized time analyses should be considered during risk-based counseling and labor management in the setting of term premature rupture of membranes.
Tran SH , Cheng YW , Kaimal AJ . Caughey AB . Length of rupture of membranes in the setting of premature rupture of membranes at term and infectious maternal morbidity. Am J Obstet Gynecol. 2008 Jun;198(6):700.e1-5. http://www.ncbi.nlm.nih.gov/pubmed/18538159
Cesarean Delivery does not improve outcome in very low birthweight vertex fetuses
OBJECTIVE: This study was undertaken to compare neonatal outcome by method of delivery in very low-birthweight less than 1500 g vertex-presenting fetuses.
STUDY DESIGN: A retrospective cohort was conducted of 2466 very low-birthweight singleton liveborn vertex-presenting fetuses in Washington State (1994-2003). The exposure considered was cesarean delivery vs vaginal delivery. The risk of neonatal demise was estimated by logistic regression. Secondary outcomes included intraventricular hemorrhage, respiratory distress, and neonatal sepsis. Analyses were stratified by birthweight, gestational age, and growth restriction to assess subgroup differences.
RESULTS: Cesarean delivery offered no survival advantage to very low-birthweight infants when compared with vaginal delivery (adjusted odds ratio [95% confidence interval]: 1.08 [0.78-1.49]). Survival benefit was noted for growth-restricted infants (adjusted odds ratio [95% confidence interval]: 0.09 [0.02-0.47]) although only 12% of such infants delivered vaginally.
CONCLUSION: For very low-birthweight vertex-presenting fetuses at risk of preterm delivery, cesarean delivery does not improve neonatal survival. Further studies are warranted to assess the potential benefit of cesarean delivery to growth-restricted very low-birthweight infants.
Wylie BJ , Davidson LL, Batra M, Reed SD. Method of delivery and neonatal outcome in very low-birthweight vertex-presenting fetuses. Am J Obstet Gynecol. 2008 Jun;198(6):640.e1-7; discussion e1-4. Epub 2008 Mar 7. http://www.ncbi.nlm.nih.gov/pubmed/18313634
Clostridium difficile-associated diarrhea: an emerging threat to pregnant women
OBJECTIVE: To estimate if Clostridium difficile-associated disease (CDAD) is increasing in peripartum women.
STUDY DESIGN: Peripartum CDAD was assessed through 1) passive surveillance collecting clinical and pathology data on severe cases and 2) survey among infectious disease consultants (ICDs) in the Emerging Infections Network.
RESULTS: Ten severe cases were collected; most had associated antibiotic use. Seven women were either admitted to the ICU or underwent colectomy. Three infants were stillborn, and 3 women died. The epidemic Clostridium difficile strain was found in 2 cases. Among 798 ICDs, 419 (52%) participated in the survey. Thirty-seven respondents (9%) recalled 55 cases, mostly in the postpartum period with 21 complications, mainly due to relapse.
CONCLUSION: Severe CDAD may be increasing in peripartum women. Clinicians should have a low threshold for testing, be aware of the potential for severe outcomes, and take steps to reduce both the risk of disease and resultant complications.
Rouphael NG , O'Donnell JA, Bhatnagar J, Lewis F, Polgreen PM, Beekmann S, Guarner J, Killgore GE, Coffman B, Campbell J, Zaki SR, McDonald LC. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008 Jun;198(6):635.e1-6. Epub 2008 Apr 8. http://www.ncbi.nlm.nih.gov/pubmed/18395693
Mycophenolate mofetil (MMF) may be linked to congenital anomalies
FDA is aware of reports of infants born with serious congenital anomalies,
including microtia and cleft lip and palate, following exposure to mycophenolate
mofetil (MMF) during pregnancy. MMF, the active drug substance in CellCept, is
an ester of the active metabolite mycophenolic acid (MPA), the active drug substance
in Myfortic. In most cases, the mothers were taking MMF following an organ transplant
to prevent organ rejection. However, some mothers taking MMF were being treated
for immune-mediated conditions such as systemic lupus erythematosus (SLE) and
erythema multiforme. Treatment began before their pregnancies and continued into
the first trimester or until the pregnancy was detected. MMF and MPA increase
the risk of spontaneous abortion in the first trimester and can cause congenital
malformations in the offspring of women who are treated during pregnancy.
FDA is continuing to work with the manufacturers of these drug products to develop
and implement means to mitigate the risks of fetal exposure. See the FDA Healthcare
Professional Information Sheet containing considerations and recommendations
for clinicians prior to prescribing MMF or MPA to women of childbearing potential.
Primary Care Discussion Forum - Ann Bullock, Cherokee, NC
How to subscribe / unsubscribe to the Primary Care Discussion Forum?
Subscribe to the Primary Care listserv
http://www.ihs.gov/cio/listserver/index.cfm?module=list&option=list&num=46&startrow=51
Unsubscribe from the Primary Care listserv
http://www.ihs.gov/cio/listserver/index.cfm?module=list&option=list&num=46&startrow=51
Questions on how to subscribe, contact ANNBULL@nc-cherokee.com
STD Corner - Lori de Ravello, National IHS STD Program
Chlamydia Positivity in American Indian/Alaska Native Women Screened in Family Planning Clinics, 1997-2004
BACKGROUND: Previous studies demonstrated high levels of Chlamydia trachomatis (CT) infections within American Indian/Alaskan Native (AI/AN) populations but there are few analyses of CT prevalence in these populations over time.
METHODS: We analyzed data from 7374 visits at which diagnostic tests for CT were collected in AI/AN women aged 15 to 24 years seen at family planning clinics associated with the Region X Infertility Prevention Project. Trends in population characteristics and test positivity were examined and compared with non-AI/AN women tested in the same setting and time period. Chlamydia positivity was adjusted for changes in diagnostic test type. Multivariable logistic regression was used to identify characteristics associated with infection.
RESULTS: Adjusted CT positivity in AI/AN women rose from 7.8% to 11.0%, which was 1.5 to 2.2 times the non-AI/AN population levels over the study period (absolute difference 2.8%-6.6%). Differences persisted after correction for test type and age. Temporal changes in positivity among AI/AN women were associated with a rise in reported risk behaviors and decline in age of the population being tested. Risk factors associated with positivity among AI/AN women were younger age, >=1 behavioral risks, >=1 clinical findings, partner with chlamydia, chlamydia in past year, and pregnancy related visit.
CONCLUSIONS: AI/AN women had consistently higher levels of chlamydia positivity than non-Native women, even after adjustment for age and diagnostic test. Further investigation of risks for chlamydia, related outcomes, access to screening, sexual networks, and enhanced surveillance would be beneficial for improving health in this vulnerable population.
Gorgos L, Fine D, Marrazzo J. Chlamydia Positivity in American Indian/Alaska Native Women Screened in Family Planning Clinics, 1997-2004. Sex Transm. Dis. 2008 Apr 29. [Epub ahead of print] http://www.ncbi.nlm.nih.gov/pubmed/18449068
Barbara Stillwater, Alaska State Diabetes Program
Stretching exercises may reduce risk of pre-eclampsia during pregnancy
Stretching exercises may be more effective at reducing the risk of pre-eclampsia than walking is for pregnant women who have already experienced the condition and who do not follow a workout routine, according to researchers at the University of North Carolina at Chapel Hill School of Nursing.
OBJECTIVES: To compare a walking exercise to a stretching exercise during pregnancy in high-risk women who were sedentary and had previously experienced pre-eclampsia.
METHODS: A randomized clinical trial of the effects of the two types of physical exercises was conducted between November 2001 and July 2006 in Washtenaw County, Michigan. Both groups engaged in the assigned exercise five times a week until the end of pregnancy.
RESULTS: Women were randomized to either the walking group (n = 41) or the stretching group (n = 38). The walkers exercised an average of 36 (SD, 6) minutes at 18 weeks gestation, 34 (SD, 7) minutes at 28 weeks gestation, and 31 (SD, 12) minutes at the last week of the intervention. On average, they exercised within target heart rate ranges 35% (SD, 32%) at 18 weeks gestation, 22% (SD, 25%) at 28 weeks gestation, and 17% (SD, 25%) at the last week of the intervention. The stretching group engaged in stretching exercises following a 40-minutes videotape. On average, the walking group exercised 4 (SD, 1) times a week at 18 weeks gestation, 4 (SD, 1) time a week at 28 weeks gestation, and 3 (SD, 1) times a week at the last week of the intervention. Equally on average, the stretching group exercised 4 (SD, 2) times a week at 18 weeks gestation, 5 (SD, 1) times a week at 28 weeks gestation, and 3 (SD, 1) times a week at the last week of the intervention. No difference between groups was observed, but both exercised significantly less frequently over the time (p 0.0001). Together, participants reported average 7,040 (SD, 2,612) steps at the beginning and 5,711 (SD, 2,739) steps at the end of the study. The walkers tracked an average 8,501 (SE, 778) steps a day at 20 weeks gestation and 7,418 (SE, 788) steps at 34 weeks gestation (n.s.). The stretchers tracked an average 6,189 (SE, 704) steps at 20 weeks gestation and 4,848 (SE, 452) steps at 34 weeks gestation (p 0.05). The incidence of pre-eclampsia was 14.6% (95% CI, 5.6 to 29.2) among the walkers and 2.6% (95% CI; 0.07 to 13.8) among the stretchers. The incidence of gestational hypertension was 22 % (95% C.I., 8.7 to 35.2) for the walkers and 40% (95% CI, 23.2 to 55.8) for the stretchers. The mean transferrin level, an antioxidant marker, was significantly higher in the stretching group mean (412 mg/dL, 95%CI, 389 to 435) than the walkers at the time of labor (mean = 368 mg/dL, 95%CI, 346 to 391) (p 0.05). No significant group differences were observed in birth outcomes.
CONCLUSION: Regular stretching exercises may promote endogenous antioxidants among women at risk for pre-eclampsia.
Yeo S , Davidge S , Ronis DL , Antonakos CL , Hayashi R , O'Leary S . A comparison of walking versus stretching exercises to reduce the incidence of preeclampsia: a randomized clinical trial. Hypertens Pregnancy. 2008;27(2):113-30. http://www.ncbi.nlm.nih.gov/pubmed/18504873
Women's Health Headlines, Carolyn Aoyama, HQE
Screening for Abuse May Be Key to Ending It
This New York Times article reviews how screening for domestic violence is different from other routine care, such as pap smears…but just as important…
http://www.nytimes.com/2008/05/20/health/20abus.html?ref=health
CDC Women’s Health Resources for Health Professionals
Video: Women’s Health Resources from the CDC
This three-minute video highlights resources that health professionals and others
can use in their clinic, practice, organization, or community. www.cdc.gov/women/videopubs
Ways to Help Improve Women's Health
What more can you do to help improve women's health? Prevention is key to keeping women healthy and safe. www.cdc.gov/women/improve
Campaigns and Programs
Learn more about selected CDC campaigns and programs that focus
on women’s
or girls’ health.
www.cdc.gov/women/campaigns
Continuing Education
Save the dates
Sexual Assault Nurse Examiner/Forensic Examiner (SANE/SAFE) Training Course
- July 21-25, 2008
- Aberdeen , South Dakota
- 40 hour didactic portion of SANE/ SAFE training
- For additional information contact Lisa Palucci, lisa.palucci@ihs.gov, at the IHS Clinical Support Center
Community Health Representative National Educational Meeting
- July 29-31, 2008
- Las Vegas , Nevada
- 40 th Anniversary of the CHR Program
- www.nachr.net/conferences/2008/
Sexual Assault Nurse Examiner/Forensic Examiner (SANE/SAFE) Training Course
- August 18-22 , 2008
- Oklahoma City , Oklahoma
- 40 hour didactic portion of SANE/ SAFE training
- For additional information contact Lisa Palucci, lisa.palucci@ihs.gov, at the IHS Clinical Support Center
Postgraduate Course on Obstetric, Neonatal and Gynecologic Care
- September 14-18, 2008
- Salt Lake City , Utah
- Comprehensive Women’s Health Update for Nurses, Advanced Practice Nurses, and Physicians
- NRP offered as pre-conference session
- Contact Yvonne Malloy, ymalloy@acog.org, for more information
International Indiginous Women’s and Children’s Health Meeting
- March 4-8, 209
- Albuquerque , NM
- Joint conference of Women’s Health and Children’s Health Providers from Canada and the United States
What's new on the ITU MCH web pages?
2007 Native Women’s Health and MCH Conference Meeting Notes
There are several upcoming Conferences
and Online CME/CEU resources, etc….
and the latest Perinatology Corners (free online CME from IHS)
…or just take a look at the What’s New page
Did you miss something in the last OB/GYN Chief Clinical Consultant Corner?
The June 2008 OB/GYN CCC Corner is available.
Hot Topics ‹ Previous
OB/GYN
Dr. Neil Murphy is the Obstetrics and Gynecology Chief Clinical Consultant (OB/GYN C.C.C.). Dr. Murphy is very interested in establishing a dialogue and/or networking with anyone involved in women's health or maternal child health, especially as it applies to Native or indigenous peoples around the world. Please don't hesitate to contact him by e-mail or phone at 907-729-3154.

