|
Chapter 13b
Electrophoretic and Immunoblot
Analysis of Staphylococcal Enterotoxins in Food
![]()
Immunoblotting can detect Staphylococcus aureus enterotoxin A in food. The method may also be adapted to other toxins in foods.
Staphylococcal enterotoxins (SE), a family of five major serological types of heat stable (1,6,9,11,18, 19,20, 21), emetic enterotoxins (SEA through SEE), are encoded by five genes, which share 50 to 85% homology at the predicted amino acid level (5,12). Enterotoxin A (SEA), a 27 kDa monomeric protein, is an extremely potent gastrointestinal toxin (2,7)and requires very sensitive methods to detect the low levels in foods (ng/g food) that can cause illness.
After antibodies to SEA were produced, immunological testing became the method of choice for SEA detection (4). Radioimmunoassay (13), microslide double diffusion and enzyme-linked immunosorbent assay (ELISA), have been used for testing food samples. ELISA is especially useful, because it is simple, sensitive (0.5 ng/ml), rapid, and available in commercial kits that use distinct antibodies, either polyclonal or monoclonal.
Cross-reaction with unrelated antigens (15,16) or endogenous peroxides in particular foods that react with colorigenic reagents may not be distinguishable from positive results by some methods without extensive controls (17). In addition, heat-treated SEA (in heat processed foods) may give negative results, because heat-treated enterotoxin may aggregate, reducing its reactivity with antibodies. However, it may retain toxicity after heat treatment (1,3).
Methods for analysis of regulatory samples of foods must resolve or avoid "false positive" and "false negative" reactions. Before antibody is applied, the SDS-PAGE immunoblot method, described below, solubilizes and separates proteins, to discriminate cross reactions to heterologous proteins that may occur.
General Principle:
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) is a common method for protein separation (10,14). An electrical field is applied so that charged molecules migrate through a polyacrylamide matrix to the electrode bearing the opposite charge. The negatively-charged detergent, SDS, denatures and strongly binds proteins. Then, SDS-bound proteins migrate to the positive pole at rates inversely proportional to their molecular weights.
In general, two-part discontinuous gels are used (10). The sample is loaded onto the upper portion (stacking gel), which has a low acrylamide concentration, low pH, and low resolving ability. When a sample runs through the stacking gel, all proteins are concentrated into a narrow band. That narrow band then enters the lower portion (resolving gel) that separates proteins by size. The acrylamide concentration chosen for the resolving gel depends on the sizes of proteins to be separated. Smaller proteins are resolved at higher acrylamide concentrations and vice versa. SEs are 25-30 kDa; 12.5% acrylamide is useful for separating proteins in that range.
Immunoblotting (also known as "Western" blotting) is widely used for analyzing proteins separated by SDS-PAGE. The proteins are transferred from the gel to a membrane. Then, the membrane is probed with an antibody ("primary antibody") against the specific antigen. To detect the antibody-antigen complex, a secondary antibody is used. Usually, this is a polyclonal antibody (e.g. anti-mouse if the primary antibody is a mouse monoclonal) tagged with a biochemically detectable marker. Some common secondary antibody tags are fluorescent molecules (e.g. FITC, rhodamine), horseradish peroxidase, alkaline phosphatase, or biotin. Then, simple colorimetric reactions are carried out to reveal the location of the complex in a band on the membrane at a position corresponding to the molecular weight of the antigen.
Immunoblots for food testing
Immunoblots have two important advantages for food testing. First, even though heat and other treatments during food processing can cause proteins to aggregate, the aggregates are solubilized and unfolded in SDS gels. Other antibody-based methods of food analysis, such as ELISA, do not have an SDS solubilization step. Instead, the sample is applied directly to the antibody, because SDS in the sample would denature the detecting antibody. Second, cross-reacting antigens usually can be distinguished from the desired antigen on the basis of molecular weight in a Western blot. In ELISA, and other assays in which samples are evaluated without separation or purification, cross-reacting antigens increase the background.
WARNING! ACRYLAMIDE IS NEUROTOXIC. ALWAYS WEAR GLOVES AND OTHER APPROPRIATE PROTECTION WHEN USING.
Commercially Prepared gels are available through suppliers (depending on apparatus).
Table 1. Lower (resolving) gel (for two 0.75mm gels)
| % Gel | 12.5% | 15% | 16% | 17% |
|---|---|---|---|---|
| Buffer A | 2.8 ml | 2.8 ml | 2.8 ml | 2.8 ml |
| H2O | 1.5 ml | 0.83 ml | 0.58 ml | 0.33 ml |
| 20% SDS | 50 µl | 50 µl | 50 µl | 50 µl |
| 10% APS | 30 µl | 30 µl | 30 µl | 30 µl |
| TEMED | 30 µl | 30 µl | 30 µl | 30 µl |
| Acrylamide solution | 3.1 ml | 3.75 ml | 4 ml | 4.25 ml |
| Total | 7.5 ml | |||
Table 2. Stacking gel (for two 0.75mm gels)
| % Gel | 4.5% |
| Buffer D | 0.625 ml |
| H2O | 1.5 ml |
| 20% SDS | 30 µl |
| 10% APS | 20 µl |
| TEMED | 20 µl |
| Acrylamide solution | 0.375 ml |
| Total | 2.57 ml |
EXAMPLES
Western blotting was tested for the ability to detect SEA in foods that are commonly associated with food poisoning. Each sample was homogenized, spiked with purified SEA (2 ng/40 µl), and applied directly to the gel.
SEA was detectable in each sample, regardless of which food was present (Fig. 1). Undiluted milk samples distorted SEA mobility (data not shown), but ten-fold diluted milk samples ran correctly. Heterologous antigens cross-reacted in several samples, because polyclonal anti-SEA antibodies reacted with components from the food matrix. For example, the antibodies recognized a 66 KDa protein in milk, whether or not SEA is present in milk (Lanes 2 and 3, Fig. 1). This unrelated band did not affect the assay for SEA, because SEA is determined by the intensity of the 27 kDa band, detected only in the "spiked" sample.

Figure 1. Western immunoblots of foods contaminated with SEA. Food samples were homogenized and spiked with purified SEA. The sample (40 µl) was then applied directly to the gel and assayed by Western blot. Milk, potato salad and meat product with or without SEA were tested. Lane 1 -- Protein Standards; Lane 2 -- milk; Lane 3 -- milk+SEA; Lane 4 -- potato salad; Lane 5 -- potato salad+SEA; Lane 6 -- meat; Lane 7 - meat+SEA.
Canned foods are problematic for ELISA because ELISA often fails to detect heat-treated SEA. Canned mushrooms were used to see if Western blots can detect heat-treated SEA in food.
The contents of a can of mushrooms (113 g in 500 ml flask) were inoculated with an overnight culture (106 cells /ml) of S. aureus (ATCC13565), then cells were grown for 6 h at 37C with shaking. Samples were taken hourly to measure bacterial growth and SEA production. Each sample was autoclaved at 121°C for 20 min to simulate canning and then assayed by Western blot. As shown in Figure 2. SEA was detected in contaminated mushrooms at 130 min (lane 3), at mid-log phase. Although there are additional cross-reacting bands, they have different molecular weights from SEA, and do not affect the analysis. There is no 27 kDa band in the uninfected control (lane 1).
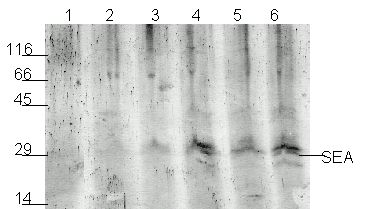
Figure 2. Detection of SEA produce by S. aureus (ATCC No. 13565) grown on mushrooms. Lane 1 -- sample after 0 min; Lane 2 -- 60 min; Lane 3 -- 130 min ; Lane 4 -- 180 min; Lane 5 -- 240 min; Lane 6 -- 300 min.
| Problem | Cause |
| Slow or no polymerization of the gel | APS is old, OR APS, TEMED or acrylamide were left out |
| No tracking dye observed | Wrong polarity |
| Smile effect or gel overheats | High voltage leads to excessive heat. |
| Sample floats in the well or diffuses out of well | Wrong concentration of glycerol in the loading dye |
| No transfer | Wrong polarity or problem with transfer buffer. |
| Transfer OK but no signal in the positive control, membrane turns purple | problem with antibodies. |
| Transfer OK but no signal in the positive control, membrane is colorless | problem with detection reagents |
| High background | problems with blocking. increase time and/or add 0.5%-1% non-fat dry milk to the Blocking Buffer |
| Diffuse /distorted marker bands | Too little SDS |
| Distorted SEA band in samples | Too much protein in sample (overloading). Dilute sample or use chromatography or immunoprecipitation to remove major proteins |
Limitations of Western blotting:
Western blotting has some limitations, which are important to recognize when applying the method to food analysis.
There are several ways to minimize this problem. One is to increase the specificity of the reaction by using monoclonal antibodies. Alternatively, several independently isolated antibodies and control samples of uncontaminated similar food can be used to determine whether the bands represent toxin or unrelated antigens.
| FDA ELECTROPHORETIC ANALYSIS REPORT | ||||||
| FDA lab: | name: | record: | date: | |||
| Sample: | ||||||
| Sample preparation: | ||||||
| Gel: % acrylamide | spacer mm | sample vol. ml | apparatus: | |||
| Running volts | time: min | remarks: | ||||
| Transfer mA | time: min | remarks: | ||||
| Blocking: | time: min | volume: ml | remarks: | |||
| Antibody 1: | time: min | volume: ml | supplier: | dilution: | ||
| Antibody 2: | time: min | volume: ml | supplier: | dilution: | ||
| Wash buffer: | vol : ml | wash#1: min | wash#2: min | wash#3: min | ||
| Developing: | vol: ml | time min | Background: | |||
| Densitometry: | resolution: dpm | setting: | ||||
| Image analysis: | software: | setting: | ||||
| Remarks: | ||||||
| Gel #1: | |||
| lane #1 | signal | lane#2 | signal |
| lane #3 | signal | lane#4 | signal |
| lane #5 | signal | lane#6 | signal |
| lane #7 | signal | lane#8 | signal |
| lane #9 | signal | lane#10 | signal |
| Gel #2: | |||
| lane #1 | signal | lane#2 | signal |
| lane #3 | signal | lane#4 | signal |
| lane #5 | signal | lane#6 | signal |
| lane #7 | signal | lane#8 | signal |
| lane #9 | signal | lane#10 | signal |
| Remarks: | |||
| Gel#1 | Gel#2 | ||
1. Anderson J.E., R.R. Beelman, and S. Doores 1986. Persistence of serological and biological activities of staphylococcal enterotoxin A in canned mushrooms. J. Food Protect. 59:1292-1299.
2. Archer, D.L., and F.E. Young. 1988. Contemporary issues: disease with a food vector. Clin. Microbiol. Rev. 1:377-398.
3. Bennett, R.W. 1992. The biomolecular temperment of staphylococcal enterotoxin in thermally processed food J. Assoc. Off. Agric. Chem. 75:6-12.
4. Bergdoll, M.S., M.J. Suargalla, and G.M. Dack. 1959. Staphylococcal enterotoxin. Identification of specific precipitating antibody with enterotoxin-neutralizing property. J. Immunol. 83:334-338.
5. Bergdoll, M.S. 1972. The enterotoxin, In: The Staphylococci ed. Jay Cohen, Wiley Interscience, pp. 301-331.
6. Denny, C.B., J.Y. Humber, and C.W. Bohrer. 1971. Effect of toxin concentration on the heat inactivation of staphylococcal enterotoxin A in beef bouillon and in phosphate buffer. Appl Microbiol 21: 1064-1066
7. Evenson, M.I., M.W. Hinds, R.S. Bernstein, and M.S. Bergdoll. 1988. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int. J. Food Microbiol. 7:311-316.
8. Frieman, S.M., J.R. Tumang, and M.K. Crow. 1993. Microbial superantigens as etiopathogenic agents in autoimmunity. Rheum. Dis. Clin. North Am. 19:207-222.
9. Fung, D.Y., D.H. Steinberg, R.D. Miller, M.J. Kurantnick, and T.F. Murphy. 1973. Thermal inactivation of staphylococcal enterotoxins B and C. Appl Microbiol 26: 938-942.
10. Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680-685.
11. Lee I.C., K.E. Stevenson, and L.G. Harmon. 1977. Effect of beef broth protein on the thermal inactivation of staphylococcal enterotoxin B1. Appl Environ Microbiol 33: 341-344
12. Marrack P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711
13. Miller, R.A., R.F. Reiser, and S.M. Bergdoll. 1976. The detection of staphylococcal enterotoxin A, B, C, D, and E in food by radioimmunoassay, using staphylococcal cells containing protein A as immunosorbent. Appl. Environ. Microbiol. 36:421-426.
14. Orden, J. A., J. Goyache, and J. Hernandez, A. Domenech, G. Suarez, and E. Gomez-Lucia. 1992. Applicability of an immunoblot technique combined with a semiautomated electrophoresis system for detection of staphylococcal enterotoxins in food extracts. Appl Environ Microbiol 58: 4083-4085
15. Park, C. E., M. Akhtar, and M. K. Rayman. 1992. Nonspecific reactions of a commercial enzyme-linked immunosorbent assay kit (TECRA) for detection of staphylococcal enterotoxins in foods. Appl Environ Microbiol 58: 2509-2512
16. Park, C. E., M. Akhtar, and M. K. Rayman. 1993. Simple solutions to false-positive staphylococcal enterotoxin assays with seafood tested with an enzyme-linked immunosorbent assay kit (TECRA). Appl. Environ. Microbiol. 59:2210-2213.
17. Park, C. E., M. Akhtar, and M. K. Rayman. 1994. Evaluation of a commercial enzyme immunoassay kit (RIDASCREEN) for detection of staphylococcal enterotoxins A, B, C, D, and E in foods. Appl. Environ. Microbiol. 60:677-681.
18. Read, Jr., R.B., and J.G. Bradshaw .1966. Staphylococcal enterotoxin B thermal inactivation in milk. J Dairy Sci 49: 202-203.
19. Read Jr., R.B., and J.G. Bradshaw 1966. Thermal inactivation of staphylococcal enterotoxin B in veronal buffer. Appl Microbiol 14: 130-132.
20. Schwabe, M., S.Notermans, R. Boot, S.R. Tatini, and J. Kramer. 1990. Inactivation of staphylococcal enterotoxins by heat and reactivation by high pH treatment. Int. J. Food Microbiol. 10:33-42.
21. Tibana, A., K.M. Rayman, M. Akhtar, and R. Szabo. 1987. Thermal stability of staphylococcal enterotoxin A, B, and C in a buffered system. J. Food Protect. 50:239-242.
Hypertext Source: Detection of Staphylococcus aureus
Enterotoxin A in food by Western Electrophoretic and Immunoblot Analysis of Staphylococcal
Enterotoxins in Food, Bacteriological Analytical Manual, 8th Edition, Revision A, 1998. Chapter 13b.
Author: Avraham Rasooly
Hypertext updated by kwg 2001-MAR-20