PART D: SCIENCE BASE
Section 4: Fats
INTRODUCTION
Fats supply energy and essential fatty acids, and serve as a carrier for the
absorption of the fat-soluble vitamins A, D, E, and K, and carotenoids. Fats
are a source of antioxidants and numerous bioactive compounds and serve as building
blocks of membranes and play a key regulatory role in numerous biological functions.
Dietary fat is found in foods derived from both plants and animals.
Fats are composed of triglycerides that consist of fatty acids and glycerol.
Individual fatty acids have different biological effects ranging from modulating
clinical markers of disease risk to regulating many intracellular biological
mechanisms due to changes in intracellular signaling and gene expression (Clarke
SD, 2004). Fatty acids modulate lipid metabolism and other physiological systems
that affect risk factors for chronic diseases. Whether these effects on health
outcomes are beneficial or harmful depend on the specific fatty acids and the
mix of fatty acids in the diet and the body. Individual fatty acids are present
in foods as mixtures. Different foods are rich sources of specific fatty acids.
Fatty acids are classified on the basis of chain length, degree of saturation
(as defined by the number of double bonds in the molecule), and position of the
first double bond from the methyl terminus. The fatty acid classes are:
-
Saturated fatty acids—Saturated fatty acids have
no double bonds. They primarily come from animal products such as meat and
dairy products. In general, animal fats are solid at room temperature. Stearic
acid is a saturated fatty acid that has different biological effects than
other saturated fatty acids. Important food sources of stearic acid are beef,
hydrogenated/partially hydrogenated vegetable oils, and chocolate.
-
Monounsaturated fatty acids—Monounsaturated fatty
acids (MUFAs) have one double bond. Plant sources that are rich in MUFAs
include vegetable oils (e.g., canola oil, olive oil, high oleic safflower
and sunflower oils) that are liquid at room temperature and nuts.
-
Polyunsaturated fatty acids—Polyunsaturated fatty
acids (PUFAs) have two or more double bonds, and may be of two types, based
on the position of the first double bond:
n-6 PUFAs. Linoleic acid, one of the n-6 fatty acids,
is required but cannot be synthesized by humans and, therefore, is considered
essential in the diet. A lack of dietary n-6 PUFAs is characterized by rough,
scaly skin and dermatitis. Primary sources are liquid vegetable oils including
soybean oil, corn oil, and safflower oil. n-3 PUFAs. α-linolenic acid is an n-3 fatty acid that
is required because it is not synthesized by humans and, therefore, is considered
essential in the diet. A lack of α-linolenic acid in the diet can result in symptoms
of a deficiency including scaly and hemorrhagic dermatitis, hemorrhagic folliculitis
of the scalp, impaired wound healing, and growth retardation. It is obtained
from plant sources including soybean oil, canola oil, walnuts, and flaxseed.
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are n-3 fatty acids
that are contained in all fish and shellfish. Fish that naturally contain more
oil (e.g., salmon, tuna, trout) (which are called fish high in n-3-fatty acids
in this report) are higher in EPA and DHA than are lean fish (e.g., cod, haddock,
flounder).
-
Trans fatty acids—Trans
fatty acids are unsaturated fatty acids that contain
at least one double bond in the trans configuration.
The partial hydrogenation of polyunsaturated oils
causes isomerization of some of the remaining double
bonds and migration of others, resulting in an increase
in the trans
fatty acid content and the hardening of the oil. Elaidic
acid (t9-C18:1) is the predominant trans fatty
acid found in processed fats. Sources of trans fatty
acids include hydrogenated/partially hydrogenated vegetable
oils that are used to make shortening and commercially
prepared baked goods, snack foods, fried foods, and margarine.
With respect to trans fatty acids,
the descriptors "hydrogenated" and "partially hydrogenated"
are used interchangeably but convey the presence
of elaidic acid in the vegetable oil that has been subjected
to the hydrogenation process. For the sake of accuracy,
in oil that is fully hydrogenated (i.e.,
the unsaturated fatty acids have been converted to stearic
acid), there are no trans unsaturated
fatty acids. Thus, fats that are hydrogentated/partially
hydrogentated have variable amounts of trans fatty
acids depending on the extent of hydrogenation. Trans fatty
acids also are present in foods that come from ruminant
animals (e.g., cattle and
sheep). Such foods include dairy products, beef, and
lamb. The predominant naturally occurring trans fatty
acid is trans-vaccenic
acid (t11-C18:1). Conjugated linoleic acid (c9, t11-C18:2)
is derived from vaccenic acid and is found to a lesser
extent in foods from ruminant animals.
-
Cholesterol is a sterol present in all animal tissues. Free cholesterol
is a component of cell membranes and serves as a precursor for steroid hormones
including estrogen, testosterone, aldosterone, and bile acids. Humans are
able to synthesize sufficient cholesterol to meet biologic requirements,
and there is no evidence for a dietary requirement for cholesterol.
The Dietary Guidelines Advisory Committee (the Committee) placed a strong focus
on fats because of the substantial body of research linking different types of
fats to blood lipid values and heart health. Lipids and lipoproteins in the blood
historically have attracted much interest because of their functions in biological
events that underlie the prevention and progression of cardiovascular disease
(see Part B, "Introduction," for further information).
Back to Top
BLOOD LIPIDS
There are different types of lipids circulating in the blood; cholesterol and
triglycerides have been most intensively studied because of the diverse mechanisms
by which they modulate risk of cardiovascular disease. Cholesterol and triglycerides
are packaged into lipoprotein particles for transport in the circulation.
The composition and biological properties of the different lipoprotein fractions
varies markedly. The predominant lipoprotein particles are: chylomicrons, very-low
density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density
lipoproteins (HDL).
-
Cholesterol is transported in the blood primarily by LDL, HDL,
and VLDL. Chylomicrons transport dietary cholesterol absorbed from
the intestine. Total serum cholesterol is the sum amount of cholesterol found
in lipoporoteins in the blood. A high total cholesterol concentration is
a risk factor for coronary heart disease (CHD).
-
Triglycerides are a naturally occurring ester of three fatty acids and glycerol.
They are the chief constituent of fats and oils and commonly circulate in
the blood in the form of lipoproteins, principally in chylomicrons and VLDL.
There is a positive relationship between serum triglyceride value and the
incidence of CHD. A high triglyceride level is one of the diagnostic criteria
for metabolic syndrome, a condition that increases risk of cardiovascular
disease. The high, and growing, prevalence of metabolic syndrome (1 in 4
individuals in the United States) has important public health implications
(Ford et al., 2002).
Blood Lipoproteins
-
Chylomicrons and VLDL are triglyceride-rich lipoproteins that transport
dietary and endogenous lipids through the circulation.
-
LDL transports about 60 to 70 percent of total serum cholesterol.
An increase in LDL cholesterol increases the risk of CHD. Lowering levels
of LDL cholesterol reduces the risk for CHD.
-
HDL carries approximately 20 to 30 percent of total serum cholesterol. A
high level of HDL cholesterol is associated with a reduced risk for CHD,
and may help prevent atherosclerosis.
Back to Top
OVERVIEW OF QUESTIONS ADDRESSED
This section addresses seven major questions related to different types of fat
and how they are related to health.
-
What are the relationships between total fat intake and health?
-
What are the relationships between saturated fat intake and health?
-
What are the relationships between trans fat intake and
health?
-
What are the relationships between cholesterol intake and cardiovascular
disease?
-
What are the relationships between n-6 PUFA intake and health?
-
What are the relationships between n-3 fatty acids and health?
-
What are the relationships between MUFA intake and health?
The general search strategies used to find the scientific evidence related to
each of these questions appears in Part C. Tables summarizing the findings were
prepared for Questions 1 (see Appendix G-3) and 5 (see Table D4-2). USDA's Center
for Nutrition Policy and Promotion conducted special analyses related to nutritional
effects of varying the percentages of total fat and of including more fish in
food intake patterns. Those analyses are described briefly under Questions 1
and 6, respectively, and in full in Appendix G-2. The Committee relied on findings
in the science-based report Dietary Reference Intakes for Energy, Carbohydrate,
Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (IOM, 2002) and
considered findings in the Third Report of the National Cholesterol Education
Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood
Cholesterol in Adults (Adult Treatment Panel III [ATP III]) (National Cholesterol
Education Program [NCEP], 2002) and the Department of Health and Human Services'
Agency for Health Care Policy and Research (AHRQ) report Effects of Omega-3 Fatty
Acids on Cardiovascular Disease (Wang et al., 2004).1
Back to Top
QUESTION 1: WHAT ARE THE RELATIONSHIPS BETWEEN TOTAL FAT INTAKE AND
HEALTH?
Conclusion
At low intakes of fat (< 20 percent of energy) and high intakes of carbohydrates
(>65 percent of energy), risk increases for inadequate intakes of vitamin
E, α-linolenic acid, and linoleic acid, and for adverse changes in HDL
cholesterol and triglycerides. At high intakes of fat (> 35 percent of energy),
the risk increases for obesity and CHD. This is because fat intakes that exceed
35 percent of energy are associated with both increased calorie and saturated
fat intakes. Total fat intake of 20 to 35 percent of calories is recommended
for adults and 25 to 35 percent for children age 4 to 18 years. A fat intake
of 30 to 35 percent of calories is recommended for children age 2 to 3 years.
Rationale
Overview. The conclusion regarding the recommended
range of total fat intake is based on the Institute of Medicine's (IOM's) Acceptable
Macronutrient Distribution Range (AMDR) of 20 percent to 35 percent of calories
from fat (IOM, 2002). As stated in Section 1, the Committee recommends that the
food guidance provided aim to achieve the most recent Recommended Dietary Allowances
(RDAs), Adequate Intakes (AIs), and AMDRs for all nutrients. Evidence concerning
the health effects of low- and high-fat intakes was obtained from the same IOM
report and from more recent publications identified by the Committee's literature
search.
The lower limit for fat intake is set at 20 percent of calories because serum
triacylglycerol concentrations increase and serum HDL cholesterol concentrations
decrease when fat intake is low and carbohydrate intake is high. This, in turn,
may increase the risk of CHD. Furthermore, it is difficult to achieve recommended
intakes of several nutrients when fat intake is below 20 percent of calories.
The upper limit on total fat intake is related to the saturated-fat content
of diets that provide more than 35 percent of calories from fat. Practical efforts
to create heart-healthy menus that provide more than 35 percent of energy from
total fat result in an unacceptably high content of saturated fatty acids. Because
saturated fatty acids are present in all fats, higher intakes of total fat are
associated with increased saturated fatty acid intakes. As discussed under Question
2, increasing the saturated fatty acid content of the diet increases the LDL
cholesterol concentration, which, in turn, increases the risk of CHD (IOM, 2002).
Other reasons for limiting total fat intake have been proposed: (1) diets with
more than 35 percent of energy from fat may increase the risk of caloric excess
and certain cancers such as breast and colorectal cancer; and (2) high-fat intakes
may promote a prothrombotic state, which may increase CHD risk. An association
between dietary fat intake and the risk for diabetes has been reported in some
epidemiologic studies, but this association may be confounded by various factors,
such as obesity (IOM, 2002).
Published Evidence. The IOM report Dietary Reference
Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein,
and Amino Acids (IOM, 2002) includes a systematic, extensive review of the
scientific literature regarding total fat and carbohydrate intake in relation
to weight change, blood lipid concentrations, and metabolic parameters for glucose
and insulin. Documentation relevant to the conclusions above is found in the
following tables:
-
11-1: Decreased Fat Intake and Body Weight Change in Non- or Moderately-Obese
Subjects
-
11-2: Fat and Carbohydrate Intake and Blood Lipid Concentrations in Healthy
Individuals
-
11-8: Interventional Studies on the Effect of Dietary Fat on the Metabolic
Parameters for Glucose and Insulin in Healthy Subjects
Evidence in Table 11-1 (IOM, 2002), which includes nine short-term and nine
long-term intervention studies, reports small losses in body weight with substantial
reductions (greater than 4 percentage points) in the percentage of energy consumed
as fat. The IOM report concludes that evidence suggests that low-fat diets (diets
with a low percentage of calories from fat) tend to be slightly hypocaloric compared
to higher fat diets in outpatient intervention trials. Data in Table 11-2 (IOM,
2002), which covers 14 intervention studies, demonstrate that decreasing fat
and increasing carbohydrate intake is associated with an increase in serum triacylglycerol
concentration and a decrease in plasma HDL cholesterol. Moreover, the reduction
in HDL cholesterol that is associated with a low fat intake results in a higher
total:HDL cholesterol ratio, which may increase the risk of CHD. Table 11-8 (IOM,
2002), which covers 13 intervention studies, reports a lack of definitive evidence
that higher fat intakes impair insulin sensitivity in humans. Collectively, the
evidence in these tables provides the rationale for the lower and upper range
for fat in the diet.
The conclusions were substantiated further by more recent publications that
reported on relationships between fat intake and the metabolic syndrome: five
clinical trials (Berrino et al., 2001; Larsson et al., 1999; Lovejoy et al.,
2001; Poppitt et al., 2002; Vessby et al., 2001), two reports from conferences
sponsored by the National Heart, Lung, and Blood Institute/American Heart Association
(Grundy, et al., 2004a), and the American Heart Association/National Heart, Lung,
and Blood Institute/American Diabetes Association (Grundy et al., 2004b), and
one review paper (Grundy et al., 2002). The evidence is convincing that better
weight control improves metabolic syndrome and that modest reductions in total
fat intake may facilitate both decreasing one's caloric intake and controlling
calories for weight control. For individuals with metabolic syndrome, an isocaloric
diet higher in total fat (30 to 35 percent of calories) with an emphasis on unsaturated
fatty acids has been shown to improve the clinical profile related to the atherogenic
dyslipidemia and insulin resistance.
Special Analyses. At the Committee's request, U.S.
Department of Agriculture's (USDA's) Center for Nutrition Policy and Promotion
used a modeling process described in Appendix G-2 to examine how changing the
percentage of calories from fat may affect the intake of other nutrients. Of
particular concern were intakes of the essential fatty acids (linoleic acid and
α-linolenic acid), protein, carbohydrates, added sugars, cholesterol, and vitamin
E. The analysis produced food patterns that showed the following:
-
At 20 percent of calories from fat, few food patterns met the AIs for both
linoleic acid and α-linolenic acid. At 25 percent of calories from fat, most
did; and at 30 percent and 35 percent, all did. In most cases, protein, fat
and carbohydrate percentages were within the AMDR.
-
At calorie levels of 2,600 or more, when only 20 percent of the calories
were supplied by fat, 66 to 68 percent of calories were supplied by carbohydrates.
To lower the carbohydrate provided by fruits, vegetables, and grains to be
consistent with the AMDR of 45 to 65 percent of calories from carbohydrate,
the proportion of calories from dietary protein could be increased.
-
At 35 percent of calories from fat, the menu modeling resulted in cholesterol
levels that were above the standard of 300 mg for energy intakes of 2,800
kcal or higher. This could pose a problem since increases in dietary cholesterol
increase LDL cholesterol, which, in turn, increases CHD risk. In a diet that
provides more than 30 percent of calories from fat, particular attention
must be paid to keeping dietary cholesterol intake at or below the recommended
limit (see Cholesterol).
-
The amount of vitamin E provided by the patterns consistently increased
with increases in the percentage of calories from fat, as well as with increases
in the energy content of the pattern. Vitamin E RDAs were met only at the
3,000 and 3,200 calorie levels.
A diet that provides 20 percent of calories from fat could be designed to meet
recommended intakes for vitamin E, linoleic, and α-linolenic acid by choosing
the foods that are better sources of these nutrients, e.g., certain liquid vegetable
oils. Exceptions might occur at the lower calorie levels (i.e., < 1,600 calories).
Positions Taken by Other Expert Groups. Using an evidence-based
approach, the National Cholesterol Education Program (NCEP) Expert Panel on Detection,
Evaluation, and Treatment of High Blood Cholesterol in Adults (2002) published
the following evidence statement and recommendation related to total fat:
Evidence Statement
The percentage of total fat in the diet, independent of caloric intake, has
not been documented to be related to body weight or risk for cancer in the general
population. Short-term studies suggest that very high fat intakes (>35 percent
calories from fat) modify metabolism in ways that could promote obesity. On the
other hand, very high carbohydrate intakes (>60 percent calories) aggravate
some of the lipid and non-lipid risk factors common in metabolic syndrome.
Recommendations
Dietary fat recommendations should emphasize a reduction in saturated fatty
acids. Furthermore, in individuals with lipid disorders or metabolic syndrome,
extremes of total fat intake – either high or low – should be avoided. In such
persons, total fat intakes should range from 25-35 percent of calories. For some
persons with the metabolic syndrome, a total fat intake of 30-35 percent may
reduce lipid and nonlipid risk factors.
(National Cholesterol Education Program Expert Panel, 2002, p. V-12)
The evidence of a relationship between total fat intake and certain cancers
is suggestive but not conclusive. The Department of Health and Human Services,
National Cancer Institute's PDQ® (Physician Data Query), published the following
evidence statements:
-
Colorectal cancer—Epidemiologic, experimental (animal), and clinical investigations
suggest that diets high in total fat [italics added], protein, calories,
alcohol, and meat (both red and white) and low in calcium and folate, are
associated with an increased incidence of colorectal cancer.
-
Prostate cancer—In general, fat of animal origin seems to be associated
with the highest risk. In a series of 384 patients with prostate cancer,
the risk of cancer progression to an advanced stage was greater in men with
a high fat intake. The announcement in 1996 that cancer mortality rates had
fallen in the United States prompted the suggestion that this may be due
to decreases in dietary fat over the same time period.
(http://www.cancer.gov/cancerinfo/pdq/prevention)
A more recent analysis of nutrition and cancer (Bingham and Riboli, 2004) details
the difficulty in assessing whether fat intake is a risk factor for breast cancer.
In particular, self-reported dietary assessment instruments may not provide an
accurate assessment of dietary fat because of measurement error biases (Prentice
and Sheppard, 1990). Based on a summary of the literature, total fat seems not
to be associated with breast cancer risk (Kushi and Giovannucci, 2002). This
conclusion is consistent with the findings of the Nurses' Health Study
(Holmes et al., 1999), which reported no association between total fat intake
and breast cancer.
An evidence-based technical report of the American Diabetes Association included
the following statement for dietary fat and diabetes, "Reduced-fat diets when
maintained long-term contribute to modest loss of weight and improvement in dyslipidemia"
(Franz et al., 2004).
Comparison of the Committee's Findings With Other Recommendations.
Both the Adult Treatment Panel (ATP) III (NCEP, 2002) and this Committee agree
on the upper limit for total fat recommendations. The basis for the difference
in the lower limit for the total fat recommendations—25 percent of calories made
by ATP III and 20 percent of calories made by this Committee—is that ATP III
focuses on recommendations for individuals at risk for CHD, such as those seeking
health care who present with an atherogenic dyslipidemia that is aggravated by
a very-low fat diet. This Committee, by contrast, targets the general public.
As stated in Section 1, the Committee is adopting Dietary Reference Intake recommendations
from the Institute of Medicine. Thus, consistent with the IOM report (IOM, 2002),
which has as a focus on healthy individuals, the 20 percent lower level of total
fat in the diet is acceptable.
Total Fat and Children's Health. Total fat intake
of 30 to 35 percent of calories is recommended for children age 2 to 3 years.
A fat intake of 25 to 35 percent of calories is recommended for children age
4 to 18 years. This is consistent with the AMDR for fat established by the IOM
(IOM, 2002). The AMDRs for fat that have been estimated for children are primarily
based on a transition from high-fat intakes that occur during infancy
to the lower fat recommendations for adults.
Evidence is less clear about whether or not low- or high-fat intakes during
childhood can lead to increased risk of chronic diseases later in life. Children
can consume fat intakes within the recommended range without compromising intakes
of energy and of essential vitamins and minerals (Nicklas and Johnson, 2004).
Two large intervention trials successfully reduced children's total fat intake
while maintaining vitamin and mineral intakes (Nicklas et al., 1996; Obarzanek
et al., 1997). In the Dietary Intervention Study in Children, the treatment group
consumed 28 percent of calories from total fat; the children experienced normal
growth and development and maintained normal nutritional biochemical values (Obarzanek
et al., 1997).
Intake Levels
Data from the Third National Health and Nutrition Examination Survey
(NHANES III) and from NHANES 1999–2000, indicate
-
For all ages of the U.S. population, the daily mean percentage of calories
from total fat was 32.7 percent (Briefel and Johnson, 2004)
-
For children age 2 to 19 years, mean fat intake was 33.5 percent of energy
(Troiano et al., 2000)
-
Among males age 12 to 19 years, fat accounted for 35.7 percent of calories
for non-Hispanic blacks, compared with 33.2 percent for non-Hispanic whites
and 34.1 percent for Mexican Americans. (Troiano et al., 2000)
-
For females age 12 to 19 years, fat intake was 36.1 percent of calories
for non-Hispanic blacks compared with 33.4 percent for non-Hispanic whites
and 34.1 percent for Mexican Americans. (Troiano et al., 2000)
Investigators using data from the Continuing Survey of Food Intake by Individuals
(CSFII) (1994–1996, 1998) reported the following additional information:
-
Fewer than 5 percent of children and adults have intakes below 20 percent
of calories from fat. However, approximately 25 percent of children and adults
have intakes greater than 35 percent of calories from fat (IOM, 2002).
-
Among children age 6 to18 years, intake of total fat was 32 percent of calories
(Gleason and Suitor, 2001).
Among adults age 20 to 74, Briefel and Johnson (2004) report that total fat
intake decreased from a mean of 36 percent of calories in 1971–1974 to 33 percent
of calories in 1999–2000 and ranged from approximately 32 to 36 percent of calories
among the different population groups surveyed. However, the absolute level of
fat intake has increased: it was 73.4 g in 1989–1991 and 76.4 g in 1994–1996
(Chanmugam et al., 2003). The concurrent increase in total fat intake means that
the decrease in the percentage of calories from fat results from an increase
in total energy intake coming mainly from carbohydrates.
Back to Top
QUESTION 2: WHAT ARE THE RELATIONSHIPS BETWEEN SATURATED FAT INTAKE
AND HEALTH?
Conclusion
The relationship between saturated fat intake and LDL cholesterol is direct
and progressive, increasing the risk of cardiovascular disease (CVD). Thus, saturated
fat consumption by adults should be as low as possible while consuming a diet
that provides 20 to 35 percent calories from fat and meets recommendations for
α-linolenic acid and linoleic acid. In particular,
-
For adults with LDL cholesterol below 130 mg/dL, less than 10 percent of
calories from saturated fatty acids is recommended.
-
For adults with an elevated LDL cholesterol ( >130
mg/dL), less than 7 percent of calories from saturated
fatty acids is recommended.2
Rationale
This conclusion concurs with the recommendation for saturated fat intake made
by the IOM, which is to decrease saturated fat intake as much as possible within
the context of a nutritionally adequate diet (IOM, 2002). The IOM recommendation
is supported by evidence from a systematic, extensive review of 27 controlled
trials. The recommendation that saturated fat be reduced to 10 percent of calories
dates back to 1977 with the publication of Dietary Goals for the United States
(U.S. Senate, 1977). Since then, the scientific evidence has supported the recommendation
that saturated fat intake be further decreased in persons with elevated LDL cholesterol
(Dixon and Ernst, 2001). The specific recommendation for less than 7 percent
of calories from saturated fat is consistent with the evidence-based recommendation
for individuals with an LDL cholesterol >130 mg/dl made by the NCEP
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol
in Adults (NCEP, 2002); and the Committee's review of 33 more recent controlled
trials on saturated fat intake and health and of a meta-analysis.
Saturated Fat and Blood Lipids. Summaries of evidence
for a positive dose-response relationship between saturated fat intake and LDL
cholesterol appear in Figures D4-1 through D4-3 shown below and in Table 11-2
of the Institute of Medicine report (IOM, 2002).
Figure D4-1 represents data from a meta-analysis of 395 dietary experiments
among 129 groups of individuals and displays the relationship between saturated
fat intake and total serum cholesterol concentrations. Figure D4-2 plots regression
equations for three meta-analyses to show calculated changes in serum LDL cholesterol
concentration in response to the change in the percentage of energy provided
by saturated fatty acids. The figures show that serum total and LDL cholesterol
concentrations increase progressively as saturated fatty acid intake increases.
Regression analysis of the data reported in Figure D4-2 demonstrates that for
each 1 percent increase in energy from saturated fatty acids, serum LDL cholesterol
concentrations increase by 1.3 to 1.8 mg/dl (Clarke et al., 1997; Hegsted et
al., 1993; Mensink and Katan, 1992). Over the range of saturated fatty acid intake
reported in the literature (2 to 33 percent of energy), serum total and LDL cholesterol
concentrations continue to increase. In addition, increasing saturated fatty
acid intake increases the LDL:HDL cholesterol ratio progressively (Figure D4-3),
which increases CHD risk. The saturated fatty acid-induced increase in the LDL:HDL
cholesterol ratio is less than that reported for trans fatty
acid (see Question 3 for more information about trans fatty acids).
The conclusions noted above were further substantiated by recent publications
examining the impact of saturated fatty acids on components of the metabolic
syndrome. Four clinical trials that replaced saturated fatty acids with MUFAs
showed that lipid profiles improved, and some beneficial effects on insulin sensitivity
were reported (Heilbronn et al., 1999; Lovejoy et al., 2002; Perez-Jimenez et
al., 2001; Vessby et al., 2001).
Magnitude of Effect. A reduction of one percentage
point in energy from saturated fat decreases serum LDL cholesterol about one
to two percent, on average (NCEP, 2002). Thus, decreasing saturated fat intake
from 12 percent of calories to less than 7 percent of calories would reduce LDL
cholesterol by about 8 to 10 percent. An LDL cholesterol lowering response of
8 to10 percent would be expected to reduce the risk of CHD by 8 to 10 percent,
since a 1 percent reduction in LDL cholesterol decreases risk for CHD events
by approximately 1 percent. This estimate of the magnitude of effect of decreasing
saturated fat intake is derived from a large sample population with inherent
variation about the mean. For example, there is evidence that the response is
greater in individuals with elevated LDL cholesterol levels and that some individuals,
especially those who are overweight or obese, are less responsive to dietary
saturated fatty acids than expected (Denke, 1995; Schaefer et al., 1997).
The recommendation to decrease saturated fat from about
12 percent of calories (estimated current intake) to less
than 7 percent of calories for adults with
an LDL cholesterol level >130 mg/dl would be expected to
decrease CHD risk by about 8 to 10 percent. Likewise, if
saturated fat intake were decreased from
12 percent of calories to 9 percent of calories in adults
who have an LDL cholesterol
< 130 mg/dl, this 3 percentage point reduction in saturated fat would decrease
LDL cholesterol about 4 to 6 percent, resulting in an approximate 5 percent reduction
in CHD risk.
Saturated Fats and Cancer. In a meta-analysis of dietary
fat and breast cancer risk, the summary relative risk for saturated fat was 1.19
(95 percent CI: 1.06,1.35), based on an analysis of 23 case-control studies and
12 cohort studies (Boyd et al., 2003). The Committee identified two case-control
studies published after that meta-analysis was completed. In a study of Korean
women that included 224 cases and 240 controls, Do et al. (2003) report that
higher breast cancer incidence was not observed with higher saturated fatty acid
intake (more than 19.5 g per day). However there was a statistically significant
trend of increasing breast cancer incidence with increasing total saturated fatty
acid intake. In the Norfolk, UK, center of the European Prospective Investigation
of Cancer, each of 186 women with breast cancer was matched with four healthy
controls (840 total participants). In this study, the risk of breast cancer was
strongly associated with the amount of saturated fat consumed. Women who consumed
more than 35 g per day of saturated fat had more than twice the risk of developing
breast cancer than that of women who consumed 10 g per day or less of saturated
fat (Bingham and Riboli, 2004).
Nutrients Provided by Diets Very Low in Saturated Fats.
Results of menu modeling activities (IOM, 2002) indicate that diets can be planned
to meet nutrient recommendations for linoleic acid and α-linolenic acid while
providing very low amounts of saturated fatty acid (3 to 5 percent of calories
from saturated fatty acid). ATP III has 10 different menu simulations for different
ethnic and gender groups that meet the recommendations of the therapeutic lifestyle
changes diet (NCEP, 2002, Diet Appendix B). That diet recommends less than 7
percent of calories from saturated fat, less than 200 mg of cholesterol per day,
1 to 2 g of stanol/sterol esters3 per day and 10 to 25 g per day of
soluble fiber. In addition, weight control and daily physical activity are recommended.
In these simulations, the saturated fatty acid content of the diet can be as
low as 4 to 6 percent of calories.
Positions Taken by Other Expert Groups. Using an evidence-based
approach, the Expert Panel on Detection, Evaluation, and Treatment of High Blood
Cholesterol in Adults published the following evidence statement and recommendation
related to saturated fat:
Evidence Statement
There is a dose response relationship between saturated fatty acids and LDL
cholesterol levels. Diets high in saturated fatty acids raise serum LDL cholesterol
levels. Reduction in intakes of saturated fatty acids lowers LDL cholesterol
levels. High intakes of saturated fatty acids are associated with high population
rates of CHD. Reduction in intake of saturated fatty acids will reduce risk for
CHD.
Recommendation
The therapeutic diet to maximize LDL cholesterol lowering should contain less
than 7 percent of total calories as saturated fatty acids.
(National Cholesterol Education Program Expert Panel, 2002, p. V-4)
In addition, an evidence-based technical report of the American Diabetes Association
included the following statement for saturated fat and diabetes:
Less than 10 percent of energy intake should be derived from saturated fats.
To lower LDL cholesterol, energy derived from saturated fat can be reduced
if weight loss is desirable or replaced with either carbohydrate or monounsaturated
fat when weight loss is not a goal.
(Franz et al., 2004, p. S39)
ATP III has defined the following categories for LDL cholesterol values (NCEP,
2002):
Recently, NCEP revised these recommendations (Grundy et al., 2004c). In high-risk
persons, the recommended LDL cholesterol goal is less than 100 mg/dl; but when
risk is very high, an LDL cholesterol goal of less than 70 mg/dl is a therapeutic
option. When risk is moderately high, the recommended LDL cholesterol goal is
less than 130 mg/dl, but an LDL cholesterol goal of less than 100 mg/dl is a
therapeutic option. This more rigorous LDL cholesterol goal likely will require
pharmacologic therapy in combination with the dietary changes.
For all adults, including those with an LDL cholesterol concentration less than
130 mg/dl, the risk of heart disease continues to decrease progressively as LDL
cholesterol decreases. Clinical trials demonstrate the efficacy of LDL-cholesterol
lowering as an important means of reducing the risk of CHD. Consequently, risk
is decreased the most when LDL is decreased most.
Saturated Fatty Acids and the Health of Children.
Research on the impact of saturated fatty acid consumption in healthy children
is lacking.
Stearic Acid
Stearic acid has attracted interest as a substitute for trans fatty
acids in prepared foods that require a solid fat. Stearic acid offers the functional
properties needed for these foods, but the question arises of how it affects
blood lipid values. Stearic acid is a unique saturated fatty acid with respect
to its effects on blood lipids and lipoproteins. Stearic acid has been shown
to have a neutral effect on serum total and LDL cholesterol concentrations (Bonanome
and Grundy, 1988; Denke, 1994; Hegsted et al., 1965; Keys, 1965; Yu et al., 1995;
Zock and Katan, 1992). A meta-analysis of 35 studies suggests that stearic acid
has a minimal effect on LDL cholesterol and no effect on HDL cholesterol (Mensink
et al., 2003). In contrast, the other long chain saturated fatty acids increase
both LDL cholesterol and HDL cholesterol (Mensink et al., 2003).
Because of the growing interest in stearic acid as a substitute for trans
fatty acids in solid fats, there is a need to assess the effects of this fatty
acid on cardiovascular disease risk factors beyond blood lipids and lipoproteins.
Only one published study provides evidence about the effects of stearic acid
on other cardiovascular disease (CVD) endpoints. In particular, Baer et al. (2004)
designed a study to evaluate the effects of individual fatty acids on hemostatic
risk factors for CVD. Compared with diets that provided 2 to 3 percent of calories
from stearic acid, a diet that provided 8 percent of calories from stearic acid
increased fibrinogen concentration by 4.4 percent. This translates to
an approximate 7 percent increase in the risk of myocardial infarction. This
study also compared the hemostatic effects of a diet that provided 4 percent
of calories from stearic acid plus 4 percent of calories from trans
fats with those of a high-carbohydrate (54.6 percent of calories from carbohydrate)
control diet. In this comparison, there was no effect on fibrinogen concentration.
Typical consumption of stearic acid in the United States is approximately 3.5
percent of calories. Thus, at intakes of stearic acid that are equal to or slightly
higher than amounts consumed in the United States, no adverse effects on fibrinogen
levels would be expected.
Saturated Fat Intake
Based on data from NHANES III and 1999–2000, reported saturated fat intake
by Americans is as follows:
-
For all ages of the U.S. population over 2 months, the daily mean percentage
of calories from saturated fat was 11.2 percent. In 1999–2000, 41 percent
of the population age 2 years and older reported intakes of less than 10
percent of calories from saturated fat (Briefel and Johnson, 2004).
-
Adult women, persons age 60 and older, Hispanics, and persons with higher
household income were more likely than others to have intakes of less than
10 percent of calories from saturated fat (Briefel and Johnson, 2004).
-
Among adults ages 20 to 74 years, mean saturated fat intake decreased from
13 percent of calories in 1971–1974 to 11 percent of calories in 1999–2000
(Briefel and Johnson, 2004).
-
For persons ages 2 to 19 years, mean saturated fat intake was 12.2 percent
of energy (Troiano et al., 2000).
-
Mean saturated fat intake ranged from 11.6 percent of energy for females
age 12 to 15 years to 12.8 percent for males age 6 to 8 years. Mean saturated
fat intake ranged from 11.7 percent of calories for non-Hispanic white females
age 12 to 19 years to 12.8 percent for Mexican American males age 6 to 11
years. (Troiano et al., 2000)
Using data from CSFII, 1994–1996, Gleason and Suitor (2001) found that mean
usual intake of saturated fat was 12 percent of calories among school-age children.
In summary, current saturated fat intake is approximately 11 to 13 percent of
calories. This represents a 1 to 2 percentage point decrease since the early
1970s for the population at large. Some population groups are consuming less
than 10 percent of calories from saturated fat.
Back to Top
QUESTION 3: WHAT ARE THE RELATIONSHIPS BETWEEN TRANS FAT INTAKE
AND HEALTH?
Conclusion
The relationship between trans fatty
acid intake and LDL cholesterol is direct and progressive,
increasing the risk of CHD. Trans
fatty acid consumption by all population groups should be
kept as low as possible, which is about 1 percent of energy
intake or less.
Rationale
Overview. This conclusion is supported by a systematic,
extensive review of the evidence conducted by the IOM (2002) covering 20 controlled
trials and 11 epidemiologic studies; the evidence-based review conducted by the
NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol
in Adults (2002); and the Committee's review of 7 more recent publications.
Summaries of evidence of relationships of trans fatty acid intake and
health from the IOM report (IOM, 2002) appear in Tables 8-9 through 8-13 of that
report and in Figure D4-3 below. Those tables cover the following topics:
-
Table 8-9: Dietary Trans Fatty Acids and Blood
Lipid Concentration: Controlled Feeding Trials
-
Table 8-10: Hydrogenated Fat Intake and Blood Lipid Concentrations: Controlled
Feeding Trials
-
Table 8-11: Dietary Trans Fatty Acids, Hydrogenated
Fat, and Blood Lipids Concentrations: Free Living Trials
-
Table 8-12: Trans Fatty Acid Intake and Blood
Clotting, Blood Pressure, and Low-Density Lipoprotein
Oxidation
-
Table 8-13: Dietary Trans Fatty Acids: Epidemiologic
Studies
Review of the Evidence
Trans Fatty Acids and Blood
Lipids. The data
reported in Tables 8-9 and 8-10 of the IOM report summarize
12 controlled feeding studies, and the data in Table
8-11 summarize 7 trials with subjects consuming
self-selected diets (IOM, 2002). These data show that, when
compared with unsaturated fatty acids, trans fatty
acids/hydrogenated fat increase LDL cholesterol concentrations.
In addition, when trans fatty acids
are substituted for saturated fatty acids, HDL cholesterol
concentration decreases;
and a dose-response effect is observed. There is a progressive
dose-dependent relationship between trans fatty
acid intake and an increase in the LDL:HDL cholesterol
ratio (Figure D4-3). This observed relationship is
progressive over the range of trans fat
intake from 0.5 to 10 percent of calories. The magnitude
of this effect is greater for trans
fatty acids than for saturated fatty acids. The saturated
fatty acids increase HDL cholesterol, albeit modestly, even
when comparisons are made at low levels
of saturated fat intake, but the dose-response relationship
for trans
fatty acid intake and the LDL:HDL cholesterol ratio begins
to become greater than that observed for saturated fatty
acids at about 2.5 percent of energy intake.
Recent clinical studies support the findings described above: both trans
fat and saturated fat increase LDL cholesterol similarly; however, saturated
fat increases HDL cholesterol whereas trans fat does not (Judd
et al., 2002; Lovejoy et al., 2002). Several of the recent studies have shown
that replacing saturated fats with trans fat decreases serum
HDL cholesterol (de Roos et al., 2001, 2002, 2003). A meta-analysis of 60 controlled
trials (Mensink et al., 2003) reported that the consumption of trans
fat significantly increased the total:HDL cholesterol ratio. Dietary trans
fatty acids also have been shown to increase small, dense LDL cholesterol proportionately
to the amount of dietary trans fatty acids (Mauger et al.,
2003).
The data reported in Table 8-12 of the IOM report (IOM, 2002) indicate that
trans fatty acids have little effect on hemostatic factors,
susceptibility of LDL cholesterol to oxidation, or blood pressure. Other clinical
studies have reported adverse effects of trans fatty acids
on other cardiovascular disease risk factors including postprandial lipids (Gatto
et al., 2003) and impaired endothelial function (de Roos et al., 2002). Recent
epidemiologic evidence from the Nurses' Health Study I and II indicates
that trans fatty acid intake is positively associated with
the systemic inflammatory markers for cardiovascular disease, with soluble tumor
necrosis factor a receptors 1 and 2 in all women, and with IL-6 and C-reactive
protein in women with higher body mass index (Mozaffarian et al., 2004).
Trans Fatty Acids and Cardiovascular Disease. Epidemiologic
evidence from 6 cohort studies (Table 8-13, IOM, 2002) suggests that a high trans
fat intake is associated with an increased risk of coronary artery disease. In
an analysis of data from the Seven Countries Study, Kromhout et al.
(1995) reported strong positive associations between 25-year death rates from
CHD and the average intake of the trans fatty acid elaidic acid (r =
0.78, P < 0.001), and the average intake of the four major long-chain saturated
fatty acids (r > 0.8, P < 0.001) and of dietary cholesterol (r = 0.55,
P < 0.05). Hu et al (1997) reported that intake of trans fat was
associated with an increased risk of coronary heart disease in women. Women in
the highest quintile of trans fat intake (2.9 percent of energy) had
a 27 percent greater risk of coronary heart disease than women in the lowest
quintile (95 percent CI: 1.03, 1.56, P = 0.02 for trend). In comparison, women
in the highest quintile of saturated fat intake had a 16 percent greater risk
of coronary heart disease than women in the lowest quintile (95 percent CI: 0.93,1.44,
P = 0.04 for trend). Similar findings were reported by Pietinen et al. (1997).
Among men in the top quintile of trans fatty acid intake (median = 6.2
g per day), the multivariate relative risk of coronary death was 1.39 (95 percent
CI: 1.09,1.78; P = 0.004) compared with men in the lowest quintile of intake
(median = 1.3 g per day); there was no association between intakes of saturated,
monounsaturated, polyunsaturated fatty acids or dietary cholesterol and the risk
of coronary death. In addition, case-control studies demonstrate an association
between trans fat intake and the risk of myocardial infarct. For example,
compared with the lowest quintile of intake, the relative risk of acute myocardial
infarction for the highest quintile of trans fatty acid intake was 2.4
(P for trend < 0.0001) (Ascherio et al., 1994).
More recent studies have reported an association between the trans
fatty acid content of adipose tissue (a biomarker of long-term fatty acid intake)
and the risk of nonfatal myocardial infarct (Baylin et al., 2003; Clifton et
al., 2004). In the study conducted by Clifton et al., both vegetable and animal
trans fat contributed to the increased risk. However, other epidemiologic
studies report a link only between the intake of hydrogenated vegetable oils
and coronary artery disease; the intake of trans fatty acids from animal
sources had no observed adverse effect (Ascherio et al., 1999; Ascherio et al.,
1996; Willett et al., 1993). Recent evidence, however, suggests that the risk
of CHD is similar between total ruminant and industrial trans fatty
acids for intakes up to 2 g per day (Weggemans et al., 2004). In a case control
study (Lemaitre et al., 2002) reported that higher total trans fatty
acids in red blood cell membranes was associated with a modest increase in the
risk of primary cardiac arrest (odds ratio for interquintile range, 1.5; 95 percent
CI, 1.0 to 2.1). Notably, higher levels of trans isomers of linoleic
acid were associated with a 3-fold increase in risk, whereas trans isomers
of oleic acid were not.
Although intakes of saturated fat, trans fat, and cholesterol all should
be decreased, because saturated fat consumption is proportionately much greater
than that of these other fats, saturated fat should be the primary focus of dietary
modification.
Positions Taken by Other Expert Groups. Using an evidence-based
approach, the NCEP Expert Panel on Detection, Evaluation, and Treatment of High
Blood Cholesterol in Adults published the following evidence statement and recommendation
related to trans fat:
Evidence Statement
Trans fatty acids raise serum LDL cholesterol levels. Consequently,
higher intakes of trans fatty acids increase risk for CHD. Prospective
studies support an association between higher intakes of trans fatty
acids and CHD incidence. However, trans fatty
acids are not classified as saturated fatty acids, nor
are they included in the quantitative recommendations
for saturated fatty acids intake of <7 percent of calories
in the TLC [Therapeutic Lifestyle Changes] diet.
Recommendation
Intakes of trans fatty acids should be as low as possible. The use
of liquid vegetable oil, soft margarine, and trans fatty acid-free margarine
are encouraged instead of butter, stick margarine, and shortening that contain
trans fat.
(National Cholesterol Education Program Expert Panel, 2002, p. V-9)
Numerous other expert groups have conducted evidence-based reviews or published
consensus statements related to trans fatty acids.
The American Diabetes Association recommends that intake of trans unsaturated
fatty acids be minimized.
(Franz et al., 2004, p. S39)
An FDA Food Advisory Committee, Nutrition Committee, recently voted (6 yes,
1 abstain) in favor of the following statement: "Although current scientific
evidence does not indicate a specific acceptable daily intake for trans
fatty acids, it is consistent with reducing trans
fatty acid intake to a level less than 1 percent of energy
(2 g per day for a 2,000 calorie diet)."
(FDA Food Advisory Committee, Nutrition Subcommittee transcripts.
April 28, 2004 p. 92)
The AHA [American Heart Association] Dietary Guidelines Revision 2000 recommends
that trans fatty acid intake be limited, and that the total intake of
cholesterol-raising fatty acids not exceed 10 percent of energy.
(Krauss et al., 2000, p. 2288)
An earlier statement issued by the American Heart Association recommended that
naturally occurring unhydrogenated oil be used when possible and attempts made
to substitute unhydrogenated oil for hydrogenated oil or saturated fat in processed
foods. Also, softer margarines should be substituted for harder margarines and
cooking fats.
(Lichtenstein, 1997, p. 2590)
The conclusion to keep trans fatty acid consumption by all population
groups as low as possible also is supported by the World Health Organization
Report (WHO) (2003), which recommends < 1 percent of energy from trans
fatty acids and the Danish Nutrition Council (Stender and Dyerbery, 2003), which
recommends that the use of industrially produced trans fatty acids in
foodstuffs be ceased as soon as possible.
Trans Fatty Acid Intake. Using 1989–1991
CSFII data, the estimated mean trans fatty acid intake for the U.S.
population ages 3 years and older was 2.6 percent of total energy intake (Allison
et al., 1999). For individuals age 20 years and older, the estimated average
daily intake of trans fat in the U.S. population is about 5.8 g per
day, which represents about 2.6 percent of total energy intake. Industrial sources
provide approximately 80 percent of trans fat in the diet, compared
to 20 percent from animal sources. The major food sources of trans fat
for U.S. adults are shown in Table D4-1.
Most trans fat comes from industrial sources of fat. However, even
if partially hydrogenated fats were removed from the food supply, the Committee
estimates that trans fats still would provide about 1 percent of the
calories because some trans fatty acids are produced in the deodorization
of vegetable oils (principally as elaidic acid), and meat and dairy products
contain naturally occurring trans fatty acids as vaccinic acid and conjugated
linoleic acid (CLA). There is emerging evidence that the naturally occurring
trans fatty acids, vaccinic acid, and conjugated
linoleic acid, have unique biological effects. In animal
studies, CLA can decrease fat deposition
and body lipid content (Wang and Jones, 2004). However, the
few human studies conducted to date have not demonstrated
a similar effect. There is also evidence
from animal studies that CLA protects against the development
and progression of atherosclerosis (Toomey et al., 2003).
Studies with both animals and cell
models demonstrate anti-carcinogenic effects of CLA and vaccinic
acid for many types of cancer (Banni et al., 2001; Corl et
al., 2003; Ip et al., 1999).
According to the Food and Drug Administration (68 Fed. Reg.
41443 (July 11, 2003)), the average trans fat intake from animal sources is 1.2 g per day. This
is approximately 0.5 percent of calories, of which conjugated linoleic acid contributes
a small quantity (151 to 212 mg per day) (IOM, 2002). trans fat from
animal products is estimated to provide less than 1 percent of calories in the
revised USDA food intake pattern (Table D1-13). Decreased consumption of foods
made with industrial sources of trans fats (see plant foods in Table
D4-1) provides the most effective means of reducing trans fat intake.
Back to Top
QUESTION 4: WHAT ARE THE RELATIONSHIPS BETWEEN CHOLESTEROL INTAKE AND
CARDIOVASCULAR DISEASE?
Conclusion
The relationship between cholesterol intake and LDL cholesterol concentrations
is direct and progressive, increasing the risk of CHD. Thus, cholesterol intake
should be kept as low as possible, within a nutritionally adequate diet. In particular,
-
For adults with an LDL cholesterol < 130 mg/dL, less than 300 mg of dietary
cholesterol per day is recommended.
-
For adults with an elevated LDL cholesterol ( >130 mg/dL), less than
200 mg of dietary cholesterol/day is recommended.
Rationale
Overview. This conclusion is supported by evidence
from a systematic, extensive review of the scientific literature by the IOM (2002)
covering 49 controlled trials and 14 observational studies; the evidence-based
review conducted by the NCEP Expert Panel on Detection, Evaluation, and Treatment
of High Blood Cholesterol in Adults (2002); and the Committee's review of 5 more
recent controlled trials.
Summaries of evidence of effects of dietary cholesterol on serum cholesterol
and CHD from the IOM report (IOM, 2002) appear in Tables 9-2 through 9-4 of that
report and Figure D4-4 below. The tables cover the following topics:
-
Table 9-2: Effects of Adding Dietary Cholesterol to Defined Diets with Strict
Control of Dietary Intake on Serum Cholesterol Concentrations
-
Table 9-3: Effects of Adding Dietary Cholesterol to Self-Selected Diets
with Strict Control of Dietary Intake on Serum Cholesterol Concentrations
-
Table 9-4: Dietary Cholesterol and Coronary Heart Disease
There is a historical basis for the cholesterol recommendation that dates back
to 1968 when the American Heart Association recommended about ~300 mg per day
to decrease the risk of CHD (American Heart Association, 1968). In 1977 the Dietary
Goals for the United States recommended that dietary cholesterol be reduced to
300 mg per day (U.S. Senate, 1977). Since then, the scientific evidence has supported
this recommendation and the more contemporary guidance that dietary cholesterol
intake be decreased further in persons with elevated LDL cholesterol (Dixon and
Ernst, 2001).
Review of the Evidence
Dietary Cholesterol and Serum Cholesterol. The data
summarized in the tables cited above show that, in most studies, as dietary cholesterol
increases there is a corresponding increase in total serum cholesterol. A meta-analysis
(Figure D4-4) of 27 controlled metabolic feeding studies of added dietary cholesterol
indicates a relationship with change in serum cholesterol that is steeper in
the range from zero to 300 to 400 mg per day of added dietary cholesterol and
less steep above this level. However, data summarized in Table 9-4 of the IOM
report (IOM, 2002) covering 15 observational studies, show a lack of consistency
in epidemiologic observations relating dietary cholesterol to clinical cardiovascular
disease and CHD endpoints. The inconsistent findings may be due to limited power
to detect effects (e.g., relatively small increases in LDL cholesterol concentration
and inaccuracy in dietary intake data), limited power to distinguish the effects
of dietary cholesterol independent of other factors (such as saturated fat, energy
intake, and fiber intake), or other factors.
This conclusion concurs with the recommendation for cholesterol intake made
by the IOM, which is to decrease cholesterol intake as much as possible within
the context of a nutritionally adequate diet (IOM, 2002). The IOM recommendation
is supported by evidence from a systematic, extensive review of the scientific
literature. The specific recommendation for less than 200 mg per day is consistent
with the evidence-based recommendation for individuals with an LDL cholesterol
greater than 130 mg/dl made by ATP III (NCEP, 2002).
Magnitude of Effect. Based on a meta-analysis of 27
controlled feeding studies, for a baseline cholesterol intake of zero, the estimated
increase in serum cholesterol is 5 mg/dl per 100 mg of added dietary cholesterol
per day—up to 400 mg of cholesterol per day. In contrast, when baseline cholesterol
intake is 300 mg per day, the estimated increase in serum cholesterol is 1.5
mg per day in response to the addition of 100 mg of dietary cholesterol per day
(Hopkins, 1992). Equations based on data from numerous studies predict that 100
mg of added dietary cholesterol per day will increase serum cholesterol by 2
to 3 mg/dl (Clarke et al., 1997; Hegsted, 1986; Howell et al., 1997). Of this
increase in total serum cholesterol, approximately 80 percent is in the LDL fraction.
For an individual with a total serum cholesterol level of 200 mg/dl, a 2 to 3
mg increase represents an approximate 1 to 1.5 percent increase in serum cholesterol
level (equivalent to a 0.8 to 1.2 percent increase in LDL cholesterol). This
increase would be expected to increase CHD risk about 1 percent (IOM, 2002; NCEP,
2002). Notably, however, the effect of added cholesterol is variable among individuals
ranging from essentially no response to a greater response. For example, a recent
study has shown that both normal weight and overweight/obese individuals who
are insulin resistant seem to have a diminished response to dietary cholesterol
compared with insulin sensitive individuals (Knopp et al., 2003). Based on the
collective evidence, the magnitude of response to dietary cholesterol is much
less than that observed for saturated and trans fat intake.
Positions Taken by Other Expert Groups. Using an evidence-based
approach, the NCEP Expert Panel on Detection, Evaluation, and Treatment of High
Blood Cholesterol in Adults published the following evidence statement and recommendation
related to cholesterol:
Evidence Statement
Higher intakes of dietary cholesterol raise serum LDL cholesterol levels in
humans. Through this mechanism, higher intakes of dietary cholesterol should
raise the risk for CHD. Reducing cholesterol intakes from high to low decreases
serum LDL cholesterol in most persons.
Recommendation
Less than 200 mg per day of cholesterol should be consumed in the TLC [Therapeutic
Lifestyle Changes] Diet to maximize the amount of LDL cholesterol lowering that
can be achieved through reduction in dietary cholesterol.
(National Cholesterol Education Program Expert Panel, 2002 p. V-4)
Using an evidence-based approach, the American Diabetes Association published
the following evidence-based nutrition principle and recommendation related to
cholesterol:
Dietary cholesterol intake should be <300 mg per day. Some individuals (i.e.,
persons with LDL cholesterol >100 mg/dl) may benefit from lowering dietary
cholesterol to <200 mg per day.
(Franz et al., 2004, p. S39)
Dietary Cholesterol and Children's Health. Research
on the impact of dietary cholesterol consumption on LDL cholesterol and other
cardiovascular disease risk factors in healthy children is lacking.
Cholesterol Intake. Mean cholesterol intake intake
is above the recommended level of 300 mg per day for adult males and below it
for adult females. For adults age 20 to 74, age-adjusted mean dietary cholesterol
intake was 341 mg in men and 242 mg in women (1999–2000) (Briefel and Johnson,
2004). Troiano et al. (2000) found an increase in cholesterol intake with age
for young males, reaching a peak of 375 mg at age 16 to 19 years. Among males
age 12 to 19 years, the mean (but not median) intake exceeded 300 mg regardless
of racial/ethnic group. Among females, the highest mean intake (233 mg) occurred
at age 9 to 11 years.
Back to Top
QUESTION 5: WHAT ARE THE RELATIONSHIPS BETWEEN n-6 PUFA INTAKE AND HEALTH?
Conclusion
An n-6 PUFA intake between 5 to 10 percent of energy may confer beneficial
effects on coronary artery disease mortality.
Rationale
Overview. The conclusion regarding the range of intake
of n-6 PUFAs is based on the IOM's AMDR for this fatty acid class (IOM, 2002).
Evidence concerning beneficial effects on coronary artery disease mortality was
obtained from the same IOM report and a systematic review of 17 published papers.
The n-6 PUFAs include linoleic acid, which accounts for about 85 percent to
90 percent of the total PUFA consumption, and arachadonic acid, which contributes
less than 2 percent of the total (IOM, 2002). A dietary source of linoleic acid
is essential for life and health. Linoleic acid serves as a precursor to eicosanoids.
A lack of dietary n-6 PUFAs is characterized by rough, scaly skin; dermatitis;
and an elevated eicosatrienoic acid:arachidonic acid (triene:tetraene) ratio.
The IOM (IOM, 2002) set an AI for linoleic acid of 17 g per day for men and 12
g per day for women. It also set an AMDR for linoleic acid of 5 to 10 percent
of calories (IOM, 2002). The lower end of the range meets the AI for linoleic
acid. The upper end of the range was based on three lines of evidence: (1) individual
dietary intakes in a North American/U.S. population rarely exceed 10 percent
of energy, (2) epidemiologic evidence for the safety of intakes greater than
10 percent of energy generally are lacking, and (3) high intakes of linoleic
acid create a pro-oxidant state that may predispose to several chronic diseases,
such as CHD and cancer.
Review of the Evidence
n-6 Fatty Acid Intake and Blood Lipids. Evidence from
six intervention studies was provided in the IOM report (IOM, 2002, see Table
11-9: Interventional Studies on n-6 Fatty Acid Intake and Blood Lipid Concentrations).
The studies included in the table demonstrate that higher n-6 polyunsaturated
fatty acid intake generally is associated with a more favorable CHD lipid risk
profile.
n-6 Fatty Acid Intake and CVD. A number of epidemiologic
studies have examined the association between n-6 PUFA intake and CVD. In two
population studies (Artaud-Wild et al., 1993; Hegsted and Ausman, 1988), PUFA
intake was negatively associated with CVD mortality after adjusting for dietary
saturated fat. Several prospective studies (Ascherio et al., 1996; Garcia-Palmieri
et al., 1980; Gordon et al., 1981; Hu et al., 1997; Shekelle et al., 1981), two
longitudinal studies (Joossens et al., 1989; Tell et al., 1994), and one cross-sectional
study (Djousse et al., 2001) reported a beneficial association of dietary PUFAs
with CVD morbidity and mortality (Table D4-2). In contrast, no significant association
was found between dietary PUFAs and CVD in the Seven Countries Study
(Keys, 1997, Kromhout et al., 1995). Similarly, other epidemiologic studies did
not find a beneficial association between PUFAs and CVD (Kark et al., 2003; Kushi
et al., 1985; Posner et al., 1991).
Adverse Effects of PUFA. In a systematic review of
research, the Committee found no studies that reported adverse effects, even
in the Jerusalem Study in which 25 percent of the population had PUFA
intakes that exceeded 12 percent of calories (Kark et al., 2003). However, as
noted previously, the upper end of the AMDR took into account that the epidemiologic
evidence for the safety of intakes greater than 10 percent of energy generally
are lacking and that high intakes of linoleic acid may create a pro-oxidant state
(IOM, 2002).
Positions Taken by Other Expert Groups. This conclusion
also was supported by the evidence-based Third Report of the National Cholesterol
Education Program Expert Panel on Detection, Evaluation, and Treatment of High
Blood Cholesterol in Adults.
Evidence Statements
Linoleic acid, a polyunsaturated fatty acid, reduces LDL cholesterol levels
when substituted for saturated fatty acids in the diet. Polyunsaturated fatty
acids also can cause small reductions in HDL cholesterol when compared with monounsaturated
fatty acids, especially when present in high amounts in the diet. Controlled
clinical trials indicate that substitution of polyunsaturated fatty acids for
saturated fatty acids reduces risk for CHD.
Recommendation
Polyunsaturated fatty acids are one form of unsaturated fatty acids that can
replace saturated fat. Most polyunsaturated fatty acids should be derived from
liquid vegetable oils, semi-liquid margarines, and other margarines low in trans
fatty acids. Intake of polyunsaturated fat can be as high as 10 percent of total
calories.
(National Cholesterol Education Program Expert Panel, 2002, p. V-11)
Using an evidence-based approach, the American Diabetes Association published
the following evidence-based nutrition principle and recommendation related to
PUFAs:
Polyunsaturated fat intake should be ≈ 10 percent
of energy intake.
(Franz et al., 2004, p. S39)
n-6 PUFA Intake. Mean n-6 PUFA intakes by Americans
fall within the AMDR. Based on CSFIII 1989-91 data, mean intakes by adults are
approximately 5 to 6 percent of total energy intake (Allison et al., 1999). Using
NHANES III data, mean intakes by children and adolescents ranged from about 6
to nearly 8 percent, depending on the age and ethnic group (Troiano et al., 2000).
Back to Top
QUESTION 6: WHAT ARE THE RELATIONSHIPS BETWEEN n-3 FATTY ACID INTAKE
AND HEALTH?
Conclusion
An α-linolenic acid intake between 0.6 to 1.2 percent of
calories will meet requirements for this fatty acid and may
afford some protection against CVD outcomes.
The consumption of two servings (approximately 8 ounces) per
week of fish high in EPA and DHA is associated with reduced
risk of both sudden death and CHD death in adults. To benefit
from the potential cardioprotective effects of EPA and DHA,
the weekly consumption of two servings of fish, particularly
fish rich in EPA and DHA, is suggested. Other sources of EPA
and DHA may provide similar benefits; however, further research
is warranted.
Rationale
α-linolenic Acid
Overview. The conclusion regarding the range of intake
of α-linolenic acid is based on the IOM's AMDR for this fatty acid (IOM, 2002).
Evidence concerning protection against CVD outcomes was obtained from the same
IOM report, several more recent studies, and data from the evidence-based report
from the HHS Agency for Health Care Policy and Research (AHRQ) entitled Effects
of Omega-3 Fatty Acids on Cardiovascular Disease (Wang et al., 2004).
A dietary source of α-linolenic acid is essential for life and health. The IOM
(IOM, 2002) set an AI for α-linolenic acid of 1.6 g per day for men
and 1.1 g per day for women. This represents approximately 0.6 percent of energy
intake for sedentary adults. The AMDR for α-linolenic acid is 0.6 percent to
1.2 percent of calories. The lower boundary of the recommended range meets the
AI for α-linolenic acid. The AI for α-linolenic acid is based on the median intakes
in the United States and Canada—countries in which an α-linolenic acid deficiency
is nonexistent in healthy individuals. The upper boundary corresponds to the
highest reported α-linolenic acid intake from foods consumed by individuals in
the United States and Canada.
Evidence Relating to Cardiovascular Disease. The IOM
(2002, pp 11-1 to 11-2) stated, "A growing body of literature suggests that higher
intakes of α-linolenic acid, EPA, and DHA may afford some degree of
protection against CHD." In addition, the recently released AHRQ report (Wang
et al., 2004) also supports the conclusion that α-linolenic acid intakes within
the AMDR range of 0.6 percent to 1.2 percent of calories may afford some protection
against cardiovascular disease outcomes. Both reports summarized the three epidemiologic
studies conducted in the United States that demonstrated that an α-linolenic
acid intake of 0.53 to 2.8 g per day reduced the risk of cardiovascular disease
events (Djousse et al., 2001), fatal ischemic heart disease (Hu et al., 1999),
and all-cause mortality (Dolecek, 1992). In addition, both reports summarized
two secondary prevention randomized controlled clinical trials (de Lorgeril et
al., 1999; Singh et al., 1997) that demonstrated a beneficial effect of α-linolenic
acid on cardiovascular events in post-myocardial infarct patients. These studies
reported that increased α-linolenic acid intake (2.0 g per day and 2.9 g per
day, respectively) reduced the risk of recurrent coronary events. These α-linolenic
acid intake values correspond to approximately 0.8 and 1.2 to 1.3 percent of
calories, respectively—values that fall within and slightly above the upper range
of the AMDR for α-linolenic acid. In these two studies, the control group consumed
0.27 percent of energy and 0.8 g per day as α-linolenic acid, respectively.
In a primary prevention study on cardiovascular disease outcomes in a population
with a high habitual fish intake conducted in Norway more than 30 years ago,
a diet that provided 5.5. g per day of α-linolenic acid from linseed oil did
not improve outcomes compared with a diet that provided 0.14 g per day of α-linolenic
acid from sunflower seed oil (Natvig et al., 1968). Notably, the two diets differed
in other ways related to the unique fatty acid profiles of linseed oil and sunflower
oil. Another primary prevention trial in subjects with multiple CVD risk factors
(Bemelmans et al., 2002) determined the 10-year estimated ischemic heart disease
risk in subjects followed for 2 years. The trial found no effect of a diet that
provided 6.3 g per day of α-linolenic acid compared with a diet that provided
1 g per day of α-linolenic acid. Possible reasons to explain a lack of effect
of these primary prevention studies may relate to study duration and sample size,
neither of which may have been sufficient to test the hypothesis adequately.
In addition, in the Natvig et al. study, the high habitual fish intake of the
Norwegian population could have blunted an α-linolenic acid effect. In the Bemelmans
et al. study (2002), the "low" α-linolenic acid intake group may have consumed
a level of α-linolenic acid sufficient to achieve a beneficial response that
was comparable to the response of the high-intake group.
There is no evidence of a beneficial effect of α-linolenic acid intake on the
incidence of stroke. Collectively, the evidence supports the hypothesis that
the consumption of α-linolenic acid reduces all-cause mortality and various cardiovascular
disease events. However, the evidence is strongest for fish or fish oil supplements,
as discussed below.
Evidence Relating to Cancer. A meta-analysis of 9
cohort and case-control studies evaluated the relationship between α-linolenic
acid and prostate cancer (Brouwer et al., 2004). In this analysis, the intake
of α-linolenic acid or the concentration of α-linolenic acid in the blood was
used to assess the relative risk of prostate cancer. The results of the individual
studies were variable. Based on meta-analysis, however, the relative risk of
prostate cancer was higher in the men with the highest intakes or highest blood
concentrations of α-linolenic acid than in men with the lowest intakes. The mean
α-linolenic acid intake in the highest intake group was 2.0 g per day, and it
was 0.8 g per day in the lowest intake group. Of the four prospective studies
evaluated in the meta-analysis, two assessed the intake of α-linolenic acid,
and two evaluated blood values of α-linolenic acid. In the two that assessed
intake, one reported a slight protective effect of α-linolenic acid intake for
prostate cancer incidence (RR 0.76; 95 percent CI: 0.66, 1.04) (Schuurman et
al., 1999). In contrast, the U.S. Health Professionals' Follow-Up Study
reported a slightly increased risk of prostate cancer with increasing α-linolenic
acid intake (RR 1.25; 95 percent CI: 0.82, 1.92) (Giovannucci et al., 1993).
In a followup to the Giovannucci et al. study, α-linolenic acid intake was unrelated
to the risk of total prostate cancer among 2965 new documented cases of total
prostate cancer, of which 448 were advanced prostate cancer (Leitzmann et al.,
2004). However, the multivariate relative risks of advanced prostate cancer from
the extreme quintiles of α-linolenic acid intake from nonanimal sources were
2.02 (95 percent CI: 1.35, 3.03, P for trend 0.0004); and from meat and dairy
products, the relative risks were 1.53 (95 percent CI: 0.88, 2.66, P for trend
0.06). In this study, the lower and upper quintiles of total α-linolenic acid
intake were less than 0.37 percent and more than 0.58 percent of energy, respectively.
Of note, the upper quintile of α-linolenic acid intake in the study by Leitzmann
et al. is comparable to the mean α-linolenic acid intake of the U.S. population
using 24-hour recall data (NHANES III), suggesting that the food frequency data
reported are underestimates of actual intake. If there is an association between
α-linolenic acid intake and prostate cancer risk, it likely would be seen at
higher intake levels than those reported. At this time, there are insufficient
data to reach a conclusion about an association between α-linolenic acid intake
and risk of prostate cancer (Astorg, 2004; Attar-Bashi et al., 2004). Thus, further
research is warranted to resolve this question.
Positions Taken by Other Expert Groups. WHO (2003)
recommends 1 to 2 percent of energy from n-3 PUFAs. The EuroDiet Core Report,
Nutrition and Diet for Healthy Lifestyles in Europe (2000) recommends
2 g of linolenic acid per day plus 200 mg of very long chain n-3 fatty acids
per day.
EPA, DHA, and Fish
Overview. The conclusion regarding fish was reached
and supported by evidence from an analysis of epidemiologic studies of the cardioprotective
effects of fish consumption among healthy populations (Dolecek, 1992; Siscovick
et al., 1995; Hu et al., 2002; Mozaffarian et al., 2003) and information from
the evidence-based AHRQ Report Effects of Omega-3 Fatty Acids on Cardiovascular
Disease (Wang et al., 2004). Information in Dietary Reference Intakes
for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and
Amino Acids (IOM, 2002) provided the starting point of the examination of
evidence. Although α-linolenic acid can be elongated to form EPA and DHA, this
conversion occurs slowly in humans and the conversion rates are incompletely
understood. Thus an important source of EPA and DHA is fish that is high in these
fatty acids. This is of significance because the evidence indicates that EPA
and DHA are responsible for the cardioprotective effects of fish consumption.
In addition, there is evidence that the nonmarine n-3 fatty acid, α-linolenic
acid, also plays a cardioprotective role.
Because the biological potency of EPA and DHA is much greater than that for
α-linolenic acid, the IOM (2002) did not recommend one AMDR to for the entire
n-3 fatty acid class. Instead, the IOM recommended that up to 10 percent of the
AMDR for α-linolenic acid can be consumed as EPA and/or DHA (0.06 to 0.12 percent
of energy). No Upper Level (UL) was established for α-linolenic acid (or for
the sum of EPA and DHA) mainly because of insufficient data to use the model
of risk assessment to set this value. With respect to health benefits of EPA
and DHA, the IOM report notes the following:
"a growing body of literature suggests that diets higher in EPA and DHA may
afford some degree of protection against CHD" (IOM, 2002, p S-6)
"n-3 polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic
acid [DHA]) have been shown to reduce the risk of CHD and stroke by a multitude
of mechanisms" (IOM, 2002, Chapter 3, pp 3–5)
After the release of the IOM Report, new evidence was published demonstrating
benefits of fish consumption on CVD among U.S. populations (Hu et al., 2002;
Mozaffarian et al., 2003).
Review of the Evidence. The AHRQ report Effects
of Omega-3 Fatty Acids on Cardiovascular Disease (Wang et al., 2004) summarizes
22 prospective cohort studies that were conducted in many parts of the world
including the United States, China, Japan, and countries in the Mediterranean
and Northern Europe. Most of the cohorts had several thousand subjects; the range
was 272 to 223,170 subjects, with most subjects at least age 40. The background
diets of the study populations from other parts of the world differed from those
of the U.S. population. Several of the large population studies in the United
States included only males or only females, with the study duration ranging from
4 to 30 years. Most of the studies used food frequency questionnaires to estimate
the dietary fish intake. Most studies provided quantitative estimates of the
amount of fish consumed (many also quantified the sum of EPA and DHA intake)
and categorized them into various quantiles (e.g., tertiles, quartiles, quintiles).
Other studies reported only the frequency of fish consumption or simply whether
fish was consumed. Despite some limitations, if viewed together, these studies
provide evidence that is highly applicable to the U.S. population. Overall the
evidence from the primary and secondary prevention studies supports the hypothesis
that the consumption of n-3 fatty acids (EPA, DHA, α-linolenic acid), fish, and
fish oil reduces all-cause mortality and various CVD outcomes. These outcomes
include sudden death and cardiac death (coronary or myocardial infarct (MI) death).
The central question is, "How much fish consumption in these studies was necessary
to elicit a cardioprotective effect?" Collectively, evidence from five U.S. epidemiologic
studies (Albert et al., 1998; Dolocek et al., 1992; Hu et al., 2002; Siscovick
et al., 2000; Mozaffarian et al., 2003) found that the average intake of EPA
and DHA (estimated from fish consumption) associated with the lowest risk of
coronary events (including CHD death, primary cardiac arrest and ischemic heart
disease death) was 496 mg per day. The range of EPA and DHA intake in the studies
that conferred the lowest risk was 246 mg per day to 919 mg per day. Because
these estimates were derived from fish consumption, it is important to put the
average of 496 mg per day of EPA and DHA per day in the context of the amount
of fish consumed to achieve this level of intake. A daily intake of 496 mg of
EPA and DHA is equivalent to about 3.5 g per week. This is approximately equivalent
to the amount of EPA and DHA in two 4-oz. servings of high n-3 fish per week,
based on an average EPA and DHA content of high n-3 fish of 1.6 g per serving
(values derived from USDA database). These data provide the rationale for the
recommendation for 2 servings of high n-3 fish per week.
There is some evidence that consuming more than two servings of fish per week
may confer further cardioprotective effects. This was observed in the Mozaffarian
et al. (2003) study that found that more than two servings of fish per week (which
contributed 919 mg per day of EPA and DHA) was associated with the lowest risk
for CHD. In addition, two recent meta-analyses report that fish consumption five
or more times per week is associated with lower CHD mortality (He et al., 2004a)
and lower incidence of stroke (He et al., 2004b). Compared with those who never
consumed fish or ate fish less than once per month, the relative risks for CHD
mortality were 0.89 for fish intake 1 to 3 times per month; 0.85 for once per
week; 0.77 for 2 to 4 times per week; and 0.62 for 5 or more times per week (He
et al., 2004a). The authors reported that for each 20 g per day increase in fish
intake there was a corresponding 7 percent lower risk of CHD mortality. Compared
with no fish intake or intake less than once per month, the relative risks for
total stroke were very slightly higher than those for CHD mortality at each level
of fish intake (He et al., 2004b). The relative risks for ischemic stroke were
lower than for total stroke: 0.69 for fish intake 1 to 3 times per month; 0.68
for once per week; 0.66 for 2 to 4 times per week; and 0.65 for 5 or more times
per week (P for trend = 0.24). Thus, the optimal quantity of fish consume is
not yet clear. Similarly, the AHRQ report (Wang et al., 2004) did not define
the optimal quantity of n-3 fatty acids to consume because of the lack of sufficient
evidence.
The AHRQ report did conclude, however, that the consumption of n-3 fatty acids
from fish or from supplements of fish oil reduces all-cause mortality and various
CVD outcomes. The available evidence indicates that the active dietary factor
in fish is EPA and DHA. The DART study (Burr et al., 1989) showed that male MI
survivors who consumed 200 to 400 g of n-3 rich fish per week, which provided
an additional 500 to 800 mg per day of n-3 fatty acids to current intake, had
the same reduction in recurrent events as did patients receiving fish oil capsules
containing 900 mg per day of EPA and DHA. In addition, the Gruppo Italiano
per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI) Prevention
Study (GISSI-Prevenzione Investigators, 1999), the largest prospective clinical
trial to test the efficacy of n-3 fatty acids for secondary prevention of CHD,
showed that subjects randomized to the EPA + DHA supplement group (850 mg per
day of omega-3 fatty acid ethyl esters) with and without 300 mg of vitamin E
per day) experienced a 15 percent reduction in the primary endpoint of death,
nonfatal MI and nonfatal stroke (P < 0.02). In addition, all-cause mortality
was reduced by 20 percent (P = 0.01) and sudden death was reduced by 45 percent
(P < 0.001) compared with the control group (vitamin E provided no benefit).
Further evidence to support the importance of EPA and DHA comes from the Indian
Experiment of Infarct Survival (Singh et al., 1997). MI survivors who were treated
with either fish oil capsules (1.8 g per day EPA + DHA) or mustard oil (2.9 g
per day α-linolenic acid) for one year had fewer total cardiac events, nonfatal
infarctions, arrhythmias and less left ventricular enlargement and angina pectoris
than did the placebo group. Only the group treated with fish oil experienced
a decrease in cardiac deaths (Singh et al., 1997). Collectively, the available
evidence from the controlled clinical trials demonstrates that EPA and DHA are
the bioactive compounds that elicit cardioprotective benefits. Thus, these results
provide an explanation for the cardioprotective effects of fish consumption reported
in the epidemiologic studies.
Although the preponderance of evidence supports a beneficial effect of fish
consumption, two studies found no association between fish consumption and health
outcomes (Kromhout et al., 1996). Differences in study findings likely relate
to differences in the definitions of endpoints and residual confounding of reference
groups with less healthy lifestyles (Guallar et al., 1999; Kromhout, 1998); variability
in the endpoints studied, the experimental design, the method of estimating fish
intake, and differences in the study populations (Sheard, 1998); or a small fraction
of the study population reporting little or no fish consumption (Albert et al.,
1998).
Fish is a good source of nutrients including protein, the B-vitamins and minerals
such as potassium, phosphorous, and selenium and also is low in calories. Since
fish is low in saturated fat, it provides a means to reduce saturated fat intake
when substituted for foods such as red meats and full-fat dairy products.
Special Analysis. At the Committee's request, USDA's
Center for Nutrition Policy and Promotion used a modeling process described in
Appendix G-2 to examine how incorporating 8 ounces per week of fish (approximately
twice that of current consumption) and/or fish high in n-3 fatty acids in the
food intake patterns would affect the nutrient profiles of patterns ranging from
1,000 to 3,200 calories per day. First, all fish items were separated into low
n-3 (LO-3) or high n-3 (HI-3) subgroups. The cutoff value specified for placement
into the LO-3 or HI-3 group was 500 mg of EPA plus DHA in a 3-ounce serving of
the fish. Using this approach, on average, 1 ounce of HI-3 fish (e.g. mackerel,
salmon, trout) contains 407 mg of EPA+DHA, and 1 ounce of LO-3 fish (e.g. cod,
haddock, snapper) contains 105 mg of EPA+DHA.
Substituting either more fish or HI-3 fish for some meat and poultry in the
food intake pattern had little impact on the amounts of other nutrients provided
by the food pattern. For most nutrients, no change was evident when expressed
as a percentage of the RDA or AI. For iron, a decrease of 2 to 4 percent was
seen in the patterns with the 8 ounces of HI-3 fish. For other nutrients, the
change was only 0 to 2 percent. The change in total fat was 0 to 1 percent of
calories, depending on the calorie level of the pattern.
Using the estimates from USDA's special analysis (see above), 8 ounces of fish
that is high in n-3 fatty acids would provide approximately 3250 mg of EPA+DHA
a week—an average of slightly less than 500 mg per day, which is about a two-fold
increase over current intake (see below). Adverse effects are not observed until
intake exceeds 3 g per day (62 Fed. Reg. 30,751 (June 5, 1997)).
Positions Taken by Other Expert Groups.
The American Heart Association—two servings of fish (preferably fatty) per
week (Krauss et al., 2000; Kris-Etherton et al., 2002).
National Cholesterol Education Program—recommends fish as a food item for
people to choose more often (NCEP, 2002; Table V.2–6).
World Health Organization—regular fish consumption (one to two servings per
week; each serving should provide the equivalent of 200 to 500 mg of EPA+DHA)
(WHO Technical Report, 2003).
European Society for Cardiology—oil fish and n-3 fatty acids have particular
protective properties for primary CVD prevention (De Backer et al 2003; Priori
et al. 2003; Van de Werf et al., 2003).
United Kingdom Scientific Advisory Committee on Nutrition—consume at least
two portions of fish per week, of which one should be oily, and provide 450
mg per day of EPA+DHA (Scientific Advisory Committee on Nutrition, 2004).
American Diabetes Association—two to three servings of fish per week provide
dietary n-3 polyunsaturated fats and can be recommended (Franz et al., 2004).
Summary
Collectively, the evidence presented above provided the basis for recommending
two servings of fish per week to decrease risk of heart disease. A conservative
estimate is that two servings of fish high in n-3 fatty acids per week may reduce
the risk of coronary death, primarily sudden death, by as much as 30 percent
(Hu et al., 2002) among adults. Fish is recommended rather than supplements because
epidemiologic and some RCT data demonstrate benefits of fish; it is a good source
of n-3 fatty acids and many other nutrients; and it is low in calories and saturated
fatty acids (see Table D4-3, EPA+DHA content of selected fish).
n-3 Fatty Acid Intake
Based on intake data from CSFII (1994–1996, 1998), the total median n-3 fatty
acid intake for men and women ranged from 1.3 to 1.8 g per day and 1.0 to 1.2
g per day, respectively (IOM, 2002, Appendix Table E-10).
Depending on age, the median intake of α-linolenic acid ranged from 1.2-1.6
g per day for men and 0.9 to 1.1 g per day for women. Estimated mean intake of
α-linolenic acid, based on over 29,000 NHANES III respondents, was 1.33 g per
day. This was equivalent to 0.55 percent of total energy intake per day (Wang
et al., 2004, Table 3.4).
For all adults, the median intakes of EPA and DHA range from 0.004 to 0.007
g per day and 0.052 to 0.093 g per day, respectively (IOM, 2002, Appendix Tables
E-12 and E-14).
Mean intake of EPA and DHA, based on analyses of a single 24-hour recall of
NHANES III data, were 0.04 and 0.07 g per day, respectively. Distributions for
EPA and DHA were very skewed and data on intakes should be used and interpreted
with caution (Wang et al., 2004, Table 1.1).
Based on NHANES 1999–2000 data, mean intake of fish is 2.92 ounces per week
(CNPP analysis, Appendix G-2). The majority of the fish consumed (63 percent)
is finfish and shellfish that contain less than 500 mg of n-3 fatty acids per
3-ounce serving. The most commonly consumed single fish is tuna (representing
22 percent of total fish consumption, with shrimp (16 percent), salmon (9 percent),
mixed fish (8 percent), and crab (7 percent) also commonly reported. Emphasis
will need to be placed on fish high in n-fatty acids to achieve the recommendation
for fish consumption.
Other sources of long chain n-3 fatty acids are currently on the market. Some
are fortified with deodorized fish oil or contain algae as the source of EPA
+ DHA. With the pending availability of agronomic crops such as corn and soybeans
that have been genetically enhanced to contain EPA and DHA, it is conceivable
that vegetable oils rich in these n-3 fatty acids will become an important plant
source of these fatty acids. EPA + DHA supplements may provide variable amounts
of these fatty acids (Consumer Reports, 2003). The α-linolenic acid from plant
sources including canola and soybean oils, walnuts and flaxseed can be converted
to a limited extent (approximately 10 percent) to EPA + DHA in the body.
Supplementary Information
See the section, "Methylmercury in Fish" in Section 9, "Food Safety" for cautions
regarding types of fish to avoid or to eat in limited amounts.
Back to Top
QUESTION 7: WHAT ARE RELATIONSHIPS BETWEEN MUFA INTAKE AND HEALTH?
Conclusion
There is an inverse relationship between the intake of MUFAs and the total
cholesterol (TC):HDL cholesterol (HDL-C) concentration ratio. If equal amounts
of MUFAs are substituted for saturated fatty acids, LDL-C decreases.
Rationale
Overview. This conclusion was supported by evidence
from the IOM (2002) review of 19 clinical trials; the evidence-based review conducted
by the Cholesterol Education Program Expert Panel on Detection, Evaluation, and
Treatment of High Blood Cholesterol in Adults (2002); and the Committee's review
of 18 more recent controlled trials.
Since humans can synthesize MUFAs from other fats and from carbohydrates, MUFAs
are not required in the diet. However, MUFAs are present in virtually all fat-containing
foods, and the dietary intake of MUFAs benefits human health by providing a vehicle
to achieve total fat recommendations within the context of recommendations for
the intakes of saturated fatty acids and PUFAs.
Implicit to a discussion of monounsaturated fats is how the level of MUFA intake
affects various biological endpoints relative to intakes of other fatty acid
classes. Table D4-4 illustrates some different ways that MUFAs can vary in the
diet. In one scenario, MUFAs could be held constant within a constant amount
of total fat, and the amount of saturated fatty acids and PUFAs would vary. Alternatively,
MUFAs could vary within a constant amount of total fat, while saturated fatty
acids and PUFAs vary. Or, MUFAs could be held constant while total fat and other
fatty acids vary. Lastly, MUFAs and total fat could vary while saturated fatty
acids and PUFAs are held constant. As shown, the MUFA content of the diet, expressed
as a percentage of total calories, can vary with the percentage of energy provided
by other fatty acids, the percentage of energy provided by total fat, and a combination
of the two. The carbohydrate and protein as a percentage of calories can vary
as well. The examples shown in the table are only a few of the many possible
combinations. Thus, the biological effects of MUFAs must be studied in the context
of the level of total fat (and other macronutrients) and the other fatty acid
classes.
Review of the Evidence
MUFAs and Blood Lipids. Figure D4-5, below, demonstrates
that an increase in MUFA intake as a percentage of total energy intake results
in a decrease in the total cholesterol:HDL-cholesterol ratio (IOM, 2002). A meta-analysis
of feeding studies estimated that the regression coefficients for the effects
of MUFAs on LDL and HDL cholesterol concentrations were -0.008 and +0.006, respectively,
suggesting a slight positive benefit (Clarke et al., 1997).
MUFAs and the Metabolic Syndrome. Recent publications
reported the following effects of MUFAs on components of the metabolic syndrome:
-
Ten clinical trials that replaced carbohydrates with MUFAs found that MUFAs
may have benefical effects on some aspects of glycemic control (Brynes et
al., 2003; Campbell et al., 1994; Garg et al., 1994, 1992; Heilbronn et al.,
1999; Parillo et al., 1992; Rasmussen et al., 1993; Scott et al., 2003; Straznicky
et al., 1999; Wien et al., 2003).
-
Four clinical trials that replaced saturated fatty acids with MUFAs showed
improvement in lipid profiles and some beneficial effects on insulin sensitivity
(Heilbronn et al., 1999; Lovejoy et al., 2002; Perez-Jimenez et al., 2001;
Vessby et al., 2001).
A summary of the available evidence demonstrates that, compared with a high-carbohydrate
diet (greater than 65 percent of calories from carbohydrate), a diet that provides
approximately 20 percent of total calories from MUFA and 35 percent from total
fat improves glycemic control in individuals with type 2 diabetes mellitus who
maintain their body weight. Specifically, such a diet may decrease triglyceride
and increase HDL cholesterol concentrations. Individuals with elevated triglycerides
or insulin levels may benefit from increasing MUFAs in the diet (by replacing
some carbohydrate calories with a comparable number of calories from MUFAs).
In addition, Krauss (2001) has shown that a moderate-fat diet that emphasizes
MUFAs may decrease the risk of expression of the atherogenic lipoprotein phenotype
(characterized by high triglycerides; low HDL cholesterol; high small-dense LDL)(Reaven,
2001). A review of 18 well-controlled clinical studies compared the effects of
substituting either MUFAs or carbohydrate for saturated fat in a blood cholesterol-lowering
diet (Kris-Etherton et al., 2000). Replacing saturated fatty acids with MUFAs
was found to reduce total and LDL cholesterol values. Compared to baseline values,
the range of serum total cholesterol concentration change was -17 to +3 percent
on the low-fat/high-carbohydrate diet, whereas the range was -20 to -3 percent
on the high-MUFA diet. The range of decrease in plasma LDL cholesterol concentration
was similar (-22 to +1 percent) among subjects on the two diets. The change in
serum triacylglycerol concentrations ranged from -23 to +37 percent for subjects
consuming the low-fat/high-carbohydrate diets and from -43 to +12 percent for
diets high in MUFAs. Changes in HDL cholesterol concentrations ranged from -25
to +2 percent for subjects on the low-fat/high-carbohydrate diets compared to
a -9 to +6 percent change for subjects on diets high in MUFAs. These data indicate
that in weight-stable individuals, a high MUFA-low saturated fatty acid diet
results in a more favorable metabolic profile with respect to total cholesterol,
HDL cholesterol, and triacylglycerol concentrations than the baseline diet or
a low-fat/high-carbohydrate diet. The evidence is clear that replacing saturated
fatty acid calories with MUFAs lowers total and LDL cholesterol levels.
Positions Taken by Other
Expert Groups. Using an evidence-based
approach, the NCEP Expert Panel on Detection, Evaluation, and Treatment of High
Blood Cholesterol in Adults published the following evidence statement and recommendation
related to MUFAs:
Evidence Statement
Monounsaturated fatty acids lower LDL cholesterol relative to saturated fatty
acids. Monounsaturated fatty acids do not lower HDL cholesterol nor raise triglycerides.
Dietary patterns that are rich in monounsaturated fatty acids provided by plant
sources and rich in fruits, vegetables, and whole grains and low in saturated
fatty acids are associated with decreased CHD risk. However, the benefits of
replacement of saturated fatty acids with monounsaturated fatty acids has not
been adequately tested in controlled clinical trials.
Recommendation
Monounsaturated fatty acids are one form of unsaturated fatty acid that can
replace saturated fatty acids. Intake of monounsaturated fatty acids can range
up to 20 percent of total calories. Most monounsaturated fatty acids should be
derived from vegetable sources, including plant oils and nuts.
(National Cholesterol Education Program ATP III Expert Panel, 2002, p. V-10)
In addition, an evidence-based technical report of the American Diabetes Association
included the following statements for MUFAs and diabetes:
For persons with elevated plasma triglycerides, reduced HDL cholesterol, and
small-dense LDL cholesterol (the metabolic syndrome), improved glycemic control,
modest weight loss, dietary saturated fat restriction, increased physical activity,
and incorporation of MUFAs may be beneficial.
Carbohydrate and MUFA together should provide 60 to 70 percent of energy
intake. However, the metabolic profile and need for weight loss should be considered
when determining the monounsaturated fat content of the diet.
To lower LDL cholesterol, energy derived from saturated fat can be reduced
if weight loss is desirable or replaced with either carbohydrate or monounsaturated
fat when weight loss is not a goal.
(Franz et al., 2004, p. S39)
MUFA Intake
Based on dietary intake data from CFSII (1994-96), median MUFA intake ranged
from 25 to 39 g per day for men and 18 to 24 g per day for women (IOM, 2002,
Appendix Table E-8). Data from the 1987-1988 Nationwide Food Consumption Survey
indicated that mean intakes of MUFAs for different age-gender groups were 13.6-14.3
percent of energy (Ganji and Betts, 1995).
In children and adolescents, MUFA intake ranged from 12.1 percent of energy
for males age 2 to 3 years and 4 to 5 years to 12.9 percent of energy for males
age 16 to 19 years. Among males age 12 to19 years, MUFAs accounted for 12.7 percent
of calories for non-Hispanic blacks compared with 12.6 percent and 12.8 percent
for non-Hispanic whites and Mexican Americans. For females age 12 to 19 years,
monounsaturated fatty acid intake was 13.5 percent for non-Hispanic blacks, compared
with 12.4 percent for non-Hispanic whites and 12.7 percent for Mexican Americans
(Troiano et al., 2000). Thus, the collective evidence from studies that have
assessed the diet of persons in the United States indicate that MUFA intake is
approximately 12 to 14 percent of calories.
Supplementary Information
Most MUFAs should be derived from plant sources rather than animal sources:
plant sources of MUFAs are lower in saturated fatty acids than are animal sources,
and plant sources contain no cholesterol. Also, some plant sources of MUFAs provide
vitamins and other compounds that may confer health benefits.
Back to Top
SUMMARY
To reduce the risks of elevated serum LDL cholesterol and of CHD, the Committee
recommends three measures:
Limiting saturated fat intake to less than 10 percent of calories Limiting trans fat intake as much as possible Limiting dietary cholesterol intake to less than 300 mg per day
To promote recommended intakes of vitamin E and essential fatty acids and to
decrease the risk of adverse changes in certain blood lipids, the Committee recommends
a total fat intake of at least 20 percent of calories. To help reduce the risk
of obesity and CHD, the Committee recommends keeping total fat intake at or below
35 percent of calories. Current mean intakes of n-6 PUFAs are within the recommended
range for essential fatty acid intake and for obtaining beneficial effects on
mortality from coronary artery disease. To reduce the risk of sudden death and
CHD death, the Committee recommends the consumption of fish twice weekly, especially
fish that are good sources of EPA and DHA. Other sources of EPA and DHA may provide
similar benefits; however, further research is warranted.
Back to Top
REFERENCES
Albert CM, Hennekens CH, O'Donnell CJ, Ajani UA, Carey VJ,
Willett WC, Ruskin JN, Manson JE. Fish consumption and risk
of sudden cardiac death. JAMA 279:23-8, 1998.
Allison DB, Egan SK, Barraj LM, Caughman C, Infante M, Heimbach
JT. Estimated Intakes of Trans Fatty and Other
Fatty Acids in the US Population. J Am Diet Assoc 99:166-74,
1999.
American Heart Association. Diet and Heart Disease.
New York, NY: American Heart Association, 1968.
Artaud-Wild SM, Connor SL, Sexton G, Connor WE. Differences
in coronary mortality can be explained by differences in cholesterol
and saturated fat intakes in 40 countries but not in France
and Finland. A paradox. Circulation 88:2771-9, 1993.
Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett
WC. Dietary intake of marine n-3 fatty acids, fish intake,
and the risk of coronary disease among men. N Engl J Med
332:977-82, 1995.
Ascherio A, Rimm EB, Giovannucci EL Spiegelman D, Stampher
M, Willett WC. Dietary fat and risk of coronary heart disease
in men: cohort follow up study in the United States. BMJ
313:84-90, 1996.
Ascherio A Katan MB, Zock PL, Stampfer MJ, Willett WC. Trans
fatty acids and coronary heart disease. N Engl J Med
340:1994-1998, 1999.
Ascherio A, Hennekens CH, Buring JE, Master C, Stampfer MJ,
Willett WC. Trans-fatty acids intake and risk of
myocardial infarction. Circulation 89:94-101, 1994.
Astorg P. Dietary N - 6 and N - 3 polyunsaturated fatty acids
and prostate cancer risk: a review of epidemiological and
experimental evidence. Cancer Causes Control 15:367-86,
2004.
Attar-Bashi NM, Frauman AG, Sinclair AJ. Alphα-linolenic
acid and the risk of prostate cancer. What is the evidence?
J Urol 171:1402-7, 2004.
Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty
acids affect plasma markers of inflammation in healthy men
fed controlled diets: a randomized crossover study. Am
J Clin Nutr 79:969-73, 2004.
Banni S, Angioni E, Murru E, Carta G, Melis MP, Bauman D, Dong Y, Ip C. Vaccenic
Acid Feeding Increases Tissue Levels of Conjugated Linoleic Acid and Suppresses
Development of Premalignant Lesions in Rat Mammary Gland Nutr Cancer 41:91-97,
2001.
Baylin A, Kabagambe EK, Ascherio A, Spiegelman D, Campos
H. High 18:2 trans-fatty acids in adipose tissue
are associated with increased risk of nonfatal acute myocardial
infarction in costa rican adults. J Nutr 133:1186-1191,
2003.
Bemelmans WJ, Broer J, Feskens EJ, et al. Effect of an increased
intake of alphα-linolenic acid and group nutritional education
on cardiovascular risk factors: the Mediterranean Alphα-linolenic
Enriched Groningen Dietary Intervention (MARGARINE) study.
Am J Clin Nutr 75:221-227, 2002.
Berrino F, Bellati C, Secreto G, Camerini E, Pala V, Panico
S, Allegro G, Kaaks R. Reducing bioavailable sex hormones
through a comprehensive change in diet: the diet and androgens
(DIANA) randomized trial. Cancer Epidemiol Biomarkers
Prev 10:25-33, 2001.
Bingham S, Riboli E. Diet & Cancer – The European Prospective
Investigation into cancer and nutrition. Nat Rev Cancer
4:206-15, 2004.
Bonanome A, Grundy SM. Effect of dietary stearic acid on
plasma cholesterol and lipoprotein levels. N Engl J Med
318:1244-8, 1988.
Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, Minkin
S. Dietary fat and breast cancer risk revisited: a meta-analysis
of the published literature. Br J Cancer 89:1672-85,
2003.
Briefel RR, Johnson CL. Secular trends in dietary intake
in the United States. Annu Rev Nutr 24:401-431, 2004.
Brouwer IA, Katan MB, Zock PL. Dietary α-linolenic acid is
associated with reduced risk of fatal coronary heart disease,
but increased prostate cancer risk: a meta-analysis. J
Nutr 134:919-22, 2004.
Brynes AE, Mark Edwards C, Ghatei MA, Dornhorst A, Morgan
LM, Bloom SR, Frost GS. A randomized four-intervention crossover
study investigating the effect of carbohydrates on daytime
profiles of insulin, glucose, non-esterified fatty acids and
triacylglycerols in middle-aged men. Br J Nutr 89:207-18,
2003.
Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam
PM, Elwood PC, Deadman NM. Effects of changes in fat, fish,
and fibre intakes on death and myocardial reinfarction: diet
and reinfarction trial (DART). Lancet 2:757-61, 1989.
Campbell LV, Marmot PE, Dyer JA, Borkman M, Storlien LH.
The high-monounsaturated fat diet as a practical alternative
for NIDDM. Diabetes Care 17:177-182, 1994.
Chanmugam P, Guthrie JF, Cecilio S, Morton JF, Basiotis PP,
Anand R. Did fat intake in the United States really decline
between 1989-1991 and 1994-1996? J Am Diet Assoc
103:867-72, 2003.
Clarke R, Frost C, Collins R, Appleby P, Peto R. Dietary
lipids and blood cholesterol: Quantitative meta-analysis of
metabolic ward studies. Br Med J 314:112–117, 1997.
Clarke SD. The multi-dimensional regulation of gene expression
by fatty acids: Polyunsaturated fats as nutrient sensors.
Curr Opin Lipidol 15:13-18, 2004.
Clifton PM, Keogh JB, Noakes M. Trans fatty acids
in adipose tissue and the food supply are associated with
myocardial infarction. J Nutr 134:874-879, 2004.
Consumer Reports. Omega-3 oil: Fish or pills? Consumer Unions
of U.S., Inc. July 2003. Corl BA, Barbano DM, Bauman DE, Ip
C. cis-9, trans-11 CLA derived endogenously from
trans-11 18:1 reduces cancer risk in rats. J
Nutr 133:2893-90, 2003.
De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova
R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia
G, Manger Cats V, Orth-Gomer K, Perk J, Pyorala K, Rodicio
JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood
D; Third Joint Task Force of European and Other Societies
on Cardiovascular Disease Prevention in Clinical Practice.
European guidelines on cardiovascular disease prevention in
clinical practice. Third Joint Task Force of European and
Other Societies on Cardiovascular Disease Prevention in Clinical
Practice. Eur Heart J 24:1601-10, 2003.
de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet,
traditional risk factors and the rate of cardiovascular complications
after myocardial infarction: Final report of the Lyon Diet
Heart Study. Circulation 99:779-785, 1999.
de Roos NM, Schouten EG, Scheek LM, van Tol A, Katan MB.
Replacement of dietary saturated fat with trans fat
reduces serum peroxidase activity in healthy men and women.
Metabolism 51:1534-37, 2002.
de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated
fatty acids by trans fatty acids lowers serum HDL
cholesterol and impairs endothelial function in healthy men
and women. Arterioscler Thromb Vasc Biol 21:1233-1237,
2001.
de Roos NM, Schouten EG, Katan MB. Trans fatty acids,
HDL-cholesterol, and cardiovascular disease. Effects of dietary
changes on vascular reactivity. Eur J Med Res 8:355-357,
2003.
Denke MA. Role of beef and beef tallow, an enriched source
of stearic acid, in a cholesterol-lowering diet. Am J
Clin Nutr 60:1044S-1049S, 1994.
Denke MA. Review of human studies evaluating individual dietary
responsiveness in patients with hypercholesterolemia. Am
J Clin Nutr 62:471S-477S, 1995.
Dixon LB, Ernst ND. Choose a diet that is low in saturated
fat and cholesterol and moderate in total fat: subtle changes
to a familiar message. J Nutr 131:510S-526S, 2001.
Djousse L, Pankow JS, Eckfeldt JH, et al. Relation between
dietary linolenic acid and coronary artery disease in the
National Heart, Lung, and Blood Institute Family Heart Study.
Am J Clin Nutr 74:612-619, 2001.
Do MH, Lee SS, Jung PJ, Lee MH. Intake of dietary fat and
vitamin in relation to breast cancer risk in Korean women:
a case-control study. J Korean Med Sci 18:534-40,
2003.
Dolecek TA. Epidemiological evidence of relationships between
dietary polyunsaturated fatty acids and mortality in the Multiple
Risk Factor Intervention Trial. Proc Soc Exp Biol Med
200:177-182, 1992.
Eurodiet Core Report. Nutrition and diet for healthy lifestyles
in Europe. Available at http://eurodiet.med.uoc.gr/.
Accessed on May 4, 2004. Additional information on the EuroDiet
Project published as supplements to Public Helath Nutrition.
Vol 4.2(A) and 2(B). 2001.
Federal Register Notice. Substances Affirmed as
Generally Recognized as Safe: Menhaden Oil Final Rule, Vol
62, No. 108, Pg 30751-30757, June 5, 1997.
Federal Register Notice. Food Labeling, Trans
Fatty Acids in Nutrition Labeling; Consumer Research to Consider
Nutrient Content and Health Claims and Possible Footnote or
Disclosure Statements; Final Rule and Proposed Rule. Vol.
68 No. 133 Pg. 41433-41506, July 11, 2003.
Food and Drug Administration (FDA) Food Advisory Committee,
Nutrition Subcommittee transcripts. April 28, 2004. Available
at http://www.fda.gov/ohrms/dockets/ac/04/transcripts/4035t1.htm.
Accessed on July 19, 2004.
Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic
syndrome among US adults: findings from the third National
Health and Nutrition Examination Survey. JAMA 287:356-359,
2002.
Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL,
Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian
AD, Purnell JQ, Wheeler M; American Diabetes Association.
Nutrition principles and recommendations in diabetes. Diabetes
Care 27 Suppl 1:S36-46, 2004.
Ganji V, Betts N. Fat, cholesterol, fiber and sodium intakes
of US population: evaluation of diets reported in 1987-88
Nationwide Food Consumption Survey. Eur J Clin Nutr
49:915-20, 1995.
Garcia-Palmieri MR, Sorlie P, Tillotson J, Costas R Jr, Cordero
E, Rodriguez M. Relationship of dietary intake to subsequent
coronary heart disease incidence: The Puerto Rico Heart Health
Program. Am J Clin Nutr 33:1818-27, 1980.
Garg A, Bantle JP, Henry RR, Coulston AM, Griver KA, Raatz
SK, Brinkley L, Chen I, Grundy SM, Huet BA, Reaven GM. Effects
of varying carbohydrate content of diet in patients with non-insulin
dependent diabetes mellitus. JAMA 271:1421-1428,
1994.
Garg A, Grundy SM, Unger RH. Comparison of effects of high
and low carbohydrate diets on plasma lipoproteins and insulin
sensitivity in patients with mild NIDDM. Diabetes
41:1278-85, 1992.
Gatto LM, Sullivan DR, Samman S. Postprandial effects of
dietary trans fatty acids on apolipoprotein(a) and
cholesteryl ester transfer. Am J Clin Nutr
77:1119-24, 2003.
Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio
A, Chute CC, Willett WC. A prospective study of dietary fat
and risk of prostate cancer. J Natl Cancer Inst 85:1571-9,
1993.
Gleason P, Suitor C. Food for thought: children's diets in the 1990s. Policy
brief. Princteon, NJ: Mathematica Policy Res, Inc. 2001.
Gordon T, Kagan A, Garcia-Palmieri M, Kannel WB, Zukel WJ,
Tillotson J, Sorlie P, Hjortland M. Diet and its relation
to coronary heart disease and death in three populations.
Circulation. 63:500-15, 1981.
Grundy SM, Abate N, Chandalia M. Diet composition and the
metabolic syndrome: what is the optimal fat intake? Am
J Med 113 Suppl 9B:25S-29S, 2002.
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant
C; American Heart Association; National Heart, Lung, and Blood
Institute. Definition of metabolic syndrome: Report of the
National Heart, Lung, and Blood Institute/American Heart Association
conference on scientific issues related to definition. Circulation.
109:433-8, 2004a.
Grundy SM, Hansen B, Smith SC Jr, Cleeman JI, Kahn RA; American
Heart Association; National Heart, Lung, and Blood Institute;
American Diabetes Association. Clinical management of metabolic
syndrome: report of the American Heart Association/National
Heart, Lung, and Blood Institute/American Diabetes Association
conference on scientific issues related to management. Circulation.
109:551-6, 2004b.
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake
DB, Pasternak RC, Smith SC Jr, Stone NJ; National Heart, Lung,
and Blood Institute; American College of Cardiology Foundation;
American Heart Association. Implications of recent clinical
trials for the National Cholesterol Education Program Adult
Treatment Panel III guidelines. Circulation. 110:227-239,
2004c.
Guallar E, Aro A, Jimenez FJ, Martin-Moreno JM, Salminen
I, van't Veer P, Kardinaal AF, Gomez-Aracena J, Martin BC,
Kohlmeier L, Kark JD, Mazaev VP, Ringstad J, Guillen J, Riemersma
RA, Huttunen JK, Thamm M, Kok FJ. Omega-3 fatty acids in adipose
tissue and risk of myocardial infarction: the EURAMIC study.
Arterioscler Thromb Vasc Biol 19:1111-8, 1999.
Hegsted DM. Serum-cholesterol response to dietary cholesterol:
a re-evaluation. Am J Clin Nutr 44:299-305, 1986.
Hegsted DM and Ausman LM. Diet, alcohol and coronary heart
disease in men. J Nutr 118:1184-9, 1988.
Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat
and serum lipids: an evaluation of the experimental data.
Am J Clin Nutr 57:875-83, 1993.
Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative
effects of dietary fat on serum cholesterol in man. Am
J Clin Nutr 17:281-95, 1965.
Heilbronn LK, Noakes M, Clifton PM. Effect of energy restriction,
weight loss, and diet composition on plasma lipids and glucose
in patients with Type 2 diabetes. Diabetes Care 22:889-895,
1999.
He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland
P. Accumulated evidence on fish consumption and coronary heart
disease mortality: a meta-analysis of cohort studies. Circulation.
109:2705-11, 2004a.
He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Goldbourt
U, Greenland P. Fish consumption and incidence of stroke:
a meta-analysis of cohort studies. Stroke. 35:1538-42,
2004. Epub May 20, 2004b.
Holmes MD, Hunter DJ, Colditz GA, Stampfer MJ, Hankinson
SE, Speizer FE, Rosner B, Willett WC. Association of dietary
intake of fat and fatty acids with risk of breast cancer.
JAMA 281:914-20, 1999.
Hopkins PN. Effects of dietary cholesterol on serum cholesterol:
a meta-analysis and review. Am J Clin Nutr 55:1060-70,
1992.
Howell WH, McNamara DJ, Tosca MA, Smith BT, Gaines JA. Plasma
lipid and lipoprotein responses to dietary fat and cholesterol:
a meta-analysis. Am J Clin Nutr 65:1747-64, 1997.
Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner
BA, Hennekens CH, Willett WC. Dietary fat intake and the risk
of coronary heart disease in women. N Engl J Med
337:1491-9, 1997.
Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert
CM, Hunter D, Manson JE.. Fish and omega-3 fatty acid intake
and risk of coronary heart disease in women. JAMA
287:1815-21, 2002.
Hu FB, Stampfer MJ, Manson JE, et al. Dietary intake of alphα-linolenic
acid and risk of fatal ischemic heart disease among women.
Am J Clin Nutr 69:890-897, 1999.
IOM (Institutes of Medicine). Dietary Reference Intakes:
Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol,
Protein, and Amino Acids. National Academy Press, Washington,
DC, 2002.
Ip C, Banni S, Angioni E, Carta G, McGinley J, Thompson HJ,
Barbano D, Bauman D. Conjugated linoleic acid-enriched butter
fat alters mammary gland morphogenesis and reduces cancer
risk in rats. J Nutr 129:2135-42, 1999.
Joossens JV, Geboers J, Kesteloot H. Nutrition and cardiovascular
mortality in Belgium. For the B.I.R.N.H. Study Group. Acta
Cardiol 44:157-82, 1989.
Judd JT, Baer DJ, Clevidence BA, Kris-Etherton PM, Muesing
RA, Iwane M. Dietary cis and trans monounsaturated
and saturated FA and plasma lipids and lipoproteins in men.
Lipids 37:123-131, 2002.
Kark JD, Kaufmann NA, Binka F, Goldberger N, Berry EM. Adipose
tissue n-6 fatty acids and acute myocardial infarction in
a population consuming a diet high in polyunsaturated fatty
acids. Am J Clin Nutr 77:796-802, 2003.
Keys A. Coronary heart disease in seven countries. 1970.
Nutrition 13:250-2, 1997.
Keys A. Effects of different dietary fats on plasma-lipid
levels. Lancet 17:318-9, 1965.
Knopp RH, Retzlaff B, Fish B, Walden C, Wallick S, Anderson
M, Aikawa K, Kahn SE. Effects of insulin resistance and obesity
on lipoproteins and sensitivity to egg feeding. Arterioscler
Thromb Vasc Biol 23:1437-43, 2003. Epub Jun 19, 2003.
Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum
RJ, Erdman JW Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA,
Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett
J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary
Guidelines: revision 2000: A statement for healthcare professionals
from the Nutrition Committee of the American Heart Association.
Circulation 102:2284-99, 2000.
Krauss RM. Atherogenic lipoprotein phenotype and diet-gene
interactions. J. Nutr 131:340S-343S, 2001.
Kris-Etherton PM, Harris W, Appel LJ. AHA Scientific Statement:
Fish consumption, fish oil, omega-3 fatty acids and cardiovascular
disease. Circulation 106:2747-57, 2002.
Kris-Etheron PM, Zhao G, Pelkman CL, Fishell VK, Coval SM.
Beneficial effects of a diet high in monounsaturated fatty
acids on risk factors for cardiovascular disease. Nutr
Clin Care 3:153-162, 2000.
Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn
H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A,
et al. Dietary saturated and trans fatty acids and
cholesterol and 25-year mortality from coronary heart disease:
the Seven Countries Study. Prev Med 24:308-15, 1995.
Kromhout D, Bloemberg BP, Feskens EJ, Hertog MG, Menotti
A, Blackburn H. Alcohol, fish, fibre and antioxidant vitamins
intake do not explain population differences in coronary heart
disease mortality. Int J Epidemiol 25:753-9, 1996.
Kromhout D. Fish consumption and sudden cardiac death. JAMA
279:65-6, 1998.
Kushi L, Giovannucci E. Dietary fat and cancer. Am J
Med 113 (Suppl 9B):63S-70S, 2002.
Kushi LH, Lew RA, Stare FJ, Ellison CR, el Lozy M, Bourke
G, Daly L, Graham I, Hickey N, Mulcahy R, et al. Diet and
20-year mortality from coronary heart disease. The Ireland-Boston
Diet-Heart Study. N Engl J Med 312:811-8, 1985.
Larsson H, Elmstahl S, Berglund G, Ahren B. Habitual dietary
intake versus glucose tolerance, insulin sensitivity and insulin
secretion in postmenopausal women. J Intern Med 245:581-91,
1999.
Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz
GC, Willett WC, Giovannucci EL. Dietary intake of n-3 and
n-6 fatty acids and the risk of prostate cancer. Am J
Clin Nutr 80:204-16, 2004.
Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann
S, Knopp RH, Copass MK, Cobb LA, Siscovick DS. Cell membrane
trans-fatty acids and the risk of primary cardiac
arrest. Circulation 105:697-701, 2002.
Lichtenstein, AH. Trans fatty acids, plasma lipid levels, and risk
of developing cardiovascular disease. A statement for healthcare professionals
from the American Heart Association.
Circulation 95:2588-90, 1997.
Lovejoy JC, Champagne CM, Smith SR, DeLany JP, Bray GA, Lefevre
M, Denkins YM, Rood JC. Relationship of dietary fat and serum
cholesterol ester and phospholipid fatty acids to markers
of insulin resistance in men and women with a range of glucose
tolerance. Metabolism 50:86-92, 2001.
Lovejoy JC, Smith SR, Champagne CM, Most MM, Lefevre M, DeLany
JP, Denkins YM, Rood JC, Veldhuis J, Bray GA. Effects of diets
enriched in saturated (palmitic), monounsaturated (oleic),
or trans (elaidic) fatty acids on insulin sensitivity
and substrate oxidation in healthy adults. Diabetes Care
25:1283-8, 2002.
Mauger JF, Lichtenstein AH, Ausman LM, Jalbert SM, Jauhiainen
M, Ehnholm C, Lamarche B. Effect of different forms of dietary
hydrogenated fats on LDL particle size. Am J Clin Nutr
78:370-5, 2003.
Mensink RP, Katan MB. Effect of dietary fatty acids on serum
lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler
Thromb 12:911-9, 1992.
Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary
fatty acids and carbohydrates on the ratio of serum total
to HDL cholesterol and on serum lipids and apolipoproteins:
a meta-analysis of 60 controlled trials. Am J Clin Nutr
77:1146-1155, 2003.
Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP,
Siscovick DS; Cardiovascular Health Study. Cardiac benefits
of fish consumption may depend on type of fish meal consumed.
Circulation 107:1372-7, 2003.
Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura
K, Willett WC, Rimm EB. Dietary intake of trans fatty acids
and systemic inflammation in women. Am J Clin Nutr
79:606-612, 2004.
National Cancer Institute PDQs Web site. Available at http://cancer.gov/cancerinfo/pdq/prevention.
Accessed on: March 15, 2004.
National Cholesterol Education Program (NCEP) Expert Panel
on Detection, Evaluation, and Treatment of High Blood Cholesterol
in Adults (Adult Treatment Panel III). Third Report of the
National Cholesterol Education Program (NCEP) Expert Panel
on Detection, Evaluation, and Treatment of High Blood Cholesterol
in Adults (Adult Treatment Panel III) final report. Circulation
106:3143-3421, 2002.
Natvig H, Borchgrevink CF, Dedichen J, et al. A controlled
trial of the effect of linolenic acid on incidence of coronary
heart disease. The Norwegian Vegetable Oil Experiment of 1965-1966.
Scand J Clin Lab Invest 105(Suppl.):1-20, 1968.
Nicklas TA, Dwyer J, Mitchell P, Zive M, Montgomery D, Lytle
L, Cutler J, Evans M, Cunningham A, Bachman K, Nichaman M,
Snyder P. Impact of fat reduction on micronutrient density
of children's diets: the CATCH Study. Prev Med 25:478-85,
1996.
Nicklas T, Johnson R; American Dietetic Association. Position
of the American Dietetic Association: Dietary guidance for
healthy children ages 2 to 11 years. J Am Diet Assoc
104:660-77, 2004.
Obarzanek E, Hunsberger SA, Van Horn L, Hartmuller VV, Barton
BA, Stevens VJ, Kwiterovich PO, Franklin FA, Kimm SY, Lasser
NL, Simons-Morton DG, Lauer RM. Safety of a fat-reduced diet:
the Dietary Intervention Study in Children (DISC). Pediatrics
100:51-9, 1997.
Perez -Jimenez F, Lopez-Miranda J, Pinillos MD, Gomez P,
Paz-Rojas E, Montilla P, Marin C, Velasco MJ, Blanco-Molina
A, Jimenez Pereperez JA, Ordovas JM. A Mediterranean and a
high-carbohydrate diet improve glucose metabolism in healthy
young persons. Diabetologia 44:2038-43, 2001.
Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC,
Albanes D, Virtamo J. Intake of fatty acids and risk of coronary
heart disease in a cohort of Finnish men. The Alpha-Tocopherol,
Beta-Carotene Cancer Prevention Study. Am J Epidemiol
145:876-87, 1997.
Poppitt SD, Keogh GF, Prentice AM, Williams DEM, Sonnemans
HMW, Valk EEJ, Robinson E, Wareham NJ. Long-term effects of
ad libitum low-fat, high-carbohydrate diets on body weight
and serum lipids in overweight subjects with metabolic syndrome.
Am J Clin Nutr 75:11-20, 2002.
Posner BM, Cobb JL, Belanger AJ, Cupples LA, D'Agostino RB,
Stokes J 3rd. Dietary lipid predictors of coronary heart disease
in men. The Framingham Study. Arch Intern Med 151:1181-7,
1991.
Prentice RL, Sheppard L. Dietary fat and cancer: consistency
of the epidemiologic data, and disease prevention that may
follow from a practical reduction in fat consumption. Cancer
Causes Control 1:81-97, 1990.
Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt
G, Brugada P, Camm JA, Cappato R, Cobbe SM, Di Mario C, Maron
BJ, McKenna WJ, Pedersen AK, Ravens U, Schwartz PJ, Trusz-Gluza
M, Vardas P, Wellens HJ, Zipes DP; European Society of Cardiology.
Update of the guidelines on sudden cardiac death of the European
Society of Cardiology. Eur Heart J 24:13-5, 2003.
Rasmussen OW, Thomsen C, Hansen KW, Vesterlund M, Winther
E, Hermansen K. Effects on blood pressure, glucose, and lipid
levels of a high-monounsaturated fat diet compared with a
high-carbohydrate diet in NIDDM subjects. Diabetes Care
16:1565-71, 1993.
Reaven G. Handbook of Physiology, Section 7: The Endocrine System. Volume 2:
The Endocrine Pancreas and Regulation of Metabolism. Oxford University Press.
Pp. 1169-97, 2001.
Schaefer EJ, Lamon-Fava S, Ausman LM, Ordovas JM, Clevidence
BA, Judd JT, Goldin BR, Woods M, Gorbach S, Lichtenstein AH.
Individual variability in lipoprotein cholesterol response
to National Cholesterol Education Program Step 2 diets.. Am
J Clin Nutr 65:823-30, 1997.
Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm
RA. Association of energy and fat intake with prostate carcinoma
risk: results from The Netherlands Cohort Study. Cancer
86:1019-27, 1999.
Scientific Advisory Committee on Nutrition. Paper FICS/04/02. March 2004.
Scott LW, Balasubramanyam A, Kimball K, Ahrens AK, Frodis
CM, Ballantyne CM. Long-term, randomized clinical trial of
two diets in the metabolic syndrome and type 2 diabetes. Diabetes
Care 28:2481-2, 2003.
Sheard NF. Fish consumption and risk of sudden cardiac death.
Nutr Rev 56:177-9, 1998.
Shekelle RB, Shryock AM, Paul O, Lepper M, Stamler J, Liu
S, Raynor WJ Jr. Diet, serum cholesterol, and death from coronary
heart disease. The Western Electric study. N Engl J Med
304:65-70, 1981.
Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri
M. Randomized, double-blind, placebo-controlled trial of fish
oil and mustard oil in patients with suspected acute myocardial
infarction: the Indian experiment of infarct survival--4.
Cardiovasc Drugs Ther 11:485-91, 1997.
Siscovick DS, Schwartz SM, Corey L, Grayston JT, Ashley R,
Wang SP, Psaty BM, Tracy RP, Kuller LH, Kronmal RA.. Dietary
intake of long chain n-3 polyunsaturated fatty acids and the
risk of primary cardiac arrest. Am J Clin Nutr 71:208-12,
2000.
Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund
KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH,
et al. Dietary intake and cell membrane levels of long-chain
n-3 polyunsaturated fatty acids and the risk of primary cardiac
arrest. JAMA 274:1363-7, 1995.
Stender S and Dyerbery J. The influence of trans fatty
acids on health. A Report from the Danish Nutrition Council.
2003.
Straznicky NE, O'Callaghan CJ, Barrington VE, Louis WJ. Hypotensive
effect of low-fat, high-carbohydrate diet can be independent
of changes in plasma insulin concentrations. Hypertension
34:580-5, 1999.
Tell GS, Evans GW, Folsom AR, Shimakawa T, Carpenter MA,
Heiss G. Dietary fat intake and carotid artery wall thickness:
the Atherosclerosis Risk in Communities (ARIC) Study. Am
J Epidemiol 139:979-89, 1994.
Toomey S, Roche H, Fitzgerald D, Belton O Regression of pre-established
atherosclerosis in the apoE-/- mouse by conjugated
linoleic acid Biochem Soc Trans 31:1075-1079, 2003.
Troiano RP, Briefel RR, Carroll MD, Bialostosky K. Energy
and fat intakes of children and adolescents in the United
Status: data from the Nacional Health and Nutrition Examination
Surveys. Am J Clin Nutr 72(suppl):1343S-53S, 2000.
Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E,
Fox KA, Julian D, Lengyel M, Neumann FJ, Ruzyllo W, Thygesen
C, Underwood SR, Vahanian A, Verheugt FW, Wijns W; Task Force
on the Management of Acute Myocardial Infarction of the European
Society of Cardiology. Management of acute myocardial infarction
in patients presenting with ST-segment elevation. The Task
Force on the Management of Acute Myocardial Infarction of
the European Society of Cardiology. Eur Heart J 24:28-66,
2003.
Vessby B, Unsitupa M, Hermansen K, Riccardi G, Rivellese
AA, Tapsell LC, Nalsen C, Berglund L, Louheranta A, Rasmussen
BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien
LH; KANWU Study. Substituting dietary saturated for monounsaturated
fat impairs insulin sensitivity in healthy men and women:
The KANWU Study. Diabetologia 44:312-9, 2001.
U.S. Senate Select Committee on Nutrition and Human Needs Dietary Goals for
the United States. 2nd ed. Washington, DC: U.S. Government Printing Office, 1977.
Wang C, Chung M, Balk E, Kupelnick B, DeVine D, Lawrence
A, Lichtenstein A, Lau J. Effects of omega-3 fatty acids
on cardiovascular disease. Evidence Report/Technology
Assessment No 04-E009-2. (Prepared by Tufts-New England Medical
Center Evidence-based Practice Center under Contract No. 290-02-0022).
AHRQ Publication No 04-E009-2. Rockville, MD: Agency for Healthcare
Research and Quality. March, 2004.
Wang Y, Jones PJH. Dietary conjugated linoleic acid and body
composition. Am J Clin Nutr 79(6 Suppl):1153S-58,
2004.
Weggemans RM, Rudrum M, Trautwein ET. Intake of ruminant
versus industrial trans fatty acids and risk of coronary
heart disease - what is the evidence? European Journal
of Lipid Science and Technology. 106:390-397,2004.
WHO Technical Report No. 916. Diet, Nutrition and the
Prevention of Chronic Diseases. Geneva, Switzerland.
2003.
Wien MA, Sabate JM, Ikle DN, Cole SE, Kandeel FR. Almonds
vs complex carbohydrates in a weight reduction program. Int
J Obes Relat Metab Disord. 27:1365-72, 2003.
Willett WC, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Rosner BA, Sampson
LA, Hennekens CH. Intake of trans fatty acids and risk of coronary heart
disease among women.
Lancet 34:581-5, 1993.
Yu S, Derr J, Etherton TD, Kris-Etherton PM. Plasma cholesterol-predictive
equations demonstrate that stearic acid is neutral and monounsaturated
fatty acids are hypocholesterolemic. Am J Clin Nutr
61:1129-39, 1995.
Zock PL, Katan MB. Hydrogenation alternatives: effects of
trans fatty acids and stearic acid versus linoleic
acid on serum lipids and lipoproteins in humans. J Lipid
Res 33:399-410, 1992.
Back to Top
1The agency for Health Care
Policy and Research (AHRQ) was developed to provide evidence-based
reports and technology assessments that could be used by
Federal and State agencies and private or public healthcare
organizations. In 1997, AHRQ, then known as the Agency for
Health Care Policy and Research (AHCPR), launched its initiative
to promote evidence-based practice in every-day care.AHRQ
established 12 Evidence-based Practice Centers (EPCs) by
awarding contracts to institutions throughout the United
States and Canada. The EPCs review relevant scientific literarure
on clinical, behavioral, and organizational topics that are
then used to develop evidence reports and technical assessments.
The EPCs are required to provide detailed documentation of
methods, rationale, and assumptions used throughout the process.
EPCs also collaborate with other medical and research organizations
in order to include a broad range of experts in the developmental
process. In March 2004, AHRQ released several evidence-based
reviews related to n-3 fatty acids, including an evidence-based
review on the effects of n-3 fatty acids on cardiovascular
disease.
2For persons with known heart
disease, medical advice and the use of ATP III Panel Guidelines
are indicated.
3Plant sterols are isolated
from soybean and tall pime-tree oils. Plant sterols can be
esterified to unsaturated fatty acids, creating sterol esters.
Hydrogenating sterols produces plant stanols and, with esterification,
stanol esters.
(NCEP, 2002)
Back to Top Table D4-1. Major Food Sources
of Trans Fat for
U.S. Adults
| Cakes, cookies, crackers, pies, bread, etc |
40 |
| Animal products |
21 |
| Margarine |
17 |
| Fried potatoes |
8 |
| Potato chips, corn chips, popcorn |
5 |
| Household shortening |
4 |
| Salad dressing |
3a |
| Breakfast cereal |
1 |
| Candy |
1 |
aUSDA analysis reported 0 grams
of trans fat in salad dressing.
Back to Top
Table D4-2. Correlation Coefficients Between Dietary
Polyunsaturated Fat (PUFA) Intake and Coronary Artery Disease
Mortality and Relative Risk or Cases
 Select icon to view table
Select icon to view table
Back to Top
Table D4-3. EPA and DHA Content of Selected Types
of Fish
| Cod, Atlantic, cooked, dry heat |
0.134 |
| Crab, Alaska king,
cooked, moist heat |
0.351
|
| Flounder, cooked, dry heat |
0.426 |
| Haddock, cooked,
dry heat |
0.202
|
| Mackerel, Pacific and jack, mixed
species, cooked, dry heat |
1.571 |
| Pollock, Atlantic,
cooked, dry heat |
0.461
|
| Salmon, Atlantic, farmed, cooked,
dry heat |
1.825 |
| Shrimp, mixed species,
cooked, moist heat |
0.268
|
| Snapper, mixed species, cooked, dry
heat |
0.273 |
| Trout, mixed species,
cooked, dry heat |
0.796 |
| Tuna, fresh, bluefin, cooked, dry
heat |
1.278 |
| Tuna, light, canned
in water, drained solids |
0.230 |
| Tuna, white, canned in water, drained
solids |
0.733 |
Back
to Top
Table D4-4. Examples of the Many Varying patterns
of Fats, Fatty Acids, and Carbohydrates Possible
Protein level can be determined
by difference [total energy percent –
(total fat percent + carbohydrate percent)
= protein percent]
 Select icon to
view table
Select icon to
view table
Back
to Top
Figure D4-1. IOM Figure 8-2: Relationship Between Serum Total Cholesterol Concentrations and Saturated Fatty Acid Intake
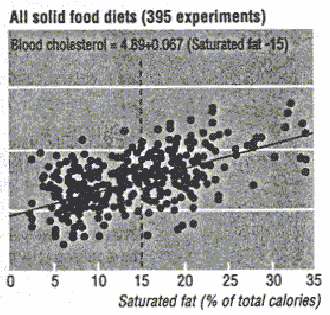 d d
Source: IOM (Institutes of Medicine).
Dietary Reference Intakes: Energy, Carbohydrates,
Fiber, Fat, Fatty Acids, Cholesterol,
Protein, and Amino Acids. Washington,
DC: National Academies Press, 2002.
Back
to Top
Figure D4-2. IOM Figure 8-3: Calculated Changes in Serum LDL Cholesterol Concentration in Response to Percent Change Dietary Saturated Fatty Acids
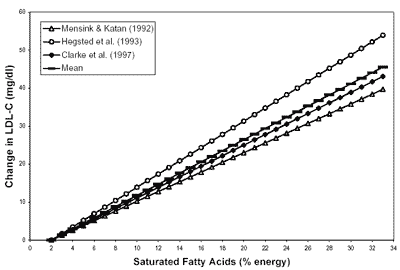 d d
Back
to Top
Figure D4-3. IOM Figure 8-4:
Change in the LDL:HDL Cholesterol Concentrations
With Increasing Energy Intake From Saturated
and Trans Fatty Acids
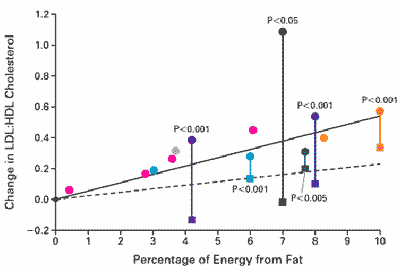 d d
Back to Top
Figure D4-4. IOM Figure 9-2: Relationship Between Change in Dietary Cholesterol (0 to 4500 mg/day) and Change in Serum Cholesterol Concentration.
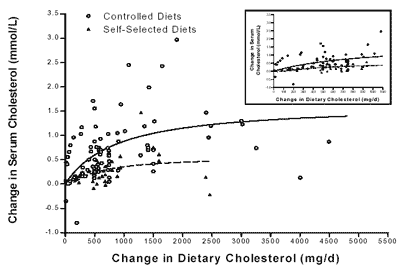 d d
Back to Top
Figure D4-5. IOM Figure11-4:
Relationship Between Monounsaturated
Fatty Acid Intake and Total Cholesterol
(TC):HDL Cholesterol (HDL-C) Concentration
Ratio
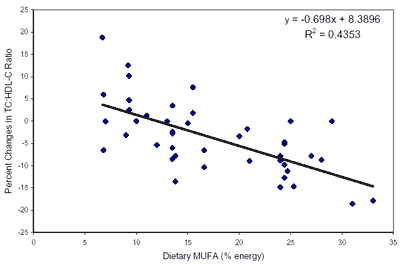 d d
Back to Top
|

