FAQs: Antimicrobial Use (AU) Option
General Submission
Q1: I’m interested in submitting data into the AU Option. Where do I start?
If your facility is not enrolled in NHSN, follow the 5-step enrollment process here: 5-Step Enrollment for Acute Care Hospitals/Facilities.
If your facility is already enrolled in NHSN and now wants to submit AU data, ensure your facility meets the requirements for NHSN AU Option participation: 1) ability to obtain data from either an electronic medication administration record (eMAR) or barcoding medication administration (BCMA) system and 2) ability to package data into Clinical Data Architecture (CDA) standardized format for upload into NHSN.
The NHSN AU Option does not allow manual data entry due to the amount of data submitted each month. Many facilities use a vendor system to package and submit AU data to NHSN in CDA format. There are a number of vendor systems listed on the Society for Infectious Disease Pharmacists websiteexternal icon that self-identify as providing services and software that allow NHSN AUR Module participation. The NHSN website also lists vendors that have an NHSN validated Antimicrobial Use (AU) reporting solution. The AU Synthetic Data Set (SDS) FAQ explains the difference between these two listings.
Your facility may already use one of these vendors so start by asking if 1) your facility already has the capability to submit AU data to NHSN using your current vendor or 2) your current vendor offers this capability as an “add on” feature. Some facilities can leverage internal information technology and informatics resources to report these data via a “homegrown” system. However, NHSN does not recommend this submission method for most facilities because it requires specialized knowledge of coding and data aggregation. If your facility is considering taking up this work internally, you can find the AU and AR CDA toolkits, which contain sample CDA files, the link to the CDA Implementation Guide, and other helpful hints here: NHSN CDA Submission Support Portal (CSSP).
You can find details about the NHSN AU Option data requirements on the NHSN AUR Module webpage, within the training slides and AUR protocol.
Q2: Do I need a SAMS card to view or submit AU data to NHSN?
Yes. If you are new to NHSN, you must complete the required NHSN training, review and accept the NHSN Rules of Behavior, and complete the SAMS enrollment process pdf icon[PDF – 250 KB]. All NHSN users must follow these steps before using the application. After you receive your SAMS card, a user with administrative rights at your facility, such as the facility administrator, must add you to the NHSN AU Option following the steps in question #7.
Q3: I received my SAMS card and now have access to my NHSN facility. How do I submit my AU data to NHSN?
Now that you have access to the NHSN AU Option, you must set up a monthly reporting plan before submitting your AU data. See question #16 for details on how to create a monthly reporting plan. CDC developed a 12-minute Quick Learn video discussing uploading CDA files into NHSN [Video – 12 min] to help facilities with this process.
Q4:Does CMS require reporting to the NHSN AUR Module? If not, could you tell me when CMS will require hospitals to report data into the AUR Module?
CMS does not require NHSN AUR Module data submission for any quality reporting program. CMS determines when to include new measures in their quality reporting programs. Users should direct questions about inclusion of AU in quality reporting programs to CMS.
However, NHSN AUR Module data submission via Clinical Document Architecture (CDA) is part of the Promoting Interoperability Program (formerly Meaningful Use Stage 3, or MU3) for Public Health Registry reporting for eligible hospitals. See questions #13-15 for more information about the Promoting Interoperability Program.
Q5: Is there a deadline submitting AU data into NHSN each month?
There are no timeline requirements for NHSN AU data submission. However, we recommend facilities upload data into NHSN within 30 days following the completion of the month to make the data most actionable. We also encourage facilities to upload retrospective data, if available, after initial AU Option implementation.
Q6: How far back can we submit retrospective AU Option data?
The business rules for monthly reporting plans determine how far back your facility can submit retrospective data. Your facility can submit AU Option data as far back as January 2012 if you have existing monthly reporting plans during that time, or January of the previous calendar year if no past monthly reporting plans exist.
Below are two examples to help explain this:
- Facility A has existing monthly reporting plans for every month going back to January 2016. They can edit the monthly reporting plans to submit retrospective AU Option data back to January 2016.
- Facility B just started submitting data to NHSN and does not have any existing monthly reporting plans (including monthly reporting plans for HAI data). They can add monthly reporting plans going back to January of the year they enrolled in NHSN or back to the January of one calendar year in the past, whichever comes first.
- If Facility B enrolled in January 2019 and it’s December 2019, they can add monthly reporting plans and submit AU Option data back to January 2019.
- If Facility B enrolled in January 2018 and it’s December 2019, they can add monthly reporting plans and submit AU Option data back to January 2018.
Q7: My pharmacist requested access to NHSN to upload data into the AU Option. How do I add him/her as a user?
A user with administrative rights, such as the facility administrator, must follow these steps to add users to the NHSN AU Option:
- From the NHSN Homepage, navigate to Users on the left-hand side menu.
- Select Add User.
- Complete all fields marked with a red asterisk (*) and select Save.
- On the next screen, assign user rights and select Save. Users need rights to the Patient Safety Component to view and submit AU data. If the user will submit AU data, NHSN recommends Administrator-level user rights. You can use the Custom Rights option to limit user rights to just AU data and no other patient safety modules. Be careful when assigning Custom Rights, as certain settings will prevent users from accessing AU data.
For complete details and instructions, please refer to the NHSN AU User Rights document pdf icon[PDF – 350 KB].
Q8: Who can see my facility’s AU data in NHSN?
Once you upload data into NHSN, only users at your NHSN facility have access to it. No outside groups have access to view your data without your permission. There is a special option for Groups (such as a corporate healthcare system or a health department) to request rights to view your data, but you must accept the request before NHSN shares your data with them. Because CMS does include these data any quality reporting programs, NHSN does not share them with CMS.
Q9: How do I delete AU data?
You can delete AU data by following the steps below:
- From the NHSN Homepage, select Summary Data then Delete AUR Data on the left-hand side menu.
- Select Antimicrobial Use Data as the Summary Data Type, then select the Location, Month, and Year you want to delete and click the Delete button.
Q10: How do I edit AU data I already imported to NHSN?
The NHSN AU Option does not allow manual data editing. To update an existing record with new information, you can use succession management. Many vendors implemented this feature by allowing users to simply export a new version of files to NHSN, but you will need to work with your vendor to determine if they offer this option.
You must regenerate data sets within NHSN after uploading the corrected CDA file to run analysis reports with the updated data. You can find additional details on succession management on the NHSN CDA Submission Support Portal (CSSP).
If your vendor does not offer this feature, you can manually delete and re-upload the data by following the steps in question #9.
Q11: I logged into my facility and can see that I have alerts for missing AU data. How do I get rid of them?
The missing summary data alerts mean that you included the AU Option in your monthly reporting plan but did not import these data into NHSN. You can clear the missing summary data alerts by:
- Submitting the AU data for those locations/months.
OR - Removing the AU Option for those locations from the monthly reporting plan(s).
The alerts are just a reminder and don’t affect your AU data in any way.
Q12: I’m from a health department. Could you tell me the names and NHSN orgIDs of the facilities submitting AU data from my jurisdiction?
NHSN’s updated Agreement to Participate and Consent allows NHSN to share specific information with State & Local Health Departments, Veterans Affairs (VA), or Department of Defense (DoD) for prevention purposes, such as the NHSN orgIDs and names of facilities submitting AU and/or AR data into NHSN.
You may use the NHSN Group function to request access to facilities’ NHSN data. Health systems and health departments commonly use this NHSN feature. The Group Administrator manages the Group centrally and, while all member facilities share data with the Group, individual facilities do not have the ability to see data from other members.
Promoting Interoperability (formerly Meaningful Use)
Q13: How do I enroll in the Promoting Interoperability Program (formerly Meaningful Use 3)?
NHSN AU and AR Option data submission via Clinical Document Architecture (CDA) are part of the Promoting Interoperability Program (formerly Meaningful Use 3, or MU3) for eligible hospitals as outlined on the NHSN CDA Submission Support Portal (CSSP) Meaningful Use page. Hospitals must submit both AU and AR Option data to qualify for the Promoting Interoperability Program. You can find additional details about the Promoting Interoperability Program here: CDC Public Health and Promoting Interoperability Programs.
Q14: On my MU3 report it shows “No” by AU and AR data, but my facility submitted data. Why is this?
Your vendor system must create CDA files using specific CDA Implementation Guide requirements to qualify for MU3 reporting. To confirm that your AUR data uploaded successfully, generate data sets within NHSN and run a line list. If your data show up in the line list for the months listed as “No” in the MU3 report, we suggest you speak with your vendor to determine why your data are not showing up on the MU3 report. If your data do not show up in the line list, they did not successfully upload to NHSN. Re-upload the data and try again.
Q15: My facility no longer plans to use AUR reporting to meet MU3. Can I remove my MU3 intent to submit?
There is not currently a way to remove your MU3 registration within NHSN. Since your facility registered its intent for MU3 within NHSN, you will still receive the automated monthly status report emails. You can disregard these emails if you do not plan to participate in MU3.
Monthly Reporting Plan
Q16: How do I include the AU Option in my monthly reporting plan?
You need to add the AU Option to your monthly reporting plan for every month you plan to submit AU data.
To add a new monthly reporting plan, if one does not already exist, follow these steps:
- From the NHSN Homepage, select Reporting Plan from the left side menu.
- Click Add to add a new monthly reporting plan.
- Select the month and year for AU data submission.
- Scroll down to the Antimicrobial Use and Resistance Module section of the plan, enter all the locations for which you’ll submit AU data that month, and check the AU box (See screen shot below for reference).
- Click the Save button at the bottom of the screen.
To edit an existing monthly reporting plan, if one already exists, follow these steps:
- From the NHSN Homepage, select Reporting Plan from the left side menu.
- Click Find to find an existing monthly reporting plan.
- Select the month and year for the AU data submission and click Find again.
- Scroll down to the bottom of the plan and click Edit.
- In edit mode, scroll down to the Antimicrobial Use and Resistance Module section of the plan, enter all the locations for which you’ll submit AU data that month, and check the AU box (See screen shot below for reference).
- Click the Save button at the bottom of the screen.

NOTE: You do not have to check the AR boxes unless you also plan to submit AR Option data.
Q17: Is a monthly reporting plan required for AU reporting?
Yes, the AU Option requires a monthly reporting plan with AU reporting indicated because facilities cannot enter AU data “off-plan”. See question #16 for how to set up a monthly reporting plan.
- Non-facility-wide inpatient (FacWideIN) locations can be the only location in the AU section of the monthly reporting plan.
- FacWideIN MUST have at least one other location added to the plan to save the monthly reporting plan.
Note: CMS does not include AUR Module data in any quality reporting program so CDC will not share AUR data with CMS, even if included in the monthly reporting plan. View the complete list of data required for each CMS Quality Reporting Program pdf icon[PDF – 1 MB].
Locations
Q18: What locations can I report AU data for?
NHSN strongly encourages AU data submission from all NHSN-defined inpatient locations, FacWideIN, and select outpatient acute care settings (specifically Emergency Department, Pediatric Emergency Department, and 24-hour Observation Area) from which your facility can accurately capture numerator and denominator data. Your facility should not submit data from locations that cannot accurately capture electronic data. Additionally, your facility should exclude these locations’ data from the FacWideIN record.
Q19: Should I include outpatient locations in the FacWideIN CDA file?
No. Your facility should only include inpatient locations (where patients stay overnight) with electronically captured numerator (antimicrobial days) and denominator (days present & admissions) data in the FacWideIN record. Your facility should not include outpatient locations such as Emergency Department and 24-hour Observation Area in the FacWideIN record.
Q20: Should I count antimicrobials administered to observation patients in my AU data?
Your facility should include antimicrobials administered to all patients physically residing in an inpatient location at any time during a given month in the location specific and FacWideIN CDA files, regardless of patient status (inpatient, outpatient, observation, etc.). While NHSN considers the 24-hour Observation Area location an outpatient location in NHSN, not all facilities designate a separate physical location for observation patients. If your facility has a 24-hour Observation Area mapped in its NHSN Location Manager, you should not include antimicrobials administered to patients physically residing in the 24-hour Observation Area in the FacWideIN record. However, if your facility does not have a separate unit for observation patients, you may include their antimicrobial administrations in the location specific and FacWideIN CDA files for the location in which they physically reside, regardless of patient status (inpatient, outpatient, observation, etc.).
Q21: Should I include AU data from the inpatient rehab facility (IRF) or inpatient psychiatric facility (IPF) physically located in my hospital with the rest of my AU data?
Your facility can include IRF and/or IPF AU data for IRFs/IPFs mapped as locations within your facility in NHSN. Your facility should also include the IRF/IPF location in your facility’s FacWideIN record. Because your facility mapped the IRF/IPF as an NHSN location, you can add it to the AUR section of the monthly reporting plan regardless of whether it has a separate CMS Certification Number (CCN) from your hospital.
If your facility enrolled the IRF/IPF as a separate NHSN facility, you cannot report its AU data with the acute care hospital, but you can report it under the separate NHSN facility. The AU Option still accepts data from an IRF or IPF enrolled in NHSN as its own facility using the rehabilitation hospital or psychiatric hospital designation. The submission process for these hospital designations is no different than a general acute care hospital.
Antimicrobial Days
Q22: Should I include topical, ocular, and aural antimicrobial administrations in my AU data?
The AU Option only accepts four routes of administration: intravenous (IV), intramuscular (IM), digestive, and respiratory. Your facility should exclude any other routes of administration (for example, topical, antibiotic locks, intraperitoneal, intraventricular, ocular, aural, or irrigation) from AU Option reporting, including total antimicrobial days and sub-stratification of the routes of administration.
Q23: If a patient receives two antimicrobials in one day, does that count as one or two antimicrobial days?
The AU Option considers each antimicrobial separately. If a patient receives two different drugs (for example, meropenem and amikacin) in the same calendar day, that patient contributes one total meropenem antimicrobial day and one total amikacin antimicrobial day to the location where the provider administered the antimicrobials. The same logic applies if the patient received antimicrobials via two different routes of administration (for example, IV meropenem and PO [digestive] amikacin).
If a patient is on a single antimicrobial (for example, ciprofloxacin) and the provider administers one dose in the morning via IV and another dose in the evening via PO, that patient contributes one IV ciprofloxacin antimicrobial day and one PO ciprofloxacin antimicrobial day. Because a single patient cannot contribute more than one antimicrobial day to the total antimicrobial days for each antimicrobial in a single calendar day, the patient only contributes one total ciprofloxacin antimicrobial day for each location.
See Appendix C starting on page 19 of the AUR Module Protocol pdf icon[PDF – 1 MB] for additional examples.
Q24: Providers administer vancomycin to patients in renal failure every other day but the drug stays in their system for two days. Does this count as one or two vancomycin antimicrobial days within the AU Option?
This counts as one vancomycin antimicrobial day because, even in the case of renal impairment, the AU Option only counts antimicrobials on the day of administration.
Q25: Why don’t the total antimicrobial days in my FacWideIN record equal the sum of antimicrobial days from all my location records?
The sum of location-specific antimicrobial days will always be greater than FacWideIN antimicrobial days because multiple administrations of an antimicrobial in separate patient care locations within a single calendar day account for one antimicrobial day within each location but only one FacWideIN antimicrobial day. For example, if a patient received one dose of vancomycin in the Medical Ward and then transferred to the Medical Intensive Care Unit (ICU), where they received another dose of vancomycin on the same day, this patient contributes one vancomycin antimicrobial day to the Medical Ward and one vancomycin antimicrobial day to the Medical ICU, but only one vancomycin antimicrobial day to FacWideIN. Patients may only contribute one antimicrobial day to each location (including FacWideIN) per day.
Q26: The sum of the routes of administration in my AU line list does not equal total antimicrobial days for some antimicrobials. Why is this?
The sum of the four routes of administration for a given antimicrobial should always be greater than or equal to the antimicrobial’s total antimicrobial days. In cases where a provider administered the antimicrobial more than once per day via multiple routes, the sum of the routes can be greater than the total antimicrobial days for that antimicrobial.
Keep in mind that the total antimicrobial days for a given antimicrobial should only include administrations via one of the four routes accepted into the AU Option (IV, IM, digestive, and respiratory). Your facility should exclude administrations via any other route from all AU Option data. Check with your vendor to make sure they only include the four routes listed above in your total antimicrobial days count.
Q27: Should partial antimicrobial administrations count towards antimicrobial days?
No. Your facility should only include completed administrations in AU data.
Days Present
Q28: Is the days present denominator used in the AU Option the same as the patient days denominator used in other parts of NHSN?
No. The days present denominator is different from the patient days denominator. Days present are the number of patients present at any time on a given day in each patient care location. Patient days are the number of patients present in each patient care location during the once daily census count.
For example, a patient admitted to the medical ward on Monday and discharged two days later, on Wednesday, will contribute three days present to the medical ward because the patient was there at some point during each of the three calendar days (Monday, Tuesday, and Wednesday). If this medical ward used midnight as the time of their daily census count, this same patient would only attribute two patient days because they were present for the midnight census count on Monday and Tuesday only.
Please note, the NHSN AU Option only counts a patient once for each location each day. For a given location or FacWideIN, the days present count should almost always be higher than the patient days count because days present take patient transfers and discharges into account while patient days do not.
Q29: If a patient transfers between locations in one calendar day, how many days present does that patient contribute to the FacWideIN record?
The patient contributes one day present to the FacWideIN record. The NHSN AU Option only counts a patient once per calendar day for the FacWideIN record.
Q30: Should my days present be equal to my patient days for a given location?
No. For a given location or FacWideIN, the days present count will almost always be higher than the patient days count because days present take patient transfers and discharges into account while patient days do not. On average, for all NHSN AU Option reporting hospitals, days present are 24% higher than patient days for the same month/location. There is some variation based on the location type. The percent difference is generally smaller in locations with longer lengths of stay and larger in locations with shorter lengths of stay. The table below provides the median percent difference between days present and patient days for select location types and the interpretation based on the average for all AU Option reporting facilities. NHSN calculated the percent difference with the following formula:
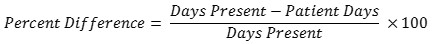
| Location Type | Median Percent Difference | Interpretation |
|---|---|---|
| FacWideIN | 24% | Days present are 24% higher than patient days for the same month/location at the FacWideIN level. |
| Adult ICUs | 25% | Days present are 25% higher than patient days for the same month/location in adult ICUs. |
| Adult Wards | 25% | Days present are 25% higher than patient days for the same month/location in adult ward locations. |
| Pediatric ICUs | 24% | Days present are 24% higher than patient days for the same month/location in pediatric ICUs. |
| Pediatric Wards | 35% | Days present are 35% higher than patient days for the same month/location in pediatric ward locations. |
| Neonatal ICUs | 8% | Days present are 8% higher than patient days for the same month/location in neonatal ICU locations. |
| Neonatal Step-Down Unit | 16% | Days present are 16% higher than patient days for the same month/location in neonatal step-down units. |
| Well Baby Nursery (Level I) | 34% | Days present are 34% higher than patient days for the same month/location in well baby nursery locations. |
| Labor, Delivery, Postpartum Units | 43% | Days present are 43% higher than patient days for the same month/location in labor and delivery locations. |
| Outpatient Locations (ED, Pediatric ED, 24 Hour Observation Locations) |
14% | Days present are 14% higher than encounters for the same month/location in outpatient locations. |
Take a closer look at your AU days present denominators to ensure your vendor system aggregates them correctly, as they can influence your SAAR calculations. Additionally, NHSN recommends validating your AU days present denominators using the methodology starting on page 10 in the AU Option Implementation Data Validation protocol pdf icon[PDF – 1 MB].
Data Import
Q31: Can I enter AU data manually?
No. Though some NHSN Modules allow for both CDA import and manual data entry, the AU Option only accepts data via CDA import due to the amount of data submitted each month. Please review the Uploading CDA Files into NHSN Quick Learn video [Video – 12 min] for additional details.
Q32: My vendor asked me to provide my facility’s OID. What is an OID and where do I get one?
An Object Identifier (OID) is a unique identifier for your NHSN facility. The AU Option uses the OID in CDA files to identify which facility submitted the files. If your facility submits data to other NHSN Modules via CDA Import, you may already have an OID. To verify if your facility has or needs an OID, navigate through the NHSN application as shown below.

If you’ve verified that your facility does not have an OID, follow the steps outlined here to obtain one: Object Identifier (OID) Entry Procedure pdf icon[PDF – 30 KB].
Q33: Can I submit AU and AR CDA files together in the same zip file?
Yes. For manual upload, all the CDA files in a zipped file must be from one facility. For DIRECT submission, the zipped file can contain CDA files for multiple facilities. For both manual and DIRECT, the zipped file can contain up to 1,000 CDA files or a maximum of 2 MB, whichever comes first. Please note: NHSN only accepts alphanumeric characters, hyphens, and underscores in CDA and zip file names. NHSN does not accept other special characters.
Q34: How many antimicrobials do I need to include in my AU CDA file?
AU Option CDA files require 85-92 antimicrobials, regardless of whether your facility used all them that month. Appendix B of the AUR Protocol pdf icon[PDF – 1 MB] lists the required antimicrobials.
Please note: Users are required to report remdesivir in AU files for summary months on and after July 2020. AU files for summary months on or after July 2020 will fail to upload into NHSN if they do not include remdesivir. Additionally, users can optionally include remdesivir in AU files for summary months January – June 2020.
CDA files containing AU data for January 2020 and later must now include meropenem/vaborbactam. CDA files can optionally include amikacin liposomal, baloxavir marboxil, colistin, eravacycline, plazomicin, and omadacycline. Users can report these optional antimicrobials for any year.
Q35: What do I do if I did not have at least one administration of all 85-92 antimicrobials each month?
You must report a value – a specific number, 0, or NA – for every antimicrobial listed in Appendix B of the AUR Protocol pdf icon[PDF – 1 MB], regardless of actual administration in your facility/location in a given month.
- Zero (0) – Use when your facility can electronically capture administrations of the antimicrobial in the eMAR/BCMA but did not administer it to any patients during the given month.
- Example: If your system can electronically capture amoxicillin via eMAR/BCMA but no providers administered it to any patients during the month, the amoxicillin total antimicrobial days count should be 0.
- The CDA file expresses this as value=”0″.
- Not applicable (NA) – Use when your facility cannot electronically capture administrations of the antimicrobial.
- Example: If a provider gives amikacin via respiratory route to patients throughout the month, but the eMAR/BCMA cannot accurately capture the administrations, the amikacin antimicrobial day respiratory count should be ‘NA’.
- The CDA file expresses this as nullFlavor=”NA”.
Q36: I don’t see an option for uploading AU data. Please help!
In NHSN, after selecting Import/Export on the navigation bar, you should see “AUR Summary Data” or “Events, Summary Data, Procedures Denominators” as an option to upload CDA files [Video – 12 min]. Both options allow you to upload AU data into NHSN. If you do not see either of these options, a user with administrative rights must change your user rights. See the NHSN AU User Rights pdf icon[PDF – 350 KB] document for more information.
Q37: How do I know if all the CDA files I submitted together in the same zip uploaded into NHSN successfully?
Sometimes, when uploading multiple AU files together, some files successfully upload and others do not. Here is a screenshot of what it looks like when you submit files together and some records pass but others fail. Note that both the Error Report and Submit buttons are enabled:

If you click on the Summary Data tab in the Validation Results table, you can see the files that passed and failed validation by looking in the Status column. In this instance, the user submitted two records – one passed validation and one failed. When you click the Submit button, only the file that successfully passed validation uploads to NHSN. Clicking the submit button generates a PDF report, which shows the file(s) that successfully imported and the file(s) that did not pass validation and did not import.
Q38: When I try to upload my AU data, I get an error message that says “Please upload files with .zip extensions only. Try again.” What does that mean?
The NHSN AU Option requires users to upload CDA files in a zip file. Try zipping the file(s) and resubmitting. Note that each zip file can contain up to 1,000 CDA files or a maximum of 2 MB, whichever comes first.
Q39: When I try to upload my AU data, I get an error message that says, “Antimicrobial Use and Resistance Module not followed for this month, year, and location.” What does that mean?
This error message means that you did not add the month, year, and location in the CDA file you’re trying to upload in the Antimicrobial Use and Resistance portion of your Monthly Reporting Plan. You must add the location(s) to the Antimicrobial Use and Resistance portion of your Monthly Reporting Plan for every month you plan to submit AU data. NHSN will not accept out-of-plan data. To edit your monthly reporting plan to include AU, follow the steps in question #16. After you edit your monthly reporting plan to include AU for the desired month, year, and location, NHSN should accept the CDA file.
If you verified you selected this location is on your monthly reporting plan, incorrect location information in your CDA file may be causing the error. AU CDA files use the exact “Your Code” value as well as the “NHSN HL7 code” from the NHSN Location Manager. To see your NHSN location manager, select Facility on the left-hand navigation bar, then Locations, and then Find. We suggest you reach out to your vendor to verify the location information in NHSN matches the information in the CDA files.
General AU Option Analysis
Q40: How do I view my AU data once I upload it into NHSN?
You can only view AU Option data using the NHSN Analysis function. You can find details on AU Option analysis in the NHSN AUR Module Protocol pdf icon[PDF – 1 MB] or in the Analysis Resources section of the NHSN AUR Module web page.
NHSN provides two-to-four-page analysis quick reference guides to assist with viewing, modifying, and interpreting AU Option data:
- Antimicrobial Use Line List pdf icon[PDF – 300 KB]
- Antimicrobial Use Rate Table – By Location pdf icon[PDF – 225 KB]
- Antimicrobial Use Rate Table – FacWideIN pdf icon[PDF – 300 KB]
- Antimicrobial Use SAAR Baseline Rate Tables pdf icon[PDF – 800 KB]
- Antimicrobial Use Bar Chart pdf icon[PDF – 300 KB]
- Antimicrobial Use Bar Chart – Selected Drugs pdf icon[PDF – 400 KB]
- Antimicrobial Use Pie Chart pdf icon[PDF – 300 KB]
- Antimicrobial Use SAAR Table pdf icon[PDF – 1 MB]
- Antimicrobial Use SAAR Table – By Location pdf icon[PDF – 750 KB]
- Antimicrobial Use – SAAR Bar Chart by Location pdf icon[PDF – 1 MB]
You must regenerate data sets within NHSN after uploading new data to run analysis reports with the most current data. See question #41 for more information on generating data sets.
Q41: I just uploaded data for a specific month but I don’t see it in the analysis reports. What happened to my data?
Newly uploaded data do not appear in the NHSN analysis outputs until you generate new data sets. Your data set is a snapshot of the data currently in your NHSN facility at the time you click the “Generate Reporting Data Sets” button. Always generate new data sets after uploading data into NHSN. See the Generating Datasets Guide pdf icon[PDF – 400 KB] for more information.
Note: Each NHSN user has their own data sets. You may not see the same data as your coworkers if you generated data sets at different times.
Standardized Antimicrobial Administration Ratio (SAAR)
Q42: What is the SAAR and how is it calculated?
The Standardized Antimicrobial Administration Ratio (SAAR) is a metric developed by CDC to analyze and report summary antimicrobial use data. NHSN calculates the SAAR by dividing observed antimicrobial use by predicted antimicrobial use. More information on how NHSN calculates the SAAR can be found in the NHSN AUR Module Protocol pdf icon[PDF – 1 MB]. Additionally, SAAR training videos can be found under Training here: NHSN AUR Training.
Q43: My facility has a SAAR over 1. Is that bad?
A high SAAR that achieves statistical significance indicates that location used more antimicrobials than predicted. A SAAR that is not statistically different from 1.0 indicates antimicrobial use is equivalent to the referent population’s antimicrobial use. A low SAAR that achieves statistical significance indicates that location used fewer antimicrobials than predicted. However, the SAAR alone is not a definitive measure of the appropriateness or judiciousness of antibacterial use. Any SAAR may warrant further investigation. For example, a SAAR above 1.0 that does not achieve statistical significance may be associated with meaningful antimicrobial over-use and require further investigation. Keep in mind, a SAAR statistically different from 1.0 may still not lead to productive investigation.
Q44: What locations do the 2017 baseline adult and pediatric SAARs include?
NHSN only generates a 2017 baseline adult or pediatric Standardized Antimicrobial Administration Ratio (SAAR) for locations mapped using one of the following thirteen CDC location types:
- Adult Medical Critical Care
- Adult Medical-Surgical Critical Care
- Adult Surgical Critical Care
- Adult Medical Ward
- Adult Medical-Surgical Ward
- Adult Surgical Ward
- Adult Oncology General Hematology-Oncology Ward
- Adult Stepdown Unit
- Pediatric Medical Critical Care
- Pediatric Medical-Surgical Critical Care
- Pediatric Medical Ward
- Pediatric Medical-Surgical Ward
- Pediatric Surgical Ward
While NHSN encourages facilities to submit AU data from all NHSN-defined inpatient locations, facility-wide inpatient (FacWideIN), and select outpatient acute-care settings (specifically, Emergency Department, Pediatric Emergency Department and 24-hour Observation Area), the only locations that can generate SAARs are those mapped to the thirteen CDC locations listed above. In the future, as more facilities submit AU data, NHSN hopes to develop SAARs for additional location types.
You can still examine antimicrobial use in other locations using line lists, rate tables, or charts. See question #40 for more information on NHSN AU Option Analysis.
You can find more information about NHSN location mapping for the SAAR in Table 5 of the AUR Protocol pdf icon[PDF – 1 MB].
Q45: What locations do the 2018 baseline neonatal SAARs include?
NHSN only generates a 2018 baseline neonatal SAAR for locations mapped using one of the following four CDC location types:
- Step down Neonatal Nursery
- Neonatal Critical Care (Level II/III)
- Neonatal Critical Care (Level III)
- Neonatal Critical Care (Level IV)
See question #44 for more information about SAAR locations.
Q46: Can I generate a SAAR report for one location and one month?
You can generate a SAAR report by month, quarter, half year, year, or cumulative time periods. You can generate a SAAR report for a specific location or location type. NHSN provides quick reference guides about each SAAR report on the NHSN AUR Module website under Analysis Resources.
Q47: What years can I generate SAARs for?
NHSN currently offers three SAAR baselines, each applicable to a select set of patient care locations and time periods. See question #44 for information about the 2017 baseline adult and pediatric SAAR locations and question #45 for information about the 2018 baseline neonatal SAAR locations. You can generate 2014 baseline adult and pediatric SAARs for data between January 1, 2014 and December 31, 2018. You can generate 2017 baseline adult and pediatric SAARs for data from January 1, 2017 going forward. You can generate 2018 baseline neonatal SAARs for data from January 1, 2018 going forward. Please note: You cannot directly compare SAARs calculated under the 2014 baseline with 2017 baseline SAAR values.

To generate a SAAR by location and month, follow the steps in the SAAR Table—by location Quick Reference Guide. pdf icon[PDF – 500 KB].
Q48: I’m not submitting data into the AU Option, but I’d like to compare my antimicrobial use against other facilities using the SAAR. Is this possible?
Currently, NHSN only makes the SAAR available for facilities reporting into the AU Option. NHSN published a paper on the 2017 SAAR methodology and baseline model details for reference: NHSN SAARs: A Progress Report and Risk Modeling Update Using 2017 Dataexternal icon.
Keep in mind, facilities not participating in the NHSN AU Option must still capture the NHSN AU Option-defined antimicrobial days and days present accurately to calculate their own SAARs.
Q49: I uploaded AU data to NHSN, generated data sets, and ensured my location is one of the SAAR locations, but I cannot generate a SAAR report. What is going on?
You may have custom user rights preventing you from viewing the SAAR reports. NHSN uses the Patient Safety Annual Facility Survey for risk adjustment in the SAARs. Without access to the survey data, you cannot view the SAAR data. Review your user rights with your NHSN Facility Administrator and, once the Facility Administrator corrects your rights, generate new data sets. Refer to the guidance document pdf icon[PDF – 350 KB] that outlines minimum AU rights.
Q50: I checked my user rights, generated data sets, and ensured my location is one of the SAAR locations, but I still cannot generate a SAAR report. What is going on?
There are a few rare circumstances under which you cannot generate SAAR reports for certain locations.
All SAARs:
- Locations reporting zero days present for the selected time period.
- Locations reporting more antimicrobial days than days present for any SAAR agent category (except for the All Antibacterial Agents adult and pediatric SAARs).
- Locations with less than one predicted antimicrobial day.
Adult SAARs:
Adult SAAR locations in a long-term acute care (LTAC) hospital, orthopedic hospital, psychiatric hospital, or rehabilitation hospital. The 2017 SAAR baseline adult referent population does not include these facility types.
Pediatric SAARs:
Pediatric SAAR locations in a critical access hospital, LTAC hospital, oncology hospital, orthopedic hospital, pediatric LTAC hospital, psychiatric hospital, rehabilitation hospital, surgical hospital, women’s hospital, or VA hospital. The 2017 SAAR baseline pediatric referent population does not include these facility types.
Neonatal SAARs:
- Neonatal SAAR locations in a critical access hospital, LTAC hospital, oncology hospital, orthopedic hospital, pediatric LTAC hospital, psychiatric hospital, rehabilitation hospital, surgical hospital, or VA hospital. The 2018 SAAR baseline neonatal referent population does not include these facility types.
- Facilities missing relevant Patient Safety Annual Survey data or responds that the facility does not care for neonates.
- Facilities reporting zero inborn and zero outborn admissions.
If you tried everything in the FAQs above and still can’t generate a SAAR, please email the NHSN Helpdesk at nhsn@cdc.gov.