COVID-19 is an emerging, rapidly evolving situation.
Get the latest public health information from CDC: https://www.coronavirus.gov
Get the latest research information from NIH: https://www.nih.gov/coronavirus
NIH staff guidance on coronavirus (NIH Only)
You are here
Small Business Grant Timeline
Small Business Grant Timeline
It takes at least 9 months after submission for a successful application to receive an award. The steps of the application and review process are detailed below to give a sense of what takes place after an application is submitted. Please note that the duration between steps is approximate. Some applications will take longer than others to go through the process.
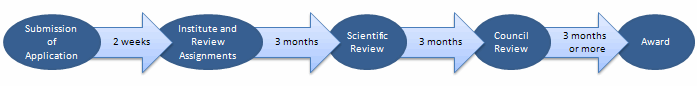
Time from application submission to institute assignment is on average 2 weeks, from institute assignment to scientific review is on average 3 months, from scientific review to council review is on average 3 months, and from council review to award is about 3 months or more.
Submission of Application – Applicants submit SBIR/STTR grant applications to NIH electronically through grants.gov by the submission deadline in the program announcement.
Institute and Review Assignments within NIH – The NIH Center for Scientific Review (CSR) assigns the application to at least one of the Institutes at NIH and to a scientific review group within 2 weeks of the submission deadline.
Scientific Review - The first level of review for NINDS small business applications takes place during the scientific review group meeting, which occurs approximately 3 months after submission. Each scored grant application is assigned a single, global score that reflects the overall impact that the project could have on the field (including commercial potential) and consideration of the five review criteria (significance, investigator(s), innovation, approach, environment). The score should appear in the Commons account within 3 business days after the review. The summary statement of the review should be available within 30 days.
Council Review – After scientific review, the applications reviewed internally by NINDS program staff. Those applications that are approved for funding are recommended to the National Advisory Neurological Disorders and Stroke (NANDS) Council. The NANDS council meets approximately 3 months after review and is made up of clinicians, research scientists, and individuals from patient advocacy groups. The recommendations of council are taken into consideration by program staff and the NINDS Director.
Award – Once NINDS program approves an application for funding, the applicant (or applicant institution) is contacted to discuss start data and funding level. NINDS will most likely request information from the applicant, including "Just in Time" information. Issuing the award is contingent on receipt of this information. When all the requirements have been completed, NINDS will send a Notice of Grant Award, detailing the funding information and the terms and conditions of the award. The absolute earliest award date is 2-3 months after council. Efforts are underway to reduce this timeline. See more details on award information.
Section 5126 of the SBIR/STTR Reauthorization Act of 2011 P.L. 112-81, signed on 12/31/2011, indicates that agencies “are encouraged to develop programs or measures to reduce the time periods between the close of an SBIR/STTR solicitation … and issuance of the Phase I and Phase II awards”. Additionally, the NIH Scientific Management Review Board (SMRB) developed recommendations for how NIH can optimize its utilization of the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs in ways that advance the NIH mission. Their final report, issued August 2013, made several recommendations; one of which was to “decrease delays between submission of the application and the disbursal of funds." As a result, HHS/NIH changed Standard Due Dates for SBIR/STTR grant applications, and is making other internal changes to allow the agency to inform SBIR/STTR grant applicants of the disposition of their applications sooner, and to make funding recommendations and awards sooner on average.










