The Fuel Cell Technologies Office's (FCTO's) applied materials-based hydrogen storage technology research, development, and demonstration (RD&D) activities focus on developing materials and systems that have the potential to meet U.S. Department of Energy (DOE) 2020 light-duty vehicle system targets with an overarching goal of meeting ultimate full-fleet, light-duty vehicle system targets.
Materials-based research is currently being pursued on metal hydride, chemical hydrogen storage, and sorbent materials.
- Metal hydride materials research focuses on improving the volumetric and gravimetric capacities, hydrogen adsorption/desorption kinetics, cycle life, and reaction thermodynamics of potential material candidates.
- Chemical hydrogen storage materials research focuses on improving volumetric and gravimetric capacity, improving transient performance, reducing release of volatile impurities, and developing efficient regeneration processes for the spent storage material.
- Sorbent materials research focuses on increasing effective adsorption temperature through increase of the dihydrogen binding energies and improving volumetric and gravimetric storage capacities through optimizing the material's pore size, increasing pore volume and surface area, and investigating effects of material densification.
A key component for advancing storage materials is the use of reliable material property measurement techniques. It is imperative to understand how the hydrogen storage properties of a material can be significantly influenced by not only individual sample characteristics—including chemical composition and distribution and microscopic and macroscopic material structure—but also pressure, temperature, and sample size. To help researchers better understand the proper measurement techniques, FCTO commissioned a best practices manual that gives a detailed overview of the recommended best practices in measuring the hydrogen storage properties of materials.
Technical Targets and Status
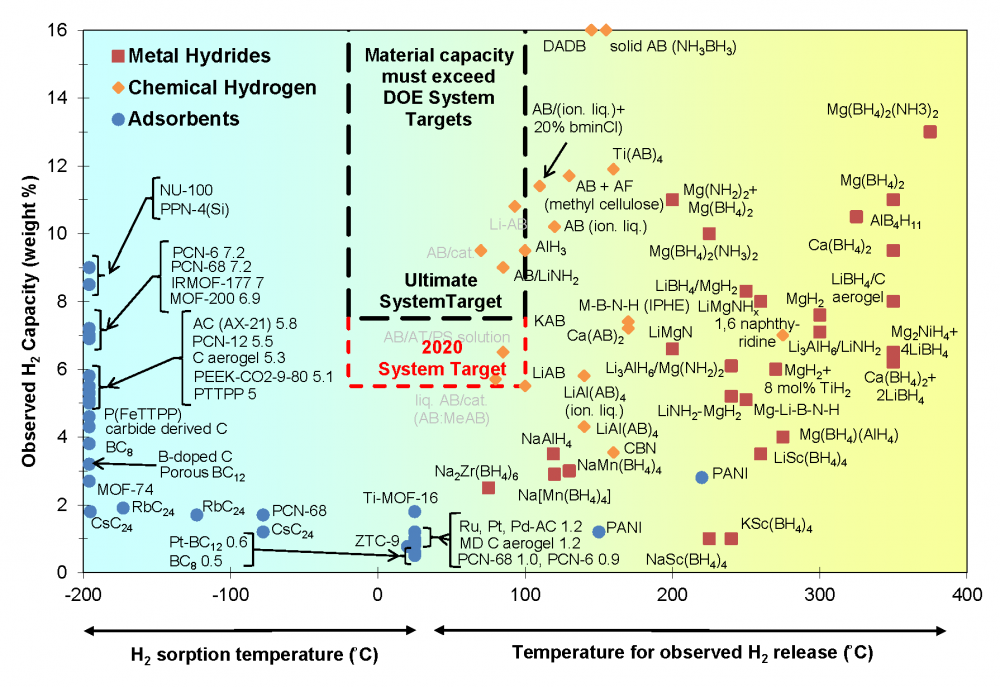
Materials-based research offers a long-term solution to the challenge of onboard automotive storage, as well as opportunities for stationary and portable power applications, with the potential to significantly reduce the required storage pressure, increase gravimetric and volumetric capacity, and reduce cost. From 2005 through 2010, the DOE Hydrogen Storage program supported three collaborative efforts—the Metal Hydride Center of Excellence, the Hydrogen Sorption Center of Excellence, and the Chemical Hydrogen Storage Center of Excellence—as well as independent projects that investigated more than 400 materials for potential use in hydrogen storage applications. Analysis activities in the Hydrogen Storage Engineering Center of Excellence (HSECoE) have determined the current status of systems using these materials. HSECoE has also developed spider charts showing three modeled systems for each material class and how they compare against all of DOE's 2020 targets.
Projected Performance and Cost of Materials-Based Automotive Hydrogen Storage Systems Compared to the 2020 and Ultimate DOE Targetsa
| Storage System Targets |
Gravimetric Density |
Volumetric Density kWh/L system (kg H2/L system) |
Cost $/kWh ($/kg H2) |
| 2020 | 1.8 (0.055) | 1.3 (0.040) | $10 ($333) |
| Ultimate | 2.5 (0.075) | 2.3 (0.070) | $8 ($266) |
| Current Status (from HSECoE) | Gravimetric Density kWh/kg system (kg H2/kg system) |
Volumetric Density kWh/L system (kg H2/L system) |
Costb $/kWh ($/kg H2) |
| Metal Hydride (MH): NaAlH4 | 0.4 (0.012) | 0.4 (0.012) | $43 ($1,430) |
| Sorbent: MOF-5, 100 bar, 80 K | 1.3 (0.038) | 0.7 (0.021) | $15 ($490) |
| Chemical Hydrogen (CH) Storage: Off-Board Regenerable (AB) | 1.5 (0.046) | 1.3 (0.040) | $17 ($550) |
| a Assumes a storage capacity of 5.6 kg of usable hydrogen. b MH reflects status at the end of phase I; CH and sorbent reflect status at the end of phase II. |
|||
The figure shows hydrogen gravimetric capacity as a function of hydrogen release temperature for many of the unique hydrogen storage materials investigated by FCTO.