Related SOP: Investigator-Initiated Clinical Trial Planning and Implementation Awards SOP
Credit: NIAID
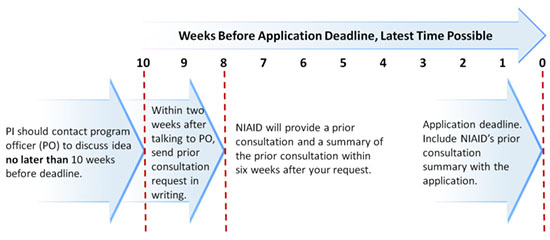
We strongly advise you to perform these steps well in advance of the dates listed.
- PI should contact program officer (PO) to discuss idea no later than 10 weeks before deadline. For the extended R01 opportunity, allow 12 weeks instead.
- Within two weeks after talking to PO, send prior consultation request in writing.
- NIAID will provide prior consultation and a summary within six weeks after your request.
- Include the NIAID prior consultation summary letter with the application.
For general information on investigator-initiated clinical trial planning and implementation awards, go to Investigator-Initiated Clinical Trial Resources.
Content last reviewed on September 25, 2015

