Table of Contents
See the main index of Decision Trees or Research Using Human Subjects
Graphical Flowchart
Credit: NIAID
Text Version of Flowchart
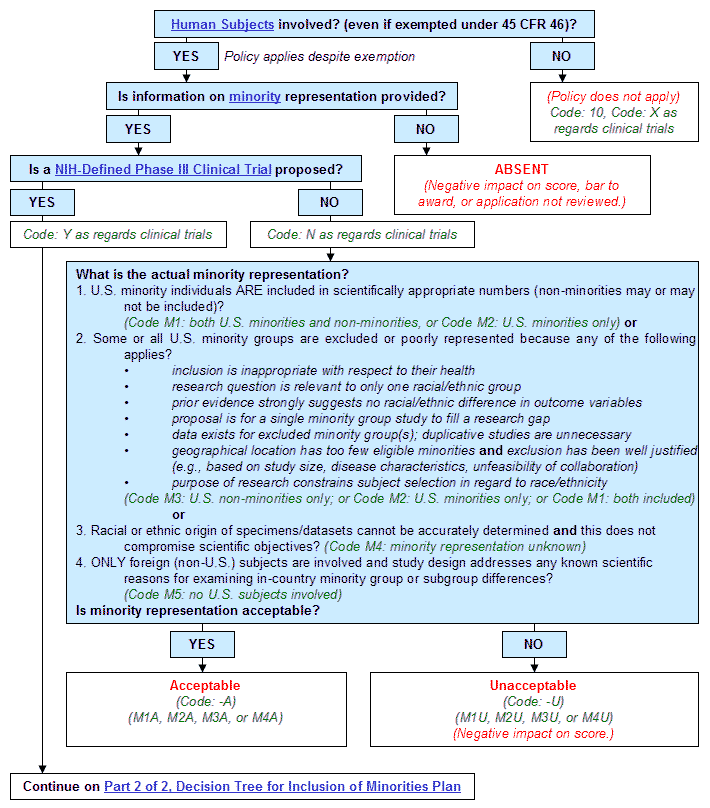
Step 1. Are Human Subjects involved (even if exempted under 45 CFR 46)?
- If yes, policy applies despite exemption. Continue to Step 2.
- If no:
- Policy does not apply.
- Code: 10, Code: X as regards clinical trials.
- End.
Step 2. Is information on minority representation provided?
- If yes, continue to Step 3.
- If no:
- Absent (Negative impact on score, bar to award, or application not reviewed.)
- End.
Step 3. Is a NIH-Defined Phase III Clinical Trial proposed?
- If yes:
- Code: Y as regards clinical trials.
- Continue on Part 2 of 2, Decision Tree for Inclusion of Minorities Plan.
- If no:
- Code: N as regards clinical trials.
- Continue to Step 4.
- What is the actual minority representation?
- U.S. minority individuals ARE included in scientifically appropriate numbers (non-minorities may or may not be included)? (Code M1: both U.S. minorities and non-minorities, or Code M2: U.S. minorities only) or
- Some or all U.S. minority groups are excluded or poorly represented because any of the following applies?
- inclusion is inappropriate with respect to their health
- research question is relevant to only one racial/ethnic group
- prior evidence strongly suggests no racial/ethnic difference in outcome variables
- proposal is for a single minority group study to fill a research gap
- data exists for excluded minority group(s); duplicative studies are unnecessary
- geographical location has too few eligible minorities and exclusion has been well justified (e.g., based on study size, disease characteristics, unfeasibility of collaboration)
- purpose of research constrains subject selection in regard to race/ethnicity (Code M3: U.S. non-minorities only; or Code M2: U.S. minorities only; or Code M1: both included) or
- Racial or ethnic origin of specimens/datasets cannot be accurately determined and this does not compromise scientific objectives? (Code M4: minority representation unknown)
- Only foreign (non-U.S.) subjects are involved and study design addresses any known scientific reasons for examining in-country minority group or subgroup differences? (Code M5: no U.S. subjects involved)
- Is minority representation acceptable?
Summary of Codes
| Minority Representation | Representation is scientifically... | |
|---|---|---|
| Acceptable | Unacceptable (bar to award) | |
| both minorities and non minorities are included | M1A | M1U |
| minorities only | M2A | M2U |
| non-minorities only | M3A | M3U |
| unknown (cannot be known) | M4A | M4U |
| only foreign (non-U.S subjects) in the study | M5A | M5U |
Clinical Trial Status
- X—Human subjects not involved (not clinical research)
- N—Clinical research, not an NIH-defined Phase III clinical trial
- Y—Clinical research, an NIH-defined Phase III clinical trial
Content last reviewed on April 19, 2016

