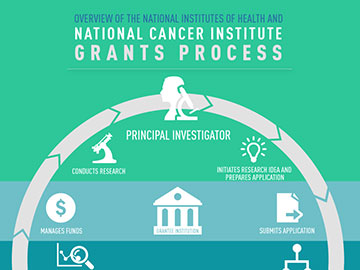
NCI Grants Process Quick Overview

The NCI grants process is designed to ensure that applications proposing the most promising scientific research projects are evaluated and awarded, and that the proposed scientific aims are completed. Here is some of the basic information you need to know when learning about or navigating this important process.
For additional history and references, download the PDF "The Grants Process, The Lifecycle of a Grant".
Definition of Roles
There are many individuals and teams involved in the NCI grants process. Learn more about the process in Application Development & Submission.
- Recipient: An organization or individual awarded a grant or cooperative agreement by the NCI that assumes legal, financial, and scientific responsibility and accountability for both the awarded funds and the performance of the grant- supported activity. Also known as awardee or grantee.
- Recipient Institution/Organization: Legally responsible and accountable to the NCI for the performance and financial aspects of the grant-supported activity.
- Institutional Business Official (BO): Person working in a research organization’s business office who has signature or other authority. That person is the same as the Grants.gov Authorized Organizational Representative (AOR) and the eRA Commons Signing Official (SO).
- Principal Investigator (PI): Individual designated by the recipient organization to direct the project or activity being supported by the grant and is responsible and accountable to recipient organization officials for the proper conduct of the project or program.
- NCI Program Directors/Program Officers: Individual responsible for the programmatic, scientific and/or technical oversight of a grant portfolio. This individual collaborates closely with Grants Management Specialists to provide oversight of the NCI grants program.
- NCI Grants Management Officer (GMO): Individual responsible for all business management aspects of grants and cooperative agreements, including review, negotiation, award, and administration.
- Grants Management Specialist: Individual selected by the GMO to oversee the business and other non-programmatic aspects of a portfolio of grants and cooperative agreements.
- Institute/Center (IC): The NIH organizational component responsible for a particular grant program or set of activities. The NCI is an IC.
Funding Types
The NCI supports cancer research that spans the continuum from basic science to clinical research to research on implementation, cancer control, and cancer care delivery through an extramural program of grants, cooperative agreements, and research and development contracts. Learn more in Research Funding Mechanisms.
- Grant: Provides federal financial assistance including money, property, or both to an eligible entity to perform approved scientific activities with little or no government involvement.
- Cooperative Agreement: A support mechanism where the NCI and extramural scientists/clinicians work together during performance of the research.
- Research and Development (R&D) Contract: Used to obtain or procure cancer research services and other resources needed by the federal government.
Application & Solicitation Types
There are nine grant application types that may be used to identify the stages in the lifecycle of a grant. The grant type defines the procedures and specifies the documents required to process the grant award.
- Type 1: New
- Type 2: Renewal (a.k.a. Competing Continuation)
- Type 3: Competing Revision /Administrative Supplement
- Type 4: Extension
- Type 5: Noncompeting Continuation
- Type 6: Successor-in-Interest and Name-Change Agreements
- Type 7: Change of Institution
- Type 8: Change of NIH awarding Institute or Center (for Type 5)
- Type 9: Change of NIH awarding Institute or Center (for Type 2)
The NCI can use three solicitation types to encourage grant application submissions through the publication of funding opportunity announcements (FOAs):
- Program Announcement (PA): A formal statement about a new or ongoing extramural activity or program
- Program Announcements Reviewed (PAR): Program Announcements with special receipt, referral, and/or review considerations
- Requests for Applications (RFAs): Issued to invite grant applications in a well-defined scientific area to accomplish specific IC program objectives
Learn more in Application Development and Submission.
Allowable Costs
Research grant funds are awarded to supplement or complement the support of research at an institution. Grant funds may be used for:
- Allowable direct costs specifically incurred in the conduct of the research project
- Facilities and administrative (F&A) costs (formerly known as indirect costs or overhead) resulting from an institution providing support services
Allowable Direct Costs
Allowable direct costs may include:
- Salaries and fringe benefits of the principal investigator, other key personnel, and supporting staff
- Expenditures for project-related equipment and supplies
- Fees and supporting costs for consultant services
- Expenses for travel beneficial to the research
- Research patient care costs
- Alterations and renovations
- Publications and other miscellaneous expenses
- Contract services
- Costs for consortium participants
Allowable Facilities and Administrative (F&A) Costs
In addition to direct costs, the HHS supports a policy of full reimbursement of F&A costs for most grant programs, with a few exceptions (e.g., training, fellowships, career programs, cancer education grants, and foreign grants). F&A costs are not readily identifiable with a particular project or activity but are necessary to the general operation of the institution and the conduct of its research activities.
Allowable F&A costs may include:
- Depreciation use allowance
- Facilities operations and maintenance
- General administration and general expense
- Departmental administration
- Sponsored project administration
- Libraries
The recipient institution assigns the costs to an F&A cost pool from which they are appropriately distributed to all organizational activities on the basis of a rate. The rate is a ratio of the F&A costs to a direct cost base. The amount awarded for F&A costs is determined by multiplying the rate by the allowable costs in the direct cost base for the project.
Rate Agreement
In order to receive reimbursement for F&A costs, the recipient institution must prepare an annual F&A cost rate proposal, which is submitted to the cognizant federal agency. The cognizant agency is generally the one that provides the largest amount of funds to a recipient over a specific period and thereby acts as a representative for all federal agencies dealing with a recipient’s common costs (e.g., F&A costs and fringe benefits). After review and negotiation of the F&A cost rate proposal, the cognizant agency establishes an accepted rate, formalized as the F&A cost rate agreement for that institution. This agreement is then made available to all other interested federal grantor agencies. The negotiated F&A cost rate is used to calculate the applicable amount of F&A costs for each award to the recipient institution.
The NIH Notice of Award (NoA)
The NoA includes both direct costs and applicable F&A costs, which are calculated by the grants management specialist. Typically, this award reflects the maximum total costs provided during the period of performance, even if a higher F&A rate is subsequently negotiated. If the amount required for F&A costs decreases because of either a new, lower negotiated rate or post-award budgetary changes in the direct costs of the grant, the excess F&A funds awarded generally may be re-budgeted to support allowable direct costs for the project, subject to specific requirements set forth in the Uniform Guidance (2 CFR 200).
Research Settings
NCI-sponsored research takes place in three settings:
- Laboratory: In the laboratory, research is pursued on the biology of cancer, the fundamental properties of cancer-causing agents and processes, and the body’s defense against and response to cancer.
- Clinic: In the clinic, patient-oriented research is carried out in prevention, detection, diagnosis, treatment, and rehabilitation.
- Community: In the community, research is carried out on the causes, risks, predispositions, incidence, and behavioral aspects of cancer within the population.
How to Submit a Grant Application
Grant Document Examples
Research Performance Progress Report (RPPR)
A RPPR is required at least annually as part of the non-competing continuation (Type 5) award process and it must be submitted through the eRA Commons. Only the PI or his/her delegate may initiate an RPPR in the eRA Commons. If there are multiple PIs, only the Contact PI (or his/her delegate) may initiate the report.
Grant Closeout
The grant closeout process is initiated as soon as grant support ends. Recipients are required to submit all closeout documentation using the eRA Commons no later than 120 days after the expiration of the project period or after the grant transfers to a new institution. If the recipient is delinquent submitting grant closeout reports, the NCI may initiate a Unilateral Closeout action. This is a serious action for the institution and can impact future funding.