Rathdrum Prairie Aquifer Water Quality
The population in the area over the Rathdrum Prairie Aquifer (RPA) is projected to potentially grow to 400,000 by the year 2060 (SPF et al, 2010). The increase in population could result in potential water quality impacts from the changing land use and urbanization. In addition, there has been an improved awareness of the interconnection of surface water and ground water along with water quality and water quantity issues. The result is increasingly complicated regulatory, political and legal issues that not only involve municipal, county and state agencies in Idaho but the equivalent involvement in Washington State.
In order to determine the current water quality and evaluate for the presence of any contaminants or use as a baseline for future water quality studies, DEQ completed a yearlong water quality investigation. The investigation included sampling numerous water wells completed in the RPA and along with the peripheral lakes. The sample locations can be seen below.
Water Quality Analytes
A number of different constituents were submitted for analysis that include; major cations and anions, nitrate-nitrogen and nitrate isotopes, arsenic, uranium, radionuclides, volatile organic compounds, semi-volatile organic compounds, deuterium and oxygen-18 isotopes along with age dating using chlorofluorocarbons and sulfur hexafluoride. General results of the investigation indicate that the drinking water quality is very good.
Nitrate-Nitrogen
Nitrate-nitrogen is an indicator of water quality impacts from surface activities such as septic effluent and fertilizer application. All the water samples obtained and analyzed had concentrations well below the drinking water standards of 10 milligrams per liter.

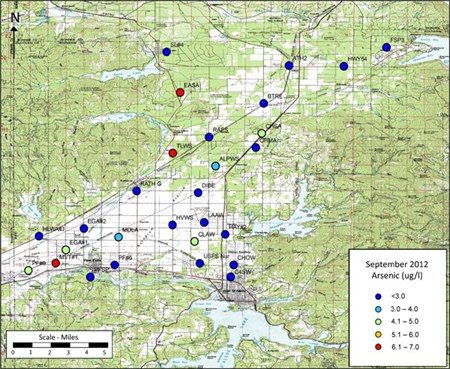
Arsenic and Uranium
Other constituents such as arsenic and uranium are naturally occurring, but can have elevated concentrations in the ground water if certain geochemical conditions are created through ground water pumping, injection of water for artificial recharge, or introduction of phosphorus. The arsenic analytical results indicated concentrations were below the allowed maximum contaminant level of 10 micrograms per liter in all wells.
The uranium analytical results also indicated concentrations were below the allowed maximum contaminant level of 30 micrograms per liter in all wells.

CFC's and SF6
During the past 50–60 years, different chemicals related to manufacturing processes and products have been released worldwide to the atmosphere such as isotopes of chlorofluorocarbons (CFCs) and sulfur hexafluoride (SF6). The average atmospheric concentrations in the northern hemisphere can be seen in the figure below. Each of the isotopes has specific time periods in which they were introduced in industrial applications and subsequent introduction into the atmosphere. CFCs are stable compounds developed in the 1930s and have been widely used in industrial applications as refrigerants, blowing agents and solvents. The three relevant isotopes of CFCs used in this study are CCl3F (CFC-11), CCl2F2 (CFC-12), and C2Cl3F3 (CFC-113). SF6 production became significant in early 1950s and was used predominantly in electrical switchgear.
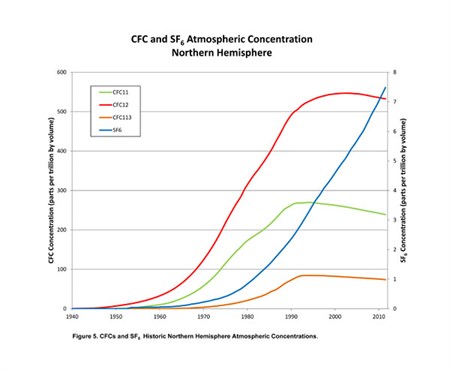
Water in contact with the atmosphere such as precipitation or surface water bodies will absorb these chemicals. The concentrations of these chemicals in the atmosphere have generally increased over time and will be reflected in the water concentration. When the water from precipitation or seepage from lakes and rivers recharges the aquifer and is no longer in contact with the atmosphere, the concentrations of CFCs and SF6 in ground water will no longer change and reflect the time of recharge. Both the CFCs and SF6 can be used for dating relatively young water generally less than 50 years.
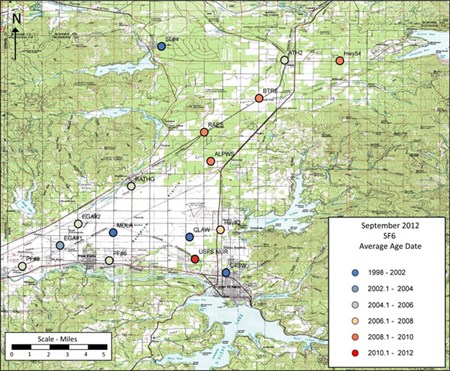
Age dating of the RPA ground water using chlorofluorocarbons (CFC-11, CFC-12, CFC-113) along with sulfur hexafluoride (SF6) showed mixed results. The chlorofluorocarbons indicated either very high concentrations indicating an external source (other than the atmosphere) or very low concentrations most likely reflecting chemical changes that take place when water is recharged through the thick unsaturated zone or from the peripheral lakes.
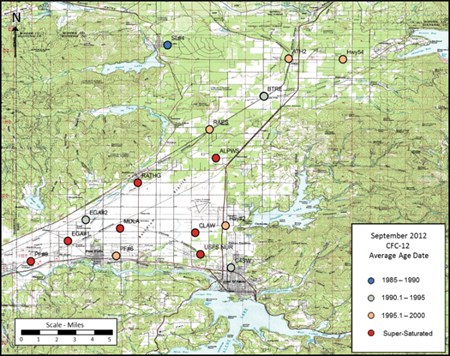
There are a number of external sources that could be the cause of the elevated CFC's in the aquifer. The three most likely sources for the Rathdrum Prairie Aquifer would be subsurface sewage disposal, landfills/dumps, and urban runoff.
The sulfur hexafluoride results are generally not affected by the same issues as the CFCs and indicate water with ages less than 15 years old.
Deuterium and Oxygen-18
Water has the chemical composition of H2O with the hydrogen component primarily composed of 1H (one-proton) and the oxygen component primarily composed of 16O (8-protons and 8-neutrons). A small fraction of water molecules will have either hydrogen composed of the 2H isotope (one proton and one-neutron) or oxygen composed of the 18O (8-protons and 10-neutrons) isotope. These isotopes do not decay and are considered stable.
The ratio of 2H and 18O in a water sample is dependent on a number of conditions relating to the history and source of the water. Generally, precipitation and ground water will have 2H/ 18O concentration ratios that will plot on a single line called the global meteoric water line (GMWL). The plotted location of the ratios will change position on the line dependent on various factors such the seasons and distance from the ocean.
The ground water samples obtained from the Rathdrum Prairie Aquifer indicated the source or sources had undergone some evaporation. The 2H and 18O concentrations in the January ground water samples indicate that the source or sources had undergone less evaporation.
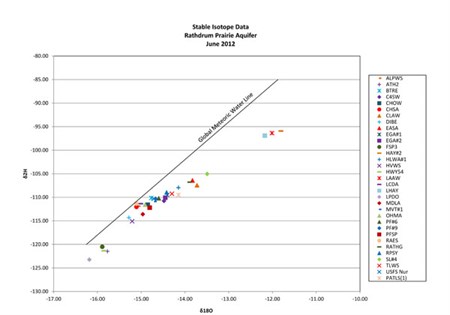
Water that has been subject to significant evaporation, such as lakes and lagoons, will show depletion in the 2H isotope and enrichment in the 18O isotope due to their molecular weight. The lighter 2H isotope will evaporate at a greater rate than the heavier 18O isotope. Waters derived from lakes and lagoons will plot on a line that deviates from the GMWL reflecting the ratios caused by evaporation. Surface water bodies such as lakes, ponds and lagoons will have ratios of these isotopes that are characteristic and maybe seen in ground water that receives significant recharge from these sources. Stable isotopes of 2H and 18O can be used to determine recharge sources, water budgets and infiltration rates particularly with surface water bodies.
The 2H and 18O analytical results from many of the ground water wells reflect ratios of the two isotopes that are very similar to the upgradient surface water bodies indicating significant recharge from the lake. The ground water wells have been grouped according to the surface water body that is providing recharge to the wells.

The wells that have significant recharge from the upgradient lakes are shown below.

For more information on the hydrogeochemical study, see the "Hydrogeochemical Investigation of the Rathdrum Prairie Aquifer" report on the Reports and Publications page.
The Panhandle Health District (PHD) has been collecting water quality information from a number of wells completed in the RPA since 1975. The water quality data consist of a variety of inorganic and organic constituents that represent general aquifer ground water quality, along with some that may indicate contamination from the surface. The emphasis of the PHD sampling program has been to detect potential water quality impacts from septic systems located over the RPA. The PHD Water Quality Database includes 33 wells distributed across the RPA. Water samples are taken from selected wells three times a year to evaluate general trends in water quality.

