Expert position paper on air pollution and cardiovascular disease
Introduction
Air pollution has wide-ranging and deleterious effects on human health and is a major issue for the global community. The Global Burden of Disease study has described the worldwide impact of air pollution with as many as 3.1 million of 52.8 million all-cause and all-age deaths being attributable to ambient air pollution in the year 2010.1 Moreover, ambient air pollution ranked ninth among the modifiable disease risk factors, being listed above other commonly recognized factors, such as low physical activity, a high-sodium diet, high cholesterol, and drug use. Finally, air pollution accounts for 3.1% of global disability-adjusted life years, an index that measures the time spent in states of reduced health.1
Although it is intuitive that air pollution is an important stimulus for the development and exacerbation of respiratory diseases, such as asthma, chronic obstructive pulmonary disease, and lung cancer, there is generally less public awareness of its substantial impact on cardiovascular disease. Historically, the 1952 Great Smog of London led to an increase in cardiovascular death as well as deaths due to respiratory disease. Subsequent studies in the 1990s, such as the Harvard Six Cities2 and American Cancer Society cohort studies,2,3 established an enduring positive association between long-term exposure to air pollution and total and cardiovascular mortality, mainly due to coronary artery disease.4 In Europe, the first study that supported this association between long-term exposure and mortality was the Netherlands Cohort Study on Diet and Cancer.5 Associations with cardiovascular morbidity and mortality are also seen with short-term (e.g. day-to-day fluctuations) pollutant exposures of residents in large urban areas worldwide, including the United States of America6 and Europe.7,8 Among multiple pathways linking air pollution to cardiovascular morbidity and mortality, the most relevant are the induction of oxidative stress, systemic inflammation, endothelial dysfunction, atherothrombosis, and arrhythmogenesis.9
Here, we present an expert consensus document on behalf of the European Society of Cardiology that explores the mechanisms and relationships between ambient air pollution and cardiovascular disease. The purpose of this document is to highlight and raise awareness of the importance and wide-ranging impact of air pollution on cardiovascular disease. It will also provide guidance to society, patients, and healthcare professionals on the potential health impacts of air pollution, and make recommendations for future public health and research priorities to manage and mitigate this avoidable cause of death and disease.
The main air pollutants
Outdoor air pollution is a complex mixture of thousands of components. From a health perspective, important components of this mixture include airborne particulate matter (PM) and the gaseous pollutants ozone, nitrogen dioxide (NO2), volatile organic compounds (including benzene), carbon monoxide (CO), and sulphur dioxide (SO2).9,10 Primary pollutants, such as soot particles, and oxides of nitrogen and sulphur, are emitted directly into the air by combustion of fossil fuels. Major sources of NO2 are motorized road traffic, power generation, industrial sources, and residential heating. Secondary pollutants are formed in the atmosphere from other components. An important example is ozone, which is formed through complex photochemical reactions of nitrogen oxides and volatile organic components.
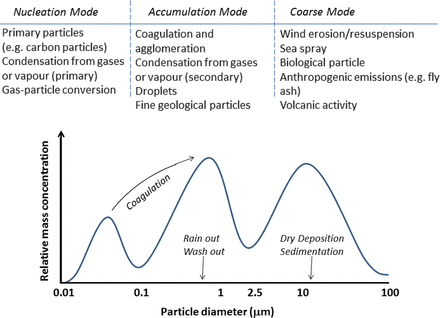
Particulate matter consists of particles from a wide variety of sources that differ in size and composition. Particles are often classified into three major size groups (Figure 1): coarse particles (diameter <10 and ≥2.5 μm), fine particles (diameter <2.5 and ≥0.1 μm), and ultrafine particles (<0.1 μm). In regulations, and consequently most monitoring networks, particles are represented by the mass concentration of particles smaller than 2.5 μm (PM2.5) and 10 μm (PM10). PM10 thus includes the fine particles and coarse particle fraction with PM2.5 generally representing ∼50–70% of the total mass of PM10. Ultrafine particles are included in PM2.5 and PM10 as well, but typically contribute a negligible fraction to the mass, whereas they dominate particle numbers. Resuspension of soil and road dust by wind or moving vehicles, as well as construction work and industrial emissions, result in coarse particles (PM10). Important sources of other primary particles typically include motorized road traffic, power plants, and industrial and residential heating using oil, coal, or wood. These combustion processes result in fine particles (PM2.5) composed of elemental carbon, transition metals, complex organic molecules, sulphate, and nitrate, with the latter components being formed in the atmosphere from volatile organic compounds, SO2 and NO2. Fine particles can travel large distances (>100 km) resulting in the potential for high background concentrations over a wide area. Outdoor air pollution varies in time and space. The European Environment Agency presents regular assessments of spatial and temporal variation of outdoor air pollution based on routine monitoring networks across Europe (http://www.eea.europa.eu/themes/air). Air pollution shows substantial variability both between areas (higher in Southern Europe) and within areas. Spatial variation is mostly related to the presence of local and regional scale sources. PM2.5 concentrations at traffic sites were on average 14% higher than at urban background locations in a large Eurpoean study.12 Larger urban-rural differences are found for soot (average 38% higher), NO2 (63% higher), and ultrafine particle numbers.
Origin and characteristics of particulate matter. Adapted from Heil.11
Temporal variation of daily average air pollution concentrations is mostly related to weather conditions affecting the dispersion of pollution and less so to variations in the strength of pollutant sources. Important factors include wind direction, wind speed and atmospheric stability. Air pollution concentrations also vary within a day. Temperature and sunlight affect chemical reaction rates such as ozone formation. Ozone concentrations are highest during the warmest, high-intensity sunlight hours of the day, often showing a broad peak from noon to about 9 pm when many people are outdoors, resulting in significant human exposure. Traffic-related pollutants, such as ultrafine particles and soot, often peak during the morning and evening rush hours, resulting in high exposures for people commuting. A study in a medium-sized city in the Netherlands found that concentrations of ultrafine particles and black carbon in transport areas more than doubled between 8 and 10 am compared with simultaneously measured concentrations at an urban background location.13
Although people in Western societies spend about 90% of their time indoors, predominantly in their own homes, outdoor air pollution (especially PM2.5) infiltrates buildings and most of the exposure typically occurs indoors. Even though household pollution is an especially prominent problem in low-income countries where solid fuels are used for cooking and heating, indoor air quality in homes, schools, working places, and other community sites is not a trivial problem in Europe. For instance, the increasing use of biomass burning to heat homes is creating major air quality problems in many Northern European cities. Concerns have also been raised about the emerging contribution of synthetically engineered nanoparticles to indoors air pollution exposure. The INDEX project, performed on behalf of the European Union, emphasized the negative role on health played by environmental tobacco smoke, organic compounds (polycyclic aromatic hydrocarbons, volatile organic substances, formaldehyde, naphthalene), carbon monoxide and benzoapyrene.14 Consequently, the World Health Organisation (WHO) and the European Commission have drafted guidelines for indoor air quality risk assessment and management (WHO 2010; European Commission 2010).15,16
Air pollution and mortality
In this section, we briefly summarize effects on mortality from all causes and the broad group of cardiovascular diseases combined. More specific cardiovascular diseases are discussed in more detail in the subsequent sections.
Short-term effects on mortality
Many single-city and multicity studies summarized in meta-analyses report an increased mortality risk associated with short-term exposure to PM, NO2, and ozone. The average percentage increase of all-cause mortality for an increment of 10 µg/m3 in short-term exposure to PM2.5 was 1.0% with substantial regional variation worldwide.17 Increases in mortality due to respiratory disease (+1.5%) and cardiovascular disease (+0.8%) were found.17 Somewhat weaker associations are observed in East Asian countries, but the overall health effects are much larger due to very high air pollution levels.18,19 In a major multi-city study combining data from Europe, the United States, and Canada, a 10 µg/m3 increase in PM10 was associated with 0.2–0.6% increases in all-cause mortality with similar effect size in the US and Europe and larger effects in Canada.20 In the Air Pollution and Health: a European Approach (APHEA) project,7 NO2 was associated with 0.3, 0.4, and 0.4% increases in total, cardiovascular, and respiratory mortality rates, respectively, per 10 µg/m3 increase in NO2 across 30 European cities.21 The APHEA study found higher effects of PM10 on daily mortality in cities with a higher temperature and a larger contribution of traffic emissions to PM.
Long-term effects on mortality
Long-term effects of air pollution on mortality have mostly been associated with ambient PM2.5 concentrations. A 2010 statement from the American Heart Association showed generally larger increases in all-cause mortality related to long-term PM2.5 exposure than for short-term exposure.22 A 2013 review reported a pooled effect of 6% (95% confidence interval 4–8%) for all-cause and 11% (95% confidence intervals 6–16%) for cardiovascular mortality for a 10 µg/m3 PM2.5 increase.23 Another recent review showed an association between all-cause mortality and long-term exposure to NO2 that was independent from PM2.5.24
In the European Study of Cohorts for Air Pollution Effects (ESCAPE) network of 22 European cohort studies (>300 000 subjects), PM2.5 effects on all-cause mortality were approximately two times larger than the previous estimates25 and associations persisted even among subjects with residential PM2.5 mean annual concentrations below 15 µg/m3. No statistically significant associations with cardiovascular mortality were found.25 The Improving Knowledge and Communication for Decision Making on Air Pollution and Health in Europe (APHEKOM) project of 25 European cities estimated that the benefit of compliance with the WHO guidelines for PM2.5 (annual mean 10 µg/m3) would add up to 22 months of life expectancy at age 30.26 The ESCAPE findings suggest that this benefit may be even greater.25
Air pollution and cardiovascular disease
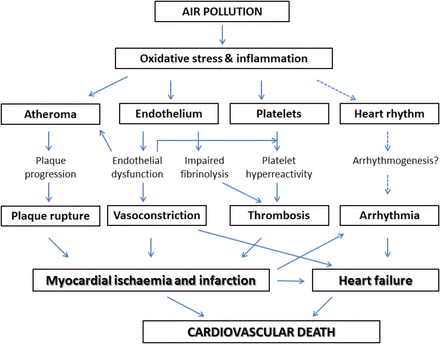
Numerous manifestations of cardiovascular disease have been associated with air pollution, involving both the arterial and venous circulations. Air pollution not only exacerbates existing heart conditions but also appears to have a role in the development of the disease (Figure 2), with particularly strong evidence for an adverse effect of PM compared with gaseous pollutants (Figure 3).
Established and unsettled clinical outcomes related to air pollution (gaseous and particulate).
Coronary artery disease
Although not a universal finding,28,29 the majority of cohort studies in diverse populations link long-term exposure to air pollution with an increased risk of incident fatal or non-fatal coronary artery disease.4,30–32 In the United States of America, an analysis of more than 65,000 post-menopausal women of the Women's Health Initiative Study showed a 21% (95% CI 4–42%) increased incidence of combined fatal and non-fatal coronary heart disease per increase of 10 µg/m3 in PM2.5.32 The ESCAPE study30 found a 12% (1–25%) increased risk per 10 µg/m3 in PM10 and a 13% (−2–30%) increased risk of coronary events per 5 µg/m3 increase in PM2.5 in more than 100 000 participants from 11 cohorts across Europe. Most importantly, positive associations were also observed below the current recommended annual European limit for PM2.5 and PM10.30
Several studies have examined associations with subclinical atherosclerosis, the underlying pathology of most coronary events. Cross-sectional studies of the carotid intima–media thickness and of coronary artery or aortic calcification have found positive associations of long-term air pollution with subclinical atherosclerosis (Table 1). Variable associations have been seen with peripheral arterial disease.33–35 More robust evidence from longitudinal studies investigating the progression of subclinical atherosclerosis is slowly accumulating (Table 1), lending strong support to the hypothesis that chronic exposure to air pollution influences the development of atherosclerosis and increases the risk for coronary artery disease.
Studies of the association between air pollution exposure and atherosclerosis
| Location of study, author | Study design | Exposure metric(s) | Major findings |
|---|---|---|---|
| Los Angeles, USA Künzli 200536 | 798 healthy subjects >40 years old without diabetes or CVD participating in unrelated clinical trials. Cross-sectional association of CIMT with exposures | Mean ambient PM2.5 exposures estimated using 2000 levels, home address, and GIS modelling Outcome: change in IMT per 10 μg/m3 ↑ in PM2.5 (range 5.2–26.9 μg/m3) | Unadjusted 5.9 (95% CI 1.0–10.9, P = 0.018) ↑ in IMT Non-significant trend for 4.4 (95% CI 0.0–9.0, P = 0.056) ↑ in IMT (adjusted for age, sex, income) Larger and more significant effects seen in subgroups of women, those older than 60 years, and subjects taking lipid lowering medications. |
| Ruhr Area, Germany Hoffmann 200737 | 4494 subjects in a population-based cohort (age 45–75 years) from the German Heinz Nixdorf Recall Study Cross-sectional association of CAC with exposures | Mean ambient PM2.5 exposures using 2002 levels, home addresses, and GIS modelling Distance between residence and major road used as a second exposure metric to traffic-related pollution Mean PM2.5 22.8 ± 1.5 μg/m3 | Trend for 17.2% ↑ in CAC (95% CI −5.6–45.5) per interdecile range (3.91 μg/m3) of PM2.5 in fully adjusted models controlling for other risk factors Significant increase in CAC of 7.0 (95% CI 0.1–14.4) per reduction in distance to major road by 50% Adjusted OR 1.45 (95% CI 1.15–1.82) for CAC >75th % for age for those living within 100 m of major roadway Stronger associations in men, younger, and less-educated subjects. |
| USA (multiple locations) Diez Roux 200838 | 5172 adults without CV disease in the MESA study. Cross-sectional associations of exposure to IMT, ABI, and CAC | Imputed prior 20-year mean ambient PM2.5 exposure levels by residential history and spatio-temporal models PM2.5 range: 12.8–24.1 μg/m3 | CIMT increased by 1 (95% CI 0–2%) for a 10–90th percentile increase in PM2.5 (12.5 μg/m3) after controlling for demographics and risk factors. CIMT increased 3 (95% CI 0–5%) per 12.5 μg/m3 increase in PM2.5 using year 2001 mean levels ABI and CAC were not related to metrics of PM2.5 exposures. |
| Ruhr Area, Germany Hoffmann 200934 | 4348 subjects in a population-based cohort (age 45–75 years) from the German Heinz Nixdorf Recall Study Cross-sectional associations of exposures to ABI and prevalence of peripheral arterial disease | Mean PM2.5 exposures estimated using year 2002 levels, home addresses, and GIS PM2.5 range: 19.8–26.9 μg/m3 Distance between residence and major road used as a second exposure metric to traffic-related pollution Mean PM2.5 levels 22.8 ± 1.5 μg/m3 | PM2.5 exposures were not related to ABI Living within 50 m of roadway after adjusting for other risk factors was associated with a 0.024 (95% CI 0.047–0.001) reduction in ABI. The OR for having an ABI <0.9 or treatment for peripheral vascular disease was 1.77 (95% CI 1.01–2.1) for individuals living within 50 m of a roadway compared with those living >200 m away. Greater effects were seen in women with no consistent associations observed in men |
| USA, multiple locations Allen 200939 | 1147 adults (age 45–84 years) without CV disease in the MESA study. Cross-sectional associations of exposures to AAC | PM2.5 exposures based upon average levels over 2 years (2000–2002). Subjects considered traffic exposed if they resided within 100 m of highway or 50 m of major roadway. PM2.5 range: 10.9 ± 0.1 to 22.8 ± 0.9 μg/m3 | Non-significant 6% (95% CI 0.96–1.16) increase in risk for presence of AAC per 10 μg/m3 increase in PM2.5. Risks for presence of AAC were stronger in those living near a monitor (RR 1.11; 95% CI 1.00–1.24) and those not employed outside home (RR 1.10; 95% CI 1.00–1.22) per 10 μg/m3 increase in PM2.5. Non-significant association with traffic exposures |
| Ruhr Area, Germany Bauer 201040 | 4814 subjects in a population-based cohort (age 45–75 years) from the German Heinz Nixdorf Recall Study Cross-sectional associations of exposure to CIMT | PM2.5 and PM10 using chemistry transport model (European Air Pollution Dispersion) with input data from emission inventories, meteorology, and regional topography. Values assigned to address. Range of PM2.5: 13.4–22.4 mg/m3 | An interdecile range increase in PM2.5 (4.2 μg/3), PM10 (6.7 μg/m3, and distance to high traffic (1939 m) associated with a 4.3 [95% confidence interval (CI): 1.9–6.7%], 1.7 (95% CI: −0.7–4.1%), and 1.2 (95% CI: −0.2–2.6%) increase in CIMT, respectively. The association was stronger in younger, obese subjects and in statin users |
| Los Angeles, USA Künzli 201041 | 1483 subjects in five treatment trials related to IMT in the Los Angeles area. Age range: 52.6 ± 8.9–63.7 ± 6.5 years. Annual rate of change of CIMT measured over 1.8 (0.4–2.4)– 3.3 (0.5–5.1) years. Longitudinal association of CIMT progression with PM2.5 and proximity to roadway | Mean ambient PM2.5 exposures in year 2000 using zip codes and GIS modelling Living <100 m to highway (n = 1.6% of all subjects) PM2.5 range: 20.12 ± 2.82–21.87 ± 1.1. | Non-significant 2.53 μm (95% CI −0.31–5.38, P = 0.081) increase in IMT progression rate per year per 10 μg/m3 elevation PM2.5 exposure in main model accounting for multiple risk factors. Significant 5.46 μm (95% CI 0.13–10.79, P = 0.044) increase in IMT progression per year for subjects living within 100 m of a highway. This is twice the average annual rate of IMT progression. Larger and significant effects observed in those with low socioeconomic status among those living <100 m from a highway. |
| Greater London, UK Tonne 201242 | 2348 participants of the Whitehall II cohort of British civil servants who had CIMT measured between 2003 and 2005. Cross-sectional association of CIMT with PM10 and PM10 oxidative potential (OP) | Weekly PM10 and PM10 OP in year prior to scan Mean ambient PM2.5 exposures in year 2000 using zip codes and GIS modelling Weekly OP predicted using measurements of antioxidant-reduced glutathione | Significant 5% increase in CIMT with 5.2 μgm/m3(interquartile range) in PM10 (95% confidence interval = 1.9–8.3%) after covariate adjustment. The association for an interquartile range change in PM10*OP (1.5 m(−3)) was weaker: 1.2 (0.2–2.2%). |
| Greater Boston, USA Wilker 201343 | CIMT 350 subjects of the Normative Aging Study between 2004 and 2008 | 1-year average black carbon (BC) using spatiotemporal models Distance to a major roadway and traffic density within a 100-m buffer of residence during the year before the first CIMT measurement. Median predicted BC at baseline for each subject was 0.29 µg/m3, IQR equivalent to 0.26 µg/m3 (25th to 75th quartile | A 0.26 µg/m3 (one IQR) increase in BC associated with a 1.1% higher CIMT (95% CI: 0.4, 1.7%) based on a fully adjusted model. Living <200 m associated with –1.4 (95% CI: –7.11, 4.6%) lower CIMT (95% CI: –5.7, 1.5%). Higher traffic density associated with higher CIMT |
| Multiple sites, USA Adar 201344 | 5660 subjects of MESA between 2000 and 2005 Cross-sectional and longitudinal associations of CIMT and its progression with PM2.5, respectively | PM2.5 estimated in year preceding and between CIMT measurements using spatio-temporal models. | A 2.5 µg/m3 higher level of residential PM2.5 during the follow-up period associated with 5.0 µm/year (95% CI 2.6–7.4 µm/year) greater IMT progression among persons in the same metropolitan area. Reduction in PM2.5 over follow-up associated with slowed IMT progression (−2.8 µm/year [95% CI −1.6−3.9 µm/year] per 1 µg/m3 reduction). |
| Ruhr Area Kälsch 201345 | Thoracic Aortic Calcification (TAC) 4814 patients from the Heinz-Nixdorf Study Cross-sectional association between TAC and PM2.5 and road noise | Exposure to PM2.5 in the year prior to TAC using chemistry transport model (EURAD-CTM), Road traffic noise using façade levels from noise models as weighted 24 h mean noise (Lden) and night-time noise (Lnight). | PM2.5 and Lnight associated with TAC-burden of 18.1 (95% CI: 6.6–30.9%)/2.4 µg/m3 PM2.5 and 3.9 (95% CI 0.0; 8.0%)/5 dB(A) Lnight, respectively, in the full model and after mutual adjustment. No effect modification of PM2.5 association by Lnight or vice versa. Low correlation between PM2.5 and noise (r = 0.07–0.10) Increase of 20% in TAC/IQR of PM2.5 corresponded to ∼1 year of older vascular age in this cohort |
| Girona, Spain Rivera 201346 | 2780 participants in the REGICOR (Registre Gironí del Cor: the Gerona Heart Register) study Cross-sectional association between ABI and CIMT and exposures | Long-term residential NO2 exposure (10 years' time-weighted average based on land-use regression and. traffic intensity). Associations with IMT and ABI using linear regression and multinomial logistic regression, | Increased NO2, 5th–95th percentiles (25 µg/m3), traffic intensity (15 000 vehicles/day), and traffic load within 100 m (7 200 000 vehicle-m/day) associated with 0.56 (95% CI: −1.5, 2.6), 2.32 (95% CI: 0.48, 4.17), and 1.91 (95% CI: −0.24, 4.06) increase in IMT, respectively. No association with CIMT in adjusted models Exposures were positively associated with an ABI of >1.3, but not <0.9. Stronger association observed among those with a high level of education and in men ≥60 years. |
PM, particulate matter; CV, cardiovascular; CIMT, carotid intima–media thickness; CAC, coronary artery calcium; CI, confidence interval; IMT, intima–media thickness; OR, odds ratio; ABI, ankle–brachial index; AAC, abdominal aortic calcification; GIS, geographic information systems.
Heart failure
Heart failure is an escalating public health issue that affects more than 23 million people worldwide. It represents the end stage of many cardiac diseases, with an annual hospitalization rate of 2% and subsequent 1-year mortality of 30%. Heart failure ranks as the most frequent reason for hospitalization and rehospitalization in older people, accounting for 5% of all hospital discharge diagnoses. The triggers of acute cardiac decompensation, especially in susceptible individuals, are therefore a major public health concern.
An English national cohort study found that long-term exposure to PM and NO2 was associated with increased incidence of heart failure.28 A systematic review and meta-analysis47 of the current evidence of the association between air pollution and heart failure showed a positive association between short-term increases in gaseous components and PM with the risk of hospitalization or death from congestive heart failure (Table 2). Subjects with pre-existing chronic heart failure, hypertension, and arrhythmia were at highest risk. However, studies on long-term exposure to elevated levels of air pollution and incidence of chronic heart failure are still missing.
Short-term association between gaseous and particulate air pollutants and hospitalization or mortality related to heart failure
| Number of events | % Increase in risk (95% CI) | |
|---|---|---|
| Gaseous pollutants | ||
| Carbon monoxide (per 1 ppm) | 1 969 500 | 3.52 (2.52–4.54) |
| Sulphur dioxide (per 10 ppb) | 771 471 | 2.36 (1.35–3.38) |
| Nitrogen dioxide (per 10 ppb) | 916 668 | 1.70 (1.25–2.16) |
| Ozone (per 10 ppb) | 887 531 | 0.46 (−0.10–1.02) |
| Particulate pollutants | ||
| PM2·5 (per 10 μg/m3) | 1 520 099 | 2.12 (1.42–2.82) |
| PM10 (per 10 μg/m3) | 896 889 | 1.63 (1.20–2.07) |
Ppm, parts per million; Ppb, parts per billion. Modified from Shah et al.47
Cardiac arrhythmias and arrest
In controlled exposure studies of healthy volunteers, air pollution does not appear to have a direct effect on arrhythmias or the frequency of ventricular ectopic beats.48 The Environmental Protection Agency has reviewed studies on patients with implantable defibrillators (six studies, one in Europe) and found inconsistent evidence for an association between arrhythmia and air pollution.49 Subsequent studies have also failed to find evidence for an association.50 In contrast, there have been some associations reported between out-of-hospital cardiac arrest and air pollution, especially PM51 and, more recently, ozone.52 Overall the current evidence suggests that air pollution may have a weak direct effect on ventricular arrhythmogenesis, in distinction to its greater effect on risk of myocardial infarction and associated ventricular fibrillation and cardiac arrest.
Cerebrovascular disease
Time-series studies from Korea have demonstrated associations between air pollution and stroke mortality.53 Consistent with this, hospital admissions for stroke have been associated with PM in the United States of America54 and Denmark.55
The Women's Health Initiative study reported that stroke and death from cerebrovascular disease increased by 35% and death from cerebrovascular disease increased by 83% per 10 µg/m3 increase in long-term PM2.5 exposure.32 Similarly, cumulative exposure to both PM10 and NO2 over a period of 12 years was associated with increased cerebrovascular mortality in China.56 The ESCAPE study found a 19% (−12–62%) increased risk of stroke per 5 µg/m3 increase in PM2.5 in nearly 100 000 participants from 11 cohorts across Europe.57 Higher risk was especially seen in subjects above age 60 and in non-smokers and was consistently observed at PM2.5 concentrations below 25 µg/m3. All in all, there is need for systematic assessment of the evidence for the role of air pollution in cerebrovascular disease.
Venous thromboembolism
The possibility that particulate air pollution enhances blood coagulability and favours thrombogenesis by impairing the venous circulation has been observed in some, but not all, studies assessing short-term exposures.22 While there have been some reports of an association with deep vein thrombosis,58 prospective studies have found no association between the risk of venous thromboembolism and traffic exposure, road proximity, or PM concentrations,59 including in a large cohort of post-menopausal women on hormone replacement therapy.60 Hence, the relationship between chronic exposure to ambient air pollution and venous thromboembolism is uncertain.
Biological mechanisms
Evidence has emerged for numerous mechanisms linking air pollution and cardiovascular disease, many of which may contribute to the risk of atherothrombotic events.
Atherosclerosis
Several epidemiological studies have demonstrated a positive association between estimated long-term exposure to PM2.5 and the burden of atherosclerosis in humans using a variety of surrogates including carotid intima–media thickness, coronary artery and aortic calcium and ankle brachial indices (Table 2). Since most of the studies are cross-sectional in nature, these findings should be interpreted with caution due to the inherent limitations of this type of analysis and not all outcomes were related to the many different metrics of air pollution exposures explored. On the other hand, errors in long-term exposure estimation or assignment, even despite the usage of sophisticated methodologies, cause a bias towards the null hypothesis. Thus, a positive association between PM2.5 exposures and atherosclerotic burden may be important and support a biological relationship between air pollution exposure and atherosclerosis. Importantly, a reduction in PM2.5 levels is associated with a decrease in carotid intima–media thickness progression suggesting biologic plausibility.44
Concentrated ambient PM2.5 potentiates plaque burden and vascular dysfunction in murine models of atherosclerosis. Exposure is also associated with features of plaque vulnerability, including enhanced innate immune cells, increased reactive oxygen species generating pathways and tissue factor expression.61,62 Similarly, diesel exhaust exposure promotes inflammation within plaque, alterations in vasomotor tone, and pro-inflammatory mediators.63 The composition of air pollution is an important determinant of these responses.
Inflammation
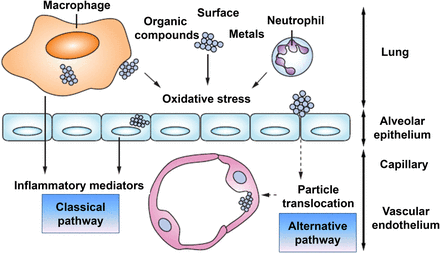
Ongoing exposure to air pollution provides a scenario where chronic low-level inflammation can arise in the lungs. During exposure to airborne pollutants, the normal phagocytes of the lung surfaces and, to an extent, the epithelial cells generate oxygen radicals and can become oxidatively stressed (Figure 4).9
Hypothetical pathways (classical and alternative) through which particulate matter determines cardiovascular effects. Reproduced with permission from Mills et al.9
Oxidative stress occurs when there is increased production of oxidizing species in cells and tissues, such as mediated by NADPH oxidase, or a significant decrease in the effectiveness of antioxidant defenses: air pollution causes oxidative stress via both of these pathways. This can be enhanced when the pollutant itself is highly oxidizing as in the case of ozone or PM2.5, which contain organic chemicals, transition metals, and high surface areas, all of which can contribute to local generation of reactive oxygen species. Oxidative stress is pro-inflammatory and many of the pro-inflammatory genes are oxidative stress-responsive. Conversely, inflammation is pro-oxidative, leading to a vicious cycle that results in high levels of oxidative stress. The extra inflammation arising from chronic exposure to air pollutants can be logically linked to the observed morbidity and mortality effects in a number of ways. The extra inflammation caused by the pollution may aggravate existing inflammatory lung disease such as chronic obstructive pulmonary disease and asthma, precipitating acute exacerbations. Systemic inflammatory effects of cytokines or oxidizing molecules emanating from the lungs may also affect atherosclerotic plaques, leading to their progression, destabilization, or rupture, precipitating acute coronary syndrome.64
Thrombosis
Short-term associations between PM exposure and cardiovascular mortality suggest a rapidly inducible effector pathway such as thrombogenicity. Exposure to traffic has been linked to triggering of myocardial infarction within hours.65,66 Experimental studies in a hamster model of arterial thrombosis have demonstrated activation of platelets within 30 min of intratracheal instillation of diesel exhaust particles.67 These findings agree with studies in healthy volunteers where inhalation of diluted diesel exhaust particulate increased thrombotic response under low- and high-shear, assessed by ex vivo flow chamber perfusion, and increased platelet-leucocyte aggregates.68 Rapid platelet sensitization results from direct contact of platelets with adsorbed diesel exhaust particulate constituents, after translocation of ultrafine PM from lung airspaces to the body. The rapid platelet activation observed in patients with diabetes briefly exposed to PM10 suggests that inhalation of polluted air activates primary haemostasis in those patients with an enhanced cardiovascular risk profile,69 including those with atherosclerotic plaques. In addition to platelet activation by translocated ultrafine PM, platelets are also sensitized by mediators released into the circulation as a result of PM-induced lung inflammation.67
The impact of air pollution on venous thrombogenicity is less evident. Experimental studies confirm the absence of venous thromboembolism and coagulation factor activation after exposure to diesel exhaust particles. On the other hand, observational studies in patients with diabetes mellitus or cardiovascular disease show some evidence for pro-thrombotic states.70 In addition, there is an association between long-term exposure and negatively charged microvesicles,71 a marker of activated coagulation associated with venous thromboembolism.72
Systemic vascular dysfunction
Observational studies suggest that exposure to air pollution may increase blood pressure, exacerbate myocardial ischaemia, and trigger myocardial infarction.9 Many of these effects may be mediated through direct or indirect effects on the systemic vasculature. Endothelial-dependent and -independent vasodilatation in peripheral resistance vessels is impaired early following controlled exposure to dilute diesel exhaust73 and can persist for up to 24 h.74 In patients with stable coronary artery disease, exercising while exposed to dilute diesel exhaust augmented myocardial ischaemia and impaired vascular fibrinolytic function.75 Concentrated ambient PM has a less consistent effect on the vasculature, likely reflecting heterogeneity in the composition of these exposures, but has been shown to cause acute arterial vasoconstriction76 and small increases in arterial blood pressure in healthy persons.77 PM is considered to be the primary mediator of systemic vascular dysfunction,78 with experimental studies suggesting oxidative stress and reduced bioavailability of nitric oxide likely to be key mechanisms.79,80
Most studies have addressed the short-term effects of exposure to air pollution through controlled exposures. However, recent observations from a large population based study suggest that long-term exposure to PM2.5 even at low concentrations in the US is associated with persistent endothelial dysfunction.81 Interestingly, reducing exposure to ambient PM in Beijing, China, where PM concentrations are 5–10 fold higher than in most European cities, lowered ambulatory blood pressure and reduced exercise-induced myocardial ischaemia.82
Mechanisms of heart failure
Increased systemic blood pressure and vasoconstriction due to short-term exposure to PM may lead to increased cardiac afterload and risk of acute decompensated heart failure. In addition, both pulmonary and right ventricular diastolic filling pressures are increased by exposure to ambient PM, suggesting a pulmonary vasoconstrictor effect of air pollution that could exacerbate congestive cardiac failure. Resulting arrhythmias could also contribute. In addition to loss of contractile capacity through myocardial infarction, inhalation of PM is associated with adverse ventricular remodelling and worsening of myocardial fibrosis.83
Epigenetic changes
Epigenetics focuses on the mechanisms of gene expression control that can be mitotically or meiotically stable, but do not directly depend on differences in DNA sequence. Air pollution affects multiple epigenetic mechanisms, as reflected in altered DNA methylation, histone modification, and microRNA expression. Because of their stability, environmentally induced epigenetic changes may accumulate over time, and persist even in the absence of factors that induced them, thereby determining deviations of health trajectories and risk of cardiovascular disease. Air pollution exposure correlates with altered blood DNA methylation profiles,84,85 which, in turn, have been shown to have the capacity to predict the 5-year incidence of, and mortality from, ischaemic heart disease and stroke.86 However, clinical evidence is still limited and faces several challenges, including use of surrogate tissues and mixed cell populations, cross-sectional designs susceptible to reverse causation, and focus on small numbers of candidate markers. Evidence in animals has suggested that some environmental pollutants cause epigenetic alterations that are transmitted for multiple generations.87 If these data were confirmed in humans, the epigenome of current generations, and potentially their health, could still be affected by historical exposures to air pollution, such as the high levels typical of industrial societies in the 1900s. This would have major repercussions since it would dramatically change our perception of the risks and heavily influence healthcare policymaking.
Interaction with traditional risk factors
A bi-directional relationship exists between air pollution and cardiovascular risk factors. Obese individuals and those with diabetes mellitus may be at higher risk of the cardiovascular effects of PM2.5.22 Concomitantly, air pollutants may acutely exacerbate and chronically instigate the development of several traditional risk factors.88,89
Concentrated PM2.5 and diesel exhaust exposures have both been implicated in acutely raising blood pressure. Numerous panel studies worldwide have also demonstrated linkages between ambient-levels of PM2.5, black carbon, and other pollutants with an elevation in blood pressure occurring within hours-to-days.88,90,91 The contributory role that these haemodynamic alterations play in mediating air pollution-associated acute cardiovascular events is unclear, although they are associated with prompting pregnancy-related blood pressure complications and emergency department attendances for hypertension.92,93 Perhaps most importantly, longer-term exposures to PM2.5 and traffic-related pollutants may promote the development of chronic hypertension per se.94
Air pollutants may impair insulin sensitivity.89 As such, long-term PM2.5 exposures may promote the development of overt diabetes mellitus potentially through systemic inflammatory responses. Whilst not all studies have shown an association, in light of the growing global epidemic of the metabolic syndrome, the public health implications that air pollution might be a ubiquitous environmental risk factor for hypertension or diabetes are enormous.
Air quality recommendations
The value of interventions targeted at reducing ambient air pollution is epitomized by a study carried out in 51 urban areas of the USA, showing that the long-term PM2.5 reduction of 10 µg/m3 is associated with an increased life expectancy of 0.61 years.95 In Europe, air quality standards have been set for the mass concentration of PM2.5 and PM10 (25 and 40 µg/m3 annual average, respectively) but there are currently no standards for PM0.1 or the chemical composition of PM. The above-mentioned APHEKOM project calculated that substantial gains in life expectancy would be achieved provided these cities decreased PM2.5 to the standards of the WHO, which recommends much lower PM2.5 and PM10 concentrations (10 and 20 µg/m3) than the European Union.26 Up to a third of Europeans living in urban areas are exposed to air pollutant levels exceeding EU air quality standards, but around 90% of them are exposed to levels deemed damaging to health according to the more stringent science-based WHO criteria. There is also convincing evidence showing that health effects are even observed below the current WHO guidelines.96
In Europe, 2013 was designated the ‘year of air’. The only significant action taken by the European Commission was the release in December of a new policy package to be implemented by the year 2030 that suggests measures to reduce harmful emissions from traffic, energy plants, and agriculture. According to the Commission, the yearly expenditure of €3.4 billion needed to achieve pollution abatement would result in 12-times higher savings of at least €40 billion per year, through decreased disease and healthcare costs, and increased working productivity. However, it is uncertain that this policy can be implemented in times of economic difficulties, particularly in the 17 countries that at the moment fail to meet the rather relaxed EU air quality standards.
Societal and personal advice
Reduction in personal and peer exposure to airborne pollutants can be achieved through simple measures such as:
travel by walking, cycling, and public transportation should be preferred to car or motorbike.97
avoid inefficient burning of biomass for domestic heating.
avoid walking and cycling in streets with high traffic intensity, particularly during rush hour traffic.
exercise in parks and gardens, but avoid major traffic roads.
limit time spent outdoors during highly polluted periods, especially infants, elderly, and those with cardiorespiratory disorders.
consider ventilation systems with filtration for homes in high-pollution areas.
In particular, individuals with or at high risk of cardiovascular disease should be advised of these measures to limit exposure to pollutants and also advised of the importance of compliance with primary or secondary prevention medication in order to combat the potential effects of air pollution exposure.
The awareness of outdoor air pollution and its adverse effect on life expectancy should not downplay the role of indoor air pollution. The Global Burden of Disease Study has estimated that household air pollution, such as occurs from biomass burning, accounts for over 3 million deaths worldwide and ranks fourth among the risk factors for all deaths.1 It should also be remembered that the burning of fossil fuels are not only a major source of air pollution but also the major source of greenhouse gases. Therefore, moving away from the use of fossil fuels for energy production will result in major benefits to human health, both from reduced exposure to air pollution and from mitigation of climate change. Individuals may have some choice over the source of their energy supply as well as the extent of their energy consumption and can apply political pressure through democratic means and lobbying to enact changes at regional and national levels that lead to reduction in air pollution exposure. Planning authorities could be encouraged or required to incentivize housing developments that are a reasonable distance from heavily congested roads and polluting industries, and to bear prevailing wind direction in mind. Patient organizations can provide a strong voice in the call for action at governmental level.
Conclusions and future research directions
There is now abundant evidence that air pollution contributes to the risk of cardiovascular disease and associated mortality, underpinned by credible evidence of multiple mechanisms that may drive this association. In light of this evidence, efforts to reduce exposure to air pollution should urgently be intensified, and supported by appropriate and effective legislation. Health professionals, including cardiologists, have an important role to play in supporting educational and policy initiatives as well as counselling their patients. Air pollution should be viewed as one of several major modifiable risk factors in the prevention and management of cardiovascular disease. Further research should explore the optimal methods of air pollution reduction and document the effects of this on the incidence of cardiovascular disease and related mortality in order to pressurize policy makers to intensify the efforts required for effective legislation on air pollution reduction.
Acknowledgements
We would like to acknowledge Dr Geriolda Topi for assistance with preparing the section on short-term mortality effects of air pollution.
Conflict of interest: O.H.F. works in ErasmusAGE, a centre for ageing research across the life course funded by Nestlé Nutrition (Nestec Ltd), Metagenics Inc., and AXA. Nestlé Nutrition (Nestec Ltd), Metagenics Inc., and AXA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. With regard to potential conflicts of interest, there is nothing to disclose. The other authors have no conflicts of interest related to this manuscript to disclose.
- Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2014. For permissions please email: journals.permissions@oup.com.