Abstract
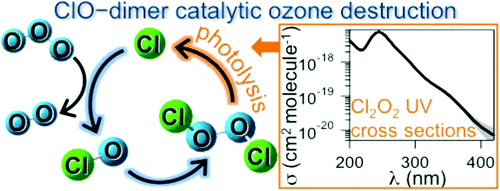
The UV photolysis of Cl2O2 (dichlorine peroxide) is a key step in the catalytic destruction of polar stratospheric ozone. In this study, the gas-phase UV absorption spectrum of Cl2O2 was measured using diode array spectroscopy and absolute cross sections, σ, are reported for the wavelength range 200−420 nm. Pulsed laser photolysis of Cl2O at 248 nm or Cl2/Cl2O mixtures at 351 nm at low temperature (200−228 K) and high pressure (∼700 Torr, He) was used to produce ClO radicals and subsequently Cl2O2 via the termolecular ClO self-reaction. The Cl2O2 spectrum was obtained from spectra recorded following the completion of the gas-phase ClO radical chemistry. The spectral analysis used observed isosbestic points at 271, 312.9, and 408.5 nm combined with reaction stoichiometry and chlorine mass balance to determine the Cl2O2 spectrum. The Cl2O2 UV absorption spectrum peaks at 244.5 nm with a cross section of 7.6−0.5+0.8 × 10−18 cm2 molecule−1 where the quoted error limits are 2σ and include estimated systematic errors. The Cl2O2 absorption cross sections obtained for wavelengths in the range 300−420 nm are in good agreement with the Cl2O2 spectrum reported previously by Burkholder et al. (J. Phys. Chem. A 1990, 94, 687) and significantly higher than the values reported by Pope et al. (J. Phys. Chem. A 2007, 111, 4322). A possible explanation for the discrepancy in the Cl2O2 cross section values with the Pope et al. study is discussed. Representative, atmospheric photolysis rate coefficients are calculated and a range of uncertainty estimated based on the determination of σCl2O2(λ) in this work. Although improvements in our fundamental understanding of the photochemistry of Cl2O2 are still desired, this work indicates that major revisions in current atmospheric chemical mechanisms are not required to simulate observed polar ozone depletion.
Supporting Information
Tabulated Cl2O2 absorption cross section data obtained in this work, parameterization of the wavelength-dependent estimated uncertainties in the Cl2O2 absorption cross section values, and simulations of isosbestic point behavior obtained using a chemical reaction mechanism. This material is available free of charge via the Internet at http://pubs.acs.org.









