Get the Lead Out!
Bioremediation research vies to eliminate subsurface heavy metal hazard
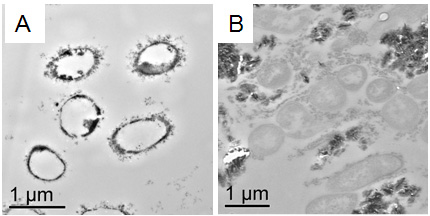
In Detox: Transmission electron microscopic images of D. desulfuricans G20 culture treated with lead (A) and without (B) soil minerals.
Heavy metal contamination of subsurface soils, groundwater and surface water is a global problem in water supplies and related ecosystem health. The Department of Energy (DOE) has long been a proponent in finding viable bioremediation solutions. In pursuit of this effort, scientists from the DOE's EMSL, South Dakota School of Mines and Technology and other universities conducted investigations of lead inhibition and detoxification in a model sulfate-reducing bacterium (SRB), D. desulfuricans G20, to improve understanding of the effects of heavy metals on SRB in the presence of soil minerals (goethite and quartz) and develop effective in situ or ex situ bioremediation technologies.
Studies of lead toxicity to sulfate-reducing bacteria are scarce with most using pure or mixed SRB in the absence of soil minerals and microbial growth media containing metal precipitants that reduce the effective soluble metal concentration available to targeted SRB. This study instead used a metal toxicity medium designed to eliminate the formation of metal precipitates and minimize metal complexation. EMSL instrumentation was essential in conducting this research. Transmission electron microscopy (see figure), energy-dispersive X-ray spectroscopy, selected area electron diffraction and X-ray diffraction analyses of thin sections of D. desulfuricans G20 treated with lead with and without the presence of soil minerals showed how the SRB interacted with lead, indentified molecular structures of secondary minerals formed during biotransformation and provided an understanding of lead detoxification in soil environments. For example, the figure shows that in the presence of soil minerals (B), the SRB detoxifies lead by sulfide formation (no lead inside the cells). However, in the absence of soil minerals (A), lead accumulates inside the cells. These results illustrate the effect of goethite and quartz in reducing lead toxicity to SRB since these soil minerals are capable of adsorbing large quantities of metals.
Scientific impact: The study demonstrated that the toxicity of lead to the SRB, D. desulfuricans G20, decreased in the presence of goethite and quartz. This study advances fundamental knowledge about SRB found in natural systems that contain lead, which will improve predictive modeling of metal immobilization processes and provide a better understanding of natural attenuation of metals or the design of active metal bioremediation strategies.
Societal impact: Lead is a major pollutant in nationwide soils, particularly among DOE sites. This study explored mechanisms of metal resistance in natural systems to determine ways to use SRB for in situ immobilization of lead. The research also impacts complex issues associated with the worldwide problem of metals in groundwater. As such, this research was actively used to educate students studying the fate and transport of toxic metals in aqueous environments about heavy metal toxicity and exploration of the metal resistance mechanisms in subsurface microbes.
Reference: Sani RK, G Rastogi, JG Moberly, A Dohnalkova, TR Ginn, N Spycher, RV Shende, and BM Peyton. 2010. "The Toxicity of Lead to Desulfovibrio Desulfuricans G20 in the Presence of Goethite and Quartz." Journal of Basic Microbiology 50(2):160-170. DOI: 10.1002/jobm.200900239.
Acknowledgment: This research was funded by the DOE's Subsurface Biogeochemical Research program, Office of Biological and Environmental Research (formerly the Environmental Remediation Science Program), as well as with grants from the National Science Foundation and South Dakota Governors 2010 seed grant program.
User Proposals: 3260 and 30457
Released: July 07, 2010

