Did you know?
Propane and butane were discovered in 1912 by Dr. Walter Snelling, a U.S. scientist. He identified these gases in gasoline, and he found that cooling and pressuring these gases changed them to liquid. He also learned that the liquefied gases could be stored and transported in pressurized containers.
Did you know?
HGL were initially considered a nuisance product but are now viewed as high-value products. Shortly before World War I, a problem in a natural gas pipeline occurred. A section of a pipeline in a natural gas field ran under a cold stream, and the low temperature caused liquids to form and sometimes block the flow of natural gas in the pipeline. This experience led engineers to treat natural gas before it entered natural gas transmission pipelines. Natural gas processing facilities were built to cool and compress natural gas, which separated the hydrocarbon gases as liquids from the natural gas. The HGL then became marketable commodities as fuels and as feedstock for making other petroleum products and petrochemicals.
What are hydrocarbon gas liquids?
Natural gas and crude oil are mixtures of different hydrocarbons. Hydrocarbons are molecules of carbon and hydrogen in various combinations. Hydrocarbon gas liquids (HGL) are hydrocarbons that occur as gases at atmospheric pressure and as liquids under higher pressures. HGL can also be liquefied by cooling. The specific pressures and temperatures at which the gases liquefy vary by the type of HGL. HGL may be described as being light or heavy according to the number of carbon atoms and hydrogen atoms in an HGL molecule.
HGL are categorized chemically as:
- Alkanes, or paraffins
- Ethane—C2H6
- Propane—C3H8
- Butanes: normal butane and isobutane—C4H10
- Natural gasoline or pentanes plus—C5H12 and heavier
- Alkenes, or olefins
- Ethylene—C2H4
- Propylene—C3H6
- Butylene and isobutylene—C4H8
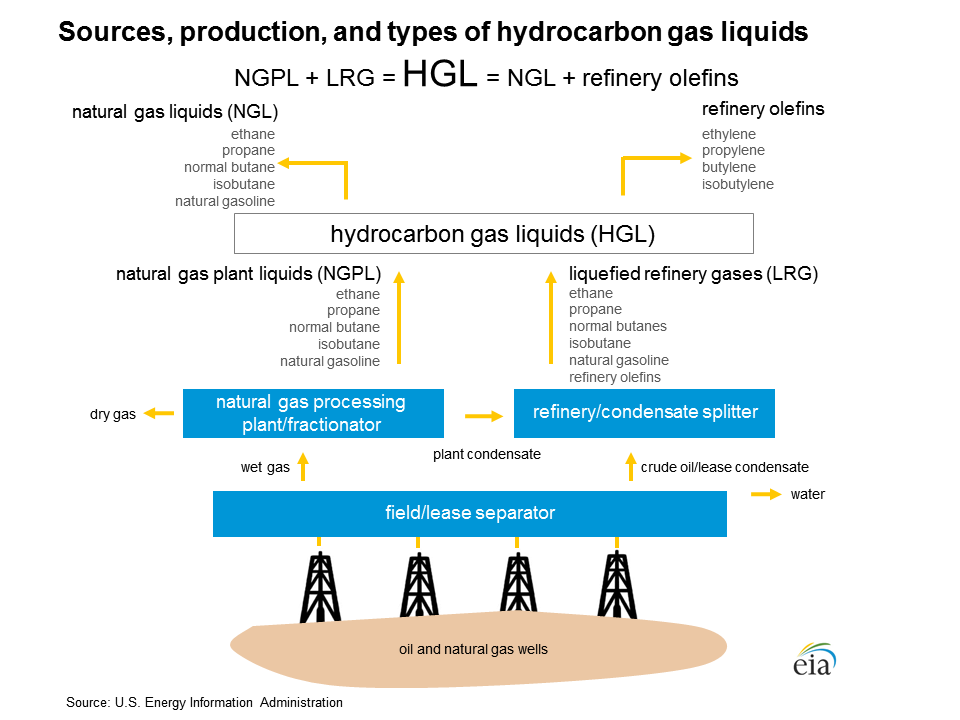
Hydrocarbon gas liquids are from natural gas and crude oil
HGL are found in raw natural gas and crude oil, and they are extracted when natural gas is processed at natural gas processing plants and when crude oil is refined into petroleum products. Natural gas plant liquids (NGPL), which account for most of HGL production in the United States, fall solely into the alkanes category. Refinery production accounts for the remainder of U.S. alkanes production, and it accounts for all of the olefins production data that are published by the U.S. Energy Information Administration (EIA). Greater volumes of olefins are produced at petrochemical plants from HGL and heavier feedstock. EIA does not collect or report petrochemical production data.
Hydrocarbon gas liquids have many uses
Because HGL straddle the gas/liquid boundary, their versatility and high energy density in liquid form make them useful for many purposes:
- Feedstock in petrochemical plants to make chemicals, plastics, and synthetic rubber
- Fuels for heating, cooking, and drying
- Fuels for transportation
- Additives for motor gasoline production
- Diluent (a diluting or thinning agent) for transportation of heavy crude oil
In 2014, total HGL use accounted for about 13% of total U.S. petroleum consumption.