Chemical & Materials Sciences Division
Research Highlights
October 2010
Who Killed the Graphite Anode?
PNNL researchers move silicon anode Li-ion battery technology forward
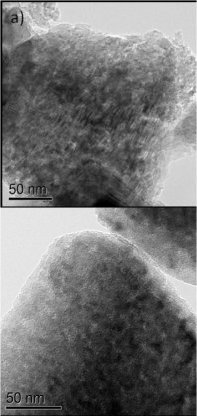
High-resolution transmission electron microscopic (TEM) images of silicon anode morphologies: a) original porous silicon and b) porous silicon coated with nano layer of carbon.
Results: Scientists at Pacific Northwest National Laboratory developed a lithium-ion (Li-ion) battery technology with reversible capacity of more than 1,600 milliamp hours per gram (mAh/g) after 40 charging/discharging cycles. This silicon-based anode technology doubles the capacity of conventional graphite anode technology used in Li-ion batteries, and may lead to Li-ion batteries with much higher energy density and capacity.
Why it matters: A future with millions of fossil-fuel powered cars displaced from American roadways by plug-in electric vehicles presents significant economic and environmental benefits. It also poses a challenge to develop a next-generation battery technology that offers far greater energy density. Silicon anodes for Li-ion batteries present a promising solution.
Today's electric vehicles rely on nickel-metal hydride batteries. They are heavy, bulky and have a specific energy that is too low, about 80 watt hours per kilogram (Wh/kg), for long-distance travel. Li-ion batteries, commonly used in handheld electronics, offer greater capacity. Composed of three main components—a graphite anode, a cathode and electrolyte (lithium salt dissolved in organic solvent)—the graphite anode has specific capacity of about 350 mAh/g. Li-ion batteries using graphite anodes exhibit a specific energy of more than 160 Wh/kg, double that of nickel-metal hydride batteries.
"If we want to increase driving distance of electric vehicles, we need to have much better capacity—at least double the capacity of graphite anodes and cathodes used in the Li-ion battery," said Dr. Jason Zhang, a PNNL scientist.
One of the limiting factors of the Li-ion battery is its anode—the graphite. Lithium is added to graphite when charging and removed as the battery is used. Graphite anodes are used in nearly all Li-ion batteries, but recent research has sought to capitalize on a better anode solution—silicon. With a theoretical capacity of more than 10 times that of graphite, silicon anodes can at least double the capacity of graphite-anode batteries. However, it is this very ability to absorb lithium and expand during charging that is the problem: The silicon breaks down quickly.
The challenge for PNNL researchers: Take advantage of silicon's high capacity while finding a way to keep it from deteriorating through repeated charging/discharging cycles.
Methods: Zhang and the PNNL research team addressed the challenge by designing a silicon particle architecture that would maintain structural integrity. Nanostructured porous silicon was used to maintain stability through repeated expansion and contraction. Next, chemical vapor deposition (CVD) of carbon coatings and highly elastic Ketjen Black (KB) carbon were used to improve the electrical conductivity throughout all cycling stages. The team placed these anodes between graphene—planar sheets of bonded carbon atoms—to maintain strong electrical contact between silicon particles.
They tested these anodes in the laboratory and found they had a reversible capacity of more than 1,600 mAh/g after 40 charging/discharging cycles. This technology more than doubles the capacity of conventional graphite anodes used in Li-ion batteries.
What's next? The PNNL research team continues to improve the performance and long-term stability of the silicon anodes from 40 to 50 charging/discharging cycles today to a goal of about 500 cycles in the future. One solution may be the development of a better binder that can maintain improved mechanical and electrical contact. This method has potential for much greater cyclability while maintaining high energy density.
Acknowledgments: This work is supported by the U.S. Department of Energy, Office of Vehicle Technologies![]() , and the Laboratory Directed Research and Development Program at Pacific Northwest National Laboratory.
, and the Laboratory Directed Research and Development Program at Pacific Northwest National Laboratory.
The research was conducted by Jie Xiao, Wu Xu, Deyu Wang, Daiwon Choi, Wei Wang, Xiaolin Li, Gordon L. Graff, Jun Liu, and Ji-Guang Zhang of Pacific Northwest National Laboratory.
Reference: Xiao J, W Xu, D Wang, D Choi, W Wang, X Li, GL Graff, J Liu, and J Zhang. 2010. "Stabilization of Silicon Anode for Li-Ion Batteries." Journal of The Electrochemical Society, 157(10):A1047-1051, doi: 10.1149/1.3464767.
