- Home
- Slides
- Home
- Tools and Resources
- Research Summaries for Consumers, Clinicians, and Policymakers
- Search for Research Summaries, Reviews, and Reports
- Research Available for Comment
- Submit a Suggestion for Research
- Submit Scientific Information Packets
- Comparative Effectiveness Research Grant and ARRA Awards
- News and Announcements
- What Is Comparative Effectiveness Research
- Who Is Involved in the Effective Health Care Program
- What Is the Effective Health Care Program
Slides
Slides: 1–12 of 56
Current Practice for Patients Undergoing PCI
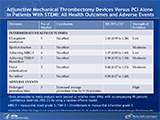
Adjunctive Mechanical Thrombectomy Devices Versus PCI Alone in Patients With STEMI: All Outcomes and Adverse Events
Comparative Effectiveness of Oral Agents: NSAIDs versus Other Agents
Presentation: Analgesics for Osteoarthritis—An Update
Keywords: comparative effectiveness | outcomes | strength of evidence | efficacy | NSAIDs | acetaminophen | glucosamine | chondroitin | pain
Knowledge Gaps and Future Research Needs (1 of 3)
Presentation: Analgesics for Osteoarthritis—An Update
Keywords: knowledge gaps | future research | CV risks | GI benefits | COX-2 selective NSAIDs
Knowledge Gaps and Future Research Needs (2 of 3)
Presentation: Analgesics for Osteoarthritis—An Update
Keywords: knowledge gaps | future research | COX-2 inhibitors | risks | CV safety | GI safety | nonselective NSAIDs | aspirin | salsalate | acetaminophen | alternative dosing
Applicability Resources
Presentation: Assessing Applicability
Other Applicability Resources
Presentation: Assessing Applicability
Keywords: applicability | population
How Individual Studies Consider Applicability
Presentation: Assessing Applicability
Keywords: applicability | transparency | efficacy trials | internal validity | outcomes
Applicability Judged for Each Question
Presentation: Assessing Applicability
Keywords: applicability | cohort study
Population and Applicability: Examples
Presentation: Assessing Applicability
Keywords: applicability | population | internal validity | exclusion criteria | inclusion criteria
Comparator and Applicability: Examples
Presentation: Assessing Applicability
Keywords: comparator | applicability
Your slide tray is being processed.


 E-mail Updates
E-mail Updates